Scroll to:
Features of central aortic pressure at patients with arterial hypertension aged 80 years and older, taking into account the presence of chronic heart failure and senile asthenia syndrome
https://doi.org/10.21886/2219-8075-2025-16-1-28-38
Abstract
Objective: to evaluate the parameters of central aortic pressure in patients with arterial hypertension (AH) depending on the presence of chronic heart failure (CHF) and senile asthenia syndrome (SSA). Materials and methods: 320 patients with AH were divided into four groups: group 1A — patients with AH, SSA and CHF (n=84), group 1B — patients with AH, SSA without CHF (n=84), group 2A — patients with AH, CHF without SSA (n=77), group 2B — patients with AH without CHF and without SSA (n=75). Central aortic pressure parameters were determined using a BPLab ABPM device using Vasotens technology (“Petr Telegin”, Nizhny Novgorod). To process the obtained data, statistical programs STATISTICA 12.0, SPSS 21.0, MedCalc 9.3.5.0 were used. Results: in patients with AH, CHF and SSA, higher average daily values of SBP ao were recorded compared to both patients with AH and CHF without SSA (p=0.004) and with AH, SSA without CHF (p=0.019). The presence of SSA led to higher AIx ao values both in patients with hypertension, SSA and CHF (p<0.001), and in patients with AH and SSA without CHF (p<0.001). In patients with AH and CHF, regardless of the presence of SSA, higher rates of PBP ao (p<0.001), AIx ao (p<0.001), ED (p<0.001) and lower rates of SERV (p<0.001) were recorded. When assessing the degree of influence of CHF or SSA, it was shown that CHF had a more pronounced effect on PBP ao (p<0.001), ED (p<0.001) and SERV (p<0.001) indicators than SSA. Conclusion: in patients with AH aged 80 years and older, the development of both SSA and, to a greater extent, the presence of CHF was accompanied by an increase in central aortic pressure. With a combination of AH, CHF and SSA, the most pronounced disturbances in the elastic properties of blood vessels were observed, which is associated with a high cardiovascular risk.
Keywords
For citations:
Safronenko V.A., Chesnikova A.I. Features of central aortic pressure at patients with arterial hypertension aged 80 years and older, taking into account the presence of chronic heart failure and senile asthenia syndrome. Medical Herald of the South of Russia. 2025;16(1):28-38. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-28-38
Introduction
Currently, the rate of population aging has increased significantly, and this trend is global. It should be noted that the number of cardiovascular diseases (CVD) increases with age. Age over 70 is the most important non-modifiable risk factor for CVD in older people [1]. In particular, elderly people make up the vast majority (about 80%) of patients with chronic heart failure (CHF), and the prevalence of arterial hypertension (AH) after the age of 80 is close to 90% [2]. A separate problem associated with older age is the clinical condition of frailty – senile asthenia syndrome (SAS). The accelerated loss of physiological reserves and frailty can lead to a wide range of health problems common among elderly patients, such as physical decline, reduced mobility and subsequent falls, decreased appetite, urinary incontinence, neuropathy, cognitive impairment, and anxiety-depressive disorders. In addition, elderly patients often have concomitant comorbidities, and the presence of SAS leads to the progression of the existing diseases [3-5].
AH and the elderly age of patients are closely related to central aortic pressure, which directly determines the load on target organs, changes in which are an independent predictor of cardiovascular complications [6][7]. It is well known that with age, central aortic pressure has a greater impact than peripheral pressure on the progression of left ventricular hypertrophy and the development of cardiovascular complications. In addition, the amplitude of the shock wave and blood pressure in the aorta directly depend on stroke volume and, conversely, on the heart rate. Accordingly, the development of CHF also leads to significant changes in central hemodynamic parameters [8][9].
Currently, there are no available data on the features of central aortic pressure in comorbid patients with AH, taking into account the presence of CHF and SAS.
The aim of the study was to evaluate central aortic pressure parameters in patients with hypertension depending on the presence of CHF and SAS.
Materials and Methods
The design and methods of this study are identical to those in our previously published work devoted to the study of vascular stiffness in patients with AH, CHF, and SAS in various combinations [10]. The study included 320 patients with AH (56.9% of women and 43.1% of men) aged 85.8±4.5 years. Patients were recruited during outpatient visits to municipal polyclinics in Rostov-on-Don.
The inclusion criteria were patients aged 80 years and older; the presence of hypertension, CHF stage IIA–IIB, and functional class (FC) II–IV. The exclusion criteria were an acute cerebrovascular event or transient ischemic attack within the last 6 months, history of ischemic heart disease, hemodynamically significant heart defects, an implanted pacemaker, severe liver (transaminase levels 5 times higher than normal) or kidney disease (glomerular filtration rate (GFR) ≤30 ml/min), and malignant neoplasms.
Depending on the presence of CHF and SAS, all patients were divided into four groups: Group 1A – patients with AH, SAS, and CHF (n=84), Group 1B – patients with AH and SAS, and without CHF (n=84), Group 2A – patients with AH and CHF, and without SAS (n=77), Group 2B – patients with AH, and without CHF and SAS (n=75).
The presence of AH was determined in accordance with national clinical guidelines [11] based on data from outpatient records, medical history, office blood pressure measurements, and 24-hour ambulatory blood pressure monitoring (ABPM).
The diagnosis of CHF was based on clinical symptoms and signs of heart failure, echocardiography data, and the level of the heart failure marker N-terminal pro-B-type natriuretic peptide (NT-proBNP) [12]. The severity of clinical signs of CHF was determined using the Clinical Status Assessment Scale (CSAS) (modified by V.Yu. Mareev, 2000), and exercise tolerance was assessed using the 6-minute walk test (6MWT).
The Age is No Barrier questionnaire, a brief set of physical functioning tests, dynamometry, and the Mini-Cog test were used for screening and diagnosis of SAS [13].
Central aortic pressure parameters were determined using a BPLab ABPM device and Vasotens technology (Petr Telegin LLC, Nizhny Novgorod). Monitoring lasted 24 hours and had to include at least 70% of successful measurements. The following central pressure parameters were assessed: SBP(Ao) – systolic blood pressure in the aorta, DBP(Ao) – diastolic blood pressure in the aorta, PBP(Ao) – pulse blood pressure in the aorta, AIX(Ao) – the augmentation index in the aorta, PPA – pulse pressure amplification, ED – the duration of ejection from the left ventricle (LV), SERV (subendocardial viability ratio) – the index of subendocardial blood flow efficiency.
Statistical analysis of the results was performed using STATISTICA 12.0 (StatSoft Inc., USA), SPSS 21.0, and MedCalc (version 9.3.5.0) software packages. The size of the representative sample characterizing the general population in terms of the prevalence of SAS was determined using the formula: n= , where n – the number of observations in the sampling; – type 1 error (at a=0.05); p – the prevalence of a feature in a population; q – reverse event frequency; D – the margin of sampling error. The hypothesis about the distribution type was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. The data are presented in the form of a confidence interval M ± SD (M – mean value, SD – standard deviation) and medians and quartiles 25% and 75% Me [Q1; Q3]. The proportions were compared using Pearson’s χ2 test with Yates’ correction for continuity. The Mann–Whitney and Kruskal–Wallis tests were used to determine differences between two independent groups. The Kruskal–Wallis ANOVA test was used to compare three or more groups of patients. Differences were considered statistically significant at p<0.05.
Results
Risk factors and comorbidities of patients included in the study are presented in Table 1 [14].
Таблица / Table 1
Анализ факторов риска и сопутствующей патологии у пациентов, включенных в исследование
Analysis of risk factors and comorbidities in patients included in the study
|
Показатель / Index |
1А группа АГ+ХСН +ССА (n=84) / Group 1A — patients with AH, SSA and CHF (n=84) |
1Б группа АГ + ССА без ХСН (n84 / Group 1B — patients with AH, SSA without CHF (n=84) |
2А группа АГ+ХСН без ССА (n=77) / Group 2A — patients with AH, CHF without SSA (n=77) |
2Б группа АГ без ССА без ХСН (n=75) / Group 2B — patients with AH without CHF and without SSA (n=75) |
р1А–1Б / р1А–1B р2А –2Б / р2А –2B р1А–2А / р1А–2А р1Б–2Б / р1B–2B |
pмг / pmg |
|
Курение, % / Smoking, % |
7,1 |
5,9 |
10,4 |
9,3 |
р1А–1Б =0, 853 р2А –2Б =0,931 р1А–2А =0, 579 р1Б –2Б =0,639 |
0,903 |
|
ИМТ, кг/м2 (М±SD) / BMI, kg/m2 (М±SD) |
23,4 ± 2,1 |
28,2±0,4 |
32,1 ± 2,0 |
30,3±0,4 |
р1А–1Б =0,062 р2А –2Б =0,319 р1А–2А =0,029 р1Б –2Б =0,823 |
0,481 |
|
Ожирение, % / Obesity, % |
9,9 |
16,7 |
23,4 |
14,7 |
р1А–1Б =0,236 р2А –2Б =0,563 р1А–2А =0,032 р1Б –2Б =0,206 |
0,582 |
|
СД 2 типа, % / Type 2 diabetes, % |
31 |
17,9 |
24,7 |
16 |
р1А–1Б =0,042 р2А –2Б =0,199 р1А–2А =0,386 р1Б –2Б =0,778 |
0,089 |
|
ХБП, % / CKD, % |
66,7 |
57,1 |
40,3 |
32 |
р1А–1Б =0,203 р2А –2Б =0,293 р1А–2А <0,001 р1Б –2Б =0,001 |
<0,001 |
|
ФП, % / AF, % |
50 |
29,8 |
24,7 |
17,3 |
р1А–1Б =0,003 р2А –2Б =0,313 р1А–2А <0,001 р1Б –2Б =0,082 |
<0,001 |
|
ОНМК, % / ACCD, % |
7,1 |
8,3 |
6,5 |
9,3 |
р1А–1Б=0,192 р2А–2Б=0,691 р1А–2А=0,199 р1Б–2Б=0,116 |
0,657 |
|
Анемия, % / Anemia, % |
23,8 |
10,7 |
15,6 |
13,3 |
р1А–1Б =0,033 р2А –2Б =0,172 р1А–2А =0,237 р1Б –2Б =0,341 |
0,193 |
Примечание: АГ — артериальная гипертензия; ХСН — хроническая сердечная недостаточность; ССА — синдром старческой астении; ФП — фибрилляция предсердий; ОНМК — острое нарушение мозгового кровообращения; ХБП — хроническая болезнь почек; СД — сахарный диабет; ИМТ — индекс массы тела; р1А-1Б – различия между 1А и 1Б группами; р2А-2Б – различия между 2А и 2Б группами; р1А-2А – различия между 1А и 2А группами; р1Б-2Б – различия между 1Б и 2Б группами; рмг – суммарное межгрупповое сравнение
Note: AH — arterial hypertension; CHF — chronic heart failure; SSA — senile asthenia syndrome; AF — atrial fibrillation; ACCD — acute cerebral circulation disorder; CKD — chronic kidney disease; DM — diabetes mellitus; BMI — body mass index; p1A-1B — differences between groups 1A and 1B; p2A-2B — differences between groups 2A and 2B; р1А-2А — differences between 1A and 2A groups; р 1B-2B — differences between groups 1B and 2B; рmg — multigroup comparison.
It should be noted that patients with AH, CHF, and SAS (group 1A) had a statistically significant higher incidence of anemia (by 13.1%, p=0.033), AF (by 20.2%, p=0.003), and type 2 diabetes melitus (by 13.1%, p=0.042) compared to patients with AH and SAS without CHF (group 1B), as well as AF (by 25.3%, p<0.001), CKD (by 26.4%, p<0.001), and a lower body mass index (p=0.049) compared to patients with AH and CHF without SAS (group 2A). In turn, patients with AH and CHF without SAS (group 2A) had a higher body mass index (BMI) (p=0.029) and a 2.4-fold higher share of patients with obesity (p=0.032) compared to patients with AH and CHF and SAS (group 1A) [10].
The duration of AH in the study groups was 22.1±2.2 years, with no significant differences (p>0.05). The occurrence rate of AH stages in patients included in the study is shown in Figure 1. All patients had a very high cardiovascular risk.
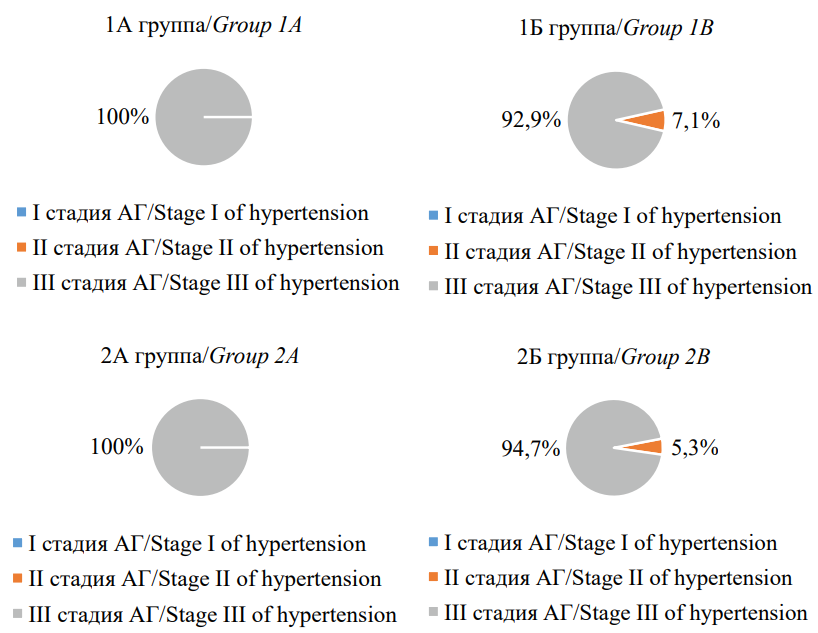
Рисунок 1. Частота встречаемости стадии артериальной гипертензии у пациентов, включенных в исследование
Figure 1. Incidence of hypertension in patients included in the study
A comparative analysis of office blood pressure and heart rate measurements in patients included in the study is presented in Figures 2 and 3. Thus, in “frail” patients with AH and CHF (group 1A), statistically significantly higher SBP values (p=0.047) and lower DBP values (p<0.001), as well as lower HR values (p=0.042) were recorded compared to “robust” patients with AH and CHF (group 2A).
As previously described in our articles, the duration of CHF in patients included in the study was 8.4±3.6 years, with no significant differences between groups (p>0.05). When assessing the stage of CHF in patients in groups 1A and 2A, no significant differences were found (p>0.05). Assessment of the occurrence rate of different FCs of CHF in the compared groups showed that there were statistically significantly more patients with CHF FC III in group 1A and with CHF FC II in group 1B (61.9% vs. 45.5% (p=0.041) and 41.6% vs. 28.6% (p=0.041), respectively). A comparative analysis of clinical manifestations of CHF according to CSAS revealed a 28.6% higher score in “frail” patients compared to robust patients (p<0.001). According to the results of the 6MWT, exercise tolerance in the group of patients with SAS was significantly lower compared to patients in the group without SAS (238.5 m [ 181.3–310.8] vs. 365.0 m [ 261.5–405.5], p <0.001) [10].
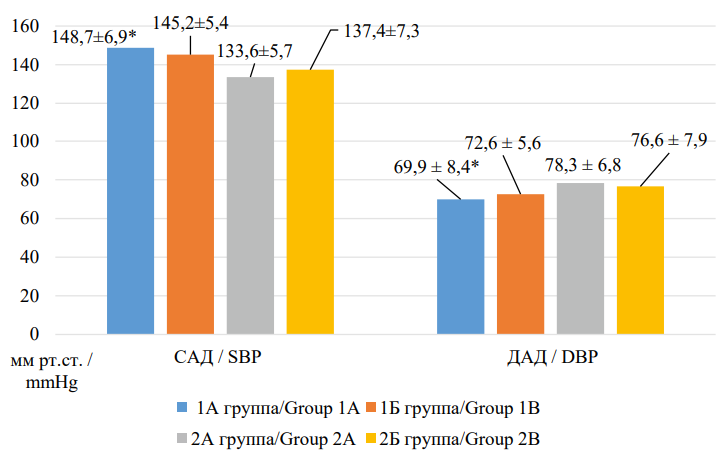
Рисунок 2. Сравнительная характеристика параметров офисного измерения артериального давления у пациентов, включенных в исследование
Figure 2. Comparative characteristics of office measurement parameters of blood pressure in patients included in the study
Примечание: САД — систолическое артериальное давление; ДАД — диастолическое артериальное давление; * - р <0,05 при сравнении с 2А группой.
Note: SBP — systolic blood pressure; DBP — diastolic blood pressure; * — p <0.05 when compared with group 2A.
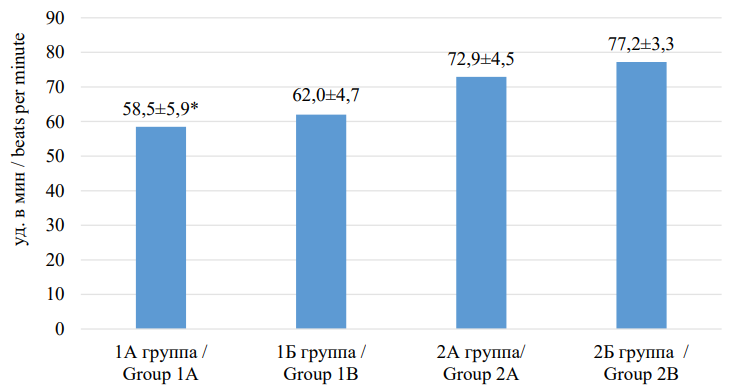
Рисунок 3. Сравнительная характеристика показателей частоты сердечных сокращений у пациентов, включённых в исследование
Figure 3. Comparative characteristics of heart rate indicators in patients included in the study
Примечание: ЧСС — частота сердечных сокращений; * — р <0,05 при сравнении с 2А группой.
Note: HR — heart rate; * — p <0.05 when compared with group 2A.
The studied patients received treatment for AH and/or CHF in accordance with existing guidelines. The frequency of prescribed different classes of drugs in the compared groups did not differ significantly (Figure 4).

Рисунок 4. Частота назначения различных классов препаратов в сравниваемых группах
Figure 4. Frequency of prescribing different classes of drugs in the compared groups
Примечание: ИАПФ — ингибиторы ангиотензинпревращающего фермента; АРАII — антагонисты рецепторов ангиотензина II типа; БАБ — бета-блокаторы; БМКК — блокаторы медленных кальциевых каналов; АМКР — антагонисты минералокортикоидных рецепторов.
Note: ACE — Аngiotensin Converting Enzyme inhibitors; ARBs — Angiotensin II Receptor Blockers; BAB — beta blockers; CCBs — slow calcium channel blockers; MCRA — mineralocorticoid receptor antagonists.
According to the results of the Morisky-Green questionnaire (8-item Morisky Medication Adherence Scale (MMAS-8)), patients in all four study groups had moderate adherence to treatment (group 1A – 6.4±1.1.2 points, group 1B – 6.6±1.1 points, group 2A – 6.4±1.1 points, group 2B – 6.5±1.2 points), which did not differ significantly (p=0.591).
The central pressure parameters for the patients included in the study are presented in Table 2.
Таблица / Table 2
Параметры центрального давления у пациентов, включённых в исследование
Parameters of central pressure in patients included in the study
|
Показатель / Index |
1А группа АГ+ХСН +ССА (n=84) / Group 1A — patients with AH, SSA and CHF (n=84) |
1Б группа АГ + ССА без ХСН (n=84) / Group 1B — patients with AH, SSA without CHF (n=84) |
2А группа АГ+ХСН без ССА (n=77) / Group 2A — patients with AH, CHF without SSA (n=77) |
2Б группа АГ без ССА без ХСН (n=75) / Group 2B — patients with AH without CHF and without SSA (n=75) |
р1А–1Б / р1А–1B р2А –2Б / р2А –2B р1А–2А / р1А–2А р1Б–2Б / р1B–2B |
pмг / pig |
|
САД ао, мм рт. ст. (Me [Q1; Q3]) / SBP ao, mmHg (Me [Q1; Q3]) |
138,6 [ 132,5–138,8] |
133,1 [ 130,8–134,9] |
131,6 [ 128,1–135,8] |
134,4 [ 131,2–137,2] |
р1А–1Б = 0,019 р2А–2Б = 0,127 р1А–2А = 0,004 р1Б–2Б = 0,493 р1Б–2А = 0,285 р1А–2Б = 0,041 |
< 0,001 |
|
ДАД ао, мм рт. ст. (Me [Q1; Q3]) / DBP ao, mmHg (Me [Q1; Q3]) |
65,5 [ 62,9–68,2] |
68,0 [ 64,4–71,4] |
69,9 [ 68,0–72,4] |
71,7 [ 69,9–74,0] |
р1А–1Б = 0,23 р2А–2Б =0,386 р1А–2А = 0,11 р1Б–2Б = 0,24 р1Б–2А = 0,491 р1А–2Б = 0,031 |
0,903 |
|
ПАД ао, мм рт. ст. (Me [Q1; Q3]) / PBP ao, mmHg (Me [Q1; Q3]) |
68,1 [ 65,6–70,9] |
63,6 [ 61,3–66,6] |
66,9 [ 64,5–68,8] |
63,3 [ 60,7–64,8] |
р1А–1Б < 0,001 р2А–2Б < 0,001 р1А–2А = 0,21 р1Б–2Б = 0,31 р1Б–2А = 0,02 р1А–2Б < 0,001 |
< 0,001 |
|
AIx ао, % (Me [Q1; Q3]) |
33,8 [ 30,9–36,9] |
22,8 [ 20,9–25,2] |
20,9 [ 17,4–22,9] |
14,6 [ 12,9–16,0] |
р1А–1Б < 0,001 р2А–2Б < 0,001 р1А–2А < 0,001 р1Б–2Б < 0,001 р1Б–2А = 0,582 р1А–2Б < 0,001 |
< 0,001 |
|
РРА, % (Me [Q1; Q3]) |
120,5 [ 117,4–124,5] |
125,7 [ 123,4–127,8] |
120,7 [ 117,1–123,2] |
123,6 [ 120,8–125,9] |
р1А–1Б = 0,17 р2А–2Б = 0,14 р1А–2А = 0,83 р1Б–2Б = 0,41 р1Б–2А = 0,19 р1А–2Б =0,125 |
< 0,485 |
|
ED, мс (Me [Q1; Q3]) |
345,5 [ 332,6–354,9] |
309,0 [ 293,3–325,3] |
340,5 [ 324,1–353,6] |
311,8 [ 296,3–326,8] |
р1А–1Б < 0,001 р2А–2Б < 0,001 р1А–2А = 0,47 р1Б–2Б = 0,94 р1Б–2А < 0,001 р1А–2Б < 0,001 |
< 0,001 |
|
SEVR, % (Me [Q1; Q3]) |
123,4 [ 119,9–127,7] |
135,1 [ 131,7–138,2] |
125,5 [ 123,1–128,1] |
135,5 [ 133,2–140,6] |
р1А–1Б < 0,001 р2А–2Б < 0,001 р1А–2А = 0,81 р1Б–2Б = 0,88 р1Б–2А < 0,001 р1А–2Б < 0,001 |
< 0,001 |
Примечание: АГ — артериальная гипертензия; ХСН — хроническая сердечная недостаточность; ССА — синдром старческой астении; САД ао — систолическое артериальное давление в аорте; ДАД ао — диастолическое артериальное давление в аорте; ПАД ао — пульсовое артериальное давление в аорте; AIx ао — индекс аугментации в аорте; РРА — амплификация пульсового давления; ED — длительность периода изгнания из левого желудочка; SEVR — индекс эффективности субэндокардиального кровотока; р1А-1Б — различия между 1А и 1Б группами; р2А-2Б — различия между 2А и 2Б группами; р1А-2А – различия между 1А и 2А группами; р1Б-2Б – различия между 1Б и 2Б группами; рмг – суммарное межгрупповое сравнение.
Note: AH — arterial hypertension; CHF — chronic heart failure; SSA — senile asthenia syndrome; SBP ao — systolic blood pressure in the aorta; DBP ao — diastolic blood pressure in the aorta; PBP ao — pulse blood pressure in the aorta; AIx ао — augmentation index in the aorta; РРА — pulse pressure amplification; ED — ejection duration from the left ventricle; SEVR — subendocardial viability ratio; p1A–1B — differences between groups 1A and 1B; p2A–2B — differences between groups 2A and 2B; р1А–2А — differences between 1A and 2A groups; р 1B–2B — differences between groups 1B and 2B; рig — summary intergroup comparison.
According to the published data, an increase in mean daily SBP above 111 mmHg is associated with an increase in asymptomatic target organ damage [15]. In our study, this indicator was exceeded in all groups studied.
To assess the impact of CHF on mean daily SBP parameters, the authors performed an intergroup comparison of groups 2A and 2B, as well as groups 1A and 1B. The results revealed no statistically significant difference in “robust” patients with AH in the presence and absence of CHF (p>0.05). At the same time, in “frail” patients with AH, the development of CHF led to significantly higher SBP parameters compared to “frail” patients with AH and without CHF (p=0.019).
When assessing the impact of SAS, a comparative analysis of groups 1B and 2B and groups 1A and 2A was performed. The obtained data revealed no significant differences in patients with AH without CHF, and with and without SAS (p=0.493). At the same time, in patients with AH and CHF, the presence of SAS led to significantly higher SBP values compared to patients with AH and CHF without SAS (p=0.004). In order to comparatively assess the degree of influence of SAS or CHF on the SBP(Ao) indicator, a comparative analysis of indicators was performed between a group of patients with AH and SAS without CHF (group 1B) and a group of patients with AH and CHF without SAS (group 2A). The results showed no significant difference (p=0.485), which suggests that CHF and SAS have a comparable effect on the mean daily SBP.
When assessing the average daily DBP(Ao), no statistically significant differences were found between the groups, regardless of the presence or absence of only CHF or SAS (p>0.05).
Analysis of the average daily PBP(Ao) values did not reveal any significant differences in the studied indicator between groups of patients with AH and CHF, as well as with AH without CHF, regardless of the presence of SAS (p>0.05). At the same time, significantly higher PBP(Ao) values were obtained in both “robust” and “frail” patients with AH and CHF (p<0.001 and p<0.001, respectively). In addition, when assessing the degree of influence of CHF or SAS on the PBP(Ao) level, significantly higher values were found in the group of patients with AH and CHF without SAS compared to the group of patients with AH and SAS without CHF (p<0.001), which suggests a greater influence of CHF on PBP(Ao) levels in patients aged 80 years and older.
Further, a comparative assessment of AIX(Ao), PPA, ED, and SEVR was conducted in patients from the study groups.
Analysis of the obtained data revealed significantly higher AIX(Ao) values in patients in group 2A (AH and CHF without SAS) compared to patients in group 2B (AH without CHF and without SAS) (p<0.001), as well as in patients in group 1A (AH, CHF, and SAS) compared to patients in group 1B (AH and SAS without CHF) (p<0.001), which allows suggesting the effect of CHF on the studied indicator regardless of the presence of SAS. When assessing the influence of SAS, significantly higher indicators of arterial stiffness were also recorded in patients in group 1B (AH and SAS without CHF) compared to patients in group 2B (AH without SAS and without CHF) (p<0.001), as well as in patients in group 1A (AH, CHF, and SAS) compared to patients in group 2A (AH and CHF without SAS) (p<0.001), which allows suggesting the influence of SAS on the studied indicator both in patients without CHF and in the presence of CHF. A comparative analysis of indicators between the group of patients with AH and SAS without CHF (group 1B) and the group with AH and CHF without SAS (group 2A) showed no significant difference (p=0.582), which makes it possible to conclude that CHF and SAS have a comparable effect on the AIX(Ao) value.
The evaluation of PPA did not show any significant differences between the compared groups (p>0.05).
Statistical analysis of ED values did not reveal any significant differences in the studied indicator between groups of patients with AH and CHF, as well as with AH without CHF, regardless of the presence of SAS (p>0.05). At the same time, significantly higher ED values were demonstrated in both “robust” and “frail” patients with AH in the presence of CHF (p<0.001 and p<0.001, respectively). In addition, when assessing the degree of influence of CHF or SAS on the ED index, significantly higher values were also found in the group of patients with AH and CHF without SAS compared to the group of patients with AH and SAS without CHF (p<0.001), which suggests a greater influence of CHF on the prolongation of blood ejection from the LV, indicating more pronounced vascular stiffness in patients with AH aged 80 years and older with CHF.
A similar situation was observed during SEVR values analysis. Thus, no significant difference in SEVR was noted between groups of patients with AH and CHF, as well as between patients with AH without CHF, regardless of the presence of SAS (p>0.05). In turn, the analysis of the obtained data made it possible to establish a significantly more pronounced effect of CHF on SEVR values in both “robust” and “frail” patients with AH. In addition, when assessing the degree of influence of CHF or SAS on SEVR, it was noted that in patients with CHF, the subendocardial blood flow efficiency index decreased significantly lower, reflecting a decrease in coronary blood flow and the development of systolic dysfunction.
It should be noted that when SAS and CHF were combined in patients with AH aged 80 years and older, the highest values of SBP (p=0.021), DBP (p=0.013), AIX(Ao) (p<0.001), ED (p<0.001) and lower values of DBP(Ao) (p<0.001) and SEVR (p<0.001) were observed, which were significantly different from similar indicators when compared with patients with AH without CHF and without SAS, indicating the most pronounced increase in vascular stiffness in this combined pathology.
Discussion
It is well known that age and elevated blood pressure are associated with vascular stiffness and other signs of remodeling of the main arteries and lead to an increase in central blood pressure, which exceeds peripheral blood pressure values. Consequently, central blood pressure readings more accurately reflect the load on the left ventricle compared to upper arm blood pressure readings [16]. Thus, a meta-analysis of 11 studies involving 5,648 patients demonstrated that an increase in central blood pressure by every 10 mmHg increased the risk of cardiovascular complications by 9% [17]. When the elastic properties of arteries decrease, blood flows into a rigid vascular system, causing the heart to generate higher systolic blood pressure, and the return of the accelerated reflected wave shifts from diastole to systole, leading to a decrease in DBP [17]. The PIUMA study demonstrated that an increase in PBP was one of the predictors of cardiovascular death in patients with AH [18]. According to a meta-analysis, an increase in PBP by 10 mmHg leads to a 14% increase in the risk of cardiovascular complications [17].
The characteristics of central hemodynamics determine not only the pressure gradient in the arterial vasculature, but also the effectiveness of the heart’s contractile activity. An increase in the pulse wave velocity leads to increased stiffness, followed by an increase in SBP. This is followed by an increase in afterload on the LV and the development of LV hypertrophy, impaired LV diastolic function, and the development of CHF [19].
On the other hand, the development of SAS in patients with age-related stiffness of the vascular wall also contributes to an increased risk of cardiovascular events in elderly patients. Thus, Qi Xue et al. (2019) showed that vascular stiffness was higher in the group of patients with SAS. According to the researchers, vascular wall stiffness was most likely associated with markers of SAS itself [20].
The results of our study also demonstrated the dependence of central aortic pressure indicators on the presence of SAS and CHF, both separately and in combination (Table 3).
Таблица / Table 3
Оценка влияния хронической сердечной недостаточности и синдрома старческой астении на пораметры центрального аортального давления у пациентов, включённых в исследование
Assessment of the influence of chronic heart failure and senile asthenia syndrome on central aortic pressure indicators in patients included in the study
|
Параметр / Parametr Критерии оценки и группы сравнения / Criteria of comparison |
САД ао / SBP ao |
ДАД ао / DBP ao |
ПАД ао / PBP ao |
Aix ао |
РРА |
ED |
SEVR |
|
ССА (1А и 2А группы; 1Б и 2Б группы) / SSA (1A and 2A groups; 1B and 2B groups) |
↑ у пациентов с ХСН / ↑ in patients with CHF |
↔ |
↔ |
↑ |
↔ |
↔ |
↔ |
|
ХСН (1А и 1Б группы; 2А и 2Б группы) / CHF (1A and 1B groups; 2A and 2B groups) |
↑ у «хрупких» пациентов / ↑ in patients with SSA |
↔ |
↑ |
↑ |
↔ |
↑ |
↓ |
|
Сравнительная оценка ССА и ХСН (1Б и 2Агруппы) / Comparative assessment of SSA and CHF (1B and 2A groups) |
↔ |
↔ |
↑ ХСН / ↑ CHF |
↔ |
↔ |
↑ ХСН / ↑ CHF |
↓ ХСН / ↓ CHF |
|
Сочетание ССА и ХСН (1А и 2Б группы) / Combination of SSA and CHF (1A and 2B groups) |
↑ |
↓ |
↑ |
↑ |
↔ |
↑ |
↓ |
Примечание: АГ — артериальная гипертензия; ХСН — хроническая сердечная недостаточность; ССА — синдром старческой астении; САД ао — систолическое артериальное давление в аорте; ДАД ао — диастолическое артериальное давление в аорте; ПАД ао — пульсовое артериальное давление в аорте; AIx ао — индекс аугментации в аорте; РРА — амплификация пульсового давления; ED — длительность периода изгнания из левого желудочка; SEVR — индекс эффективности субэндокардиального кровотока. Представлены результаты оценки влияния хронической сердечной недостаточности и синдрома старческой астении на показатели центрального аортального давления у пациентов, включённых в исследование: ↑ — статистически значимо «больше»; ↓ — статистически значимо «меньше»; ↔ — различия статистически незначимы.
Note: AH — arterial hypertension; CHF — chronic heart failure; SSA — senile asthenia syndrome; SBP ao — systolic blood pressure in the aorta; DBP ao — diastolic blood pressure in the aorta; PBP ao — pulse blood pressure in the aorta; AIx ао — augmentation index in the aorta; РРА — pulse pressure amplification; ED — ejection duration from the left ventricle; SEVR — subendocardial viability ratio. The results of assessing the effect of chronic heart failure and senile asthenia syndrome on central aortic pressure in patients included in the study are presented: ↑ — statistically significant “more”; ↓ — statistically significant “less”; ↔ — differences are statistically insignificant.
Thus, in patients with AH aged 80 years and older with SAS, both in patients with CHF and without CHF, negative dynamics of vascular stiffness parameters were recorded. In turn, CHF also demonstrated a negative effect on vascular stiffness indicators in “frail” and “robust” patients. A more detailed analysis revealed that in patients with AH and CHF, the presence of SAS led to significantly higher average daily SBP values compared to patients with AH, CHF without SAS (p=0.004), as well as a significantly higher AIX(Ao) index in patients with AH regardless of the presence of CHF (p<0.001), reflecting more pronounced vascular stiffness and, consequently, a higher risk of developing cardiovascular complications. Patients with AH and CHF, regardless of the presence of SAS, had significantly higher PBP(Ao) (p<0.001), AIX(Ao) (p<0.001), and ED (p<0.001) values and significantly lower SERV values (p<0.001). In addition, when assessing the degree of influence, the presence of CHF demonstrated a significantly more pronounced effect on PBP(Ao) (p<0.001), ED (p<0.001), and SERV (p<0.001) compared to SAS, indicating a more pronounced effect of CHF on vascular stiffness parameters in patients with AH aged 80 years and older.
It is important to emphasize that the most significant changes in all analyzed indicators of central aortic pressure, making it possible to suggest the most pronounced vascular stiffness and, consequently, a higher risk of cardiovascular complications, were observed in patients with a combination of AH, CHF, and SAS. The results of the study indicate the need to optimize antihypertensive and lipid-lowering therapy to achieve target blood pressure levels and lipid profile indicators, control and correction of existing risk factors in order to reduce the severity of vascular stiffness and improve the prognosis in patients with AH aged 80 years and older in combination with CHF and SAS.
Conclusion
The presence of both SAS and CHF in patients with AH aged 80 years and older was accompanied by an increase in central aortic pressure and, as a result, progression of vascular stiffness. However, CHF led to more pronounced vascular changes. When AH, CHF, and SAS were combined, the most pronounced disturbances in the elastic properties of the vascular wall were observed, indicating a higher risk of fatal and non-fatal cardiovascular events in this combined pathology.
References
1. Fadah K., Hechanova А., Mukherjee D. Epidemiology, pathophysiology and management of coronary artery disease in the elderly. Int J Angiol. 2022;31(4):244-250. https://doi.org/10.1055/s-0042-1751234.
2. Tkacheva O.N., Belenkov Yu.N., Karpov Yu.A., Zyryanov S.K. Gerontology Issues in Cardiology Practice. Kardiologiia. 2019;59(12):54-63. (In Russ.) https://doi.org/10.18087/cardio.2019.12.n876
3. Davidov E.L., Тihonova N.V., Glushanko V.S., Shulmin A.V., Zakharova A.S. Asthenic syndrome in elderly people: diagnostic, treatment and rehabilitation trends. Siberian Medical Review. 2020;(5):40-48. (In Russ.) https://doi.org/10.20333/2500136-2020-5-40-48
4. Khazova E.V., Smetanina E.D., Malkova M.I. Senile asthenia syndrome in patients with cardiovascular diseases: issues of epidemiology, diagnosis, prognosis. Medical almanac. 2023;3(76):98-106. (In Russ.). eLIBRARY ID: 54678736 EDN: YELHVQ
5. Díez-Villanueva P, Jiménez-Méndez C, Bonanad C, GarcíaBlas S, Pérez-Rivera Á, et al. Risk Factors and Cardiovascular Disease in the Elderly. Rev Cardiovasc Med. 2022;23(6):188. https://doi.org/10.31083/j.rcm2306188
6. Kuznetsov A.A., Tsvetkova E.E., Denisova D.V., Ragino Yu.I., Voevoda M.I. Central Aortic Pressure: Reference and Diagnostic Values. Kardiologiia. 2019;59(3):11-17. (In Russ.) https://doi.org/10.18087/cardio.2019.3.10235
7. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press. 2009;18(6):308-347. https://doi.org/10.3109/08037050903450468
8. Kotovskaya Yu.V., Kobalava Zh.D. Aortic pressure: modern ideas about the clinical and prognostic significance of its indicators. Medical advice. 2013;9:26-33. (In Russ.). eLIBRARY ID: 21168715 EDN: RVGCRL
9. Radova N.F., Nunuparova M.M. The central aortic blood pressure. Clinical value and the forecast. Medical alphabet. 2017;3(39):21-27. (In Russ.). eLIBRARY ID: 32368069 EDN: YNMLVO
10. Safronenko V.A., Chesnikova A.I., Sementsova N.A. Features of vascular rigidity in patients with arterial hypertension in combination with chronic heart failure and senile asthenia syndrome. "Arterial’naya Gipertenziya" ("Arterial Hypertension"). 2022;28(6):659-668. (In Russ.) https://doi.org/10.18705/1607-419X-2022-28-6-659-668
11. Kobalava Zh.D., Konradi A.O., Nedogoda S.V., Shlyakhto E.V., Arutyunov G.P., et al. 2024 Clinical practice guidelines for Hypertension in adults. Russian Journal of Cardiology. 2024;29(9):6117. (In Russ.) https://doi.org/10.15829/1560-4071-2024-6117. EDN: GUEWLU
12. Galyavich A.S., Tereshchenko S.N., Uskach T.M., Ageev F.T., Aronov D.M., et al. 2024 Clinical practice guidelines for Chronic heart failure. Russian Journal of Cardiology. 2024;29(11):6162. (In Russ.) https://doi.org/10.15829/1560-4071-2024-6162. EDN: WKIDLJ
13. Clinical practice guidelines for Senile asthenia. (In Russ.). Accessed 02.09.2024. URL: https://diseases.medelement.com/disease/%D1%81%D1%82%D0%B0%D1%80%D1%87%D0%B5%D1%81%D0%BA %D0%B0%D1%8F-%D0%B0%D1%81%D1%82%D0%B5%D0 %BD%D0%B8%D1%8F-%D0%BA%D0%BF-%D1%80%D1%84-2024/18021
14. Safronenko V.A., Chesnikova A.I., Safronenko A.V., Skarzhinskaya N.S., Kuznetsov I.I., Nasytko A.D. Clinical traits of chronic heart failure in patients with arterial hypertension and senile asthenia syndrome: an observational cross-sectional study. Kuban Scientific Medical Bulletin. 2021;28(4):25- 40. (In Russ.) https://doi.org/10.25207/1608-6228-2021-28-4-25-40
15. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, et al. Relation of Central Arterial Stiffness to Incident Heart Failure in the Community. J Am Heart Assoc. 2015;4(11):e002189. https://doi.org/10.1161/JAHA.115.002189
16. Kotovskaya Yu.V., Rogoza A.N., Orlova Ya.A., Posokhov I.N. Ambulatory pulse wave monitoring: current and future. Opinion paper of Russian Experts. Cardiovascular Therapy and Prevention. 2018;17(6):95-109. (In Russ.) https://doi.org/10.15829/1728-8800-2018-6-95-109
17. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865-1871. https://doi.org/10.1093/eurheartj/ehq024
18. Verdecchia P, Reboldi G, Mazzotta G, Angeli F. The Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) Study. Panminerva Med. 2021;63(4):464-471. https://doi.org/10.23736/S0031-0808.21.04383-4
19. Teregulov Yu.E., Mayanskaya S.D., Teregulova E.T. Changes in elastic properties of arteries and hemodynamic processes. Practical medicine. 2017;(2):14-20.(In Russ.). eLIBRARY ID: 29044730 EDN: YLPFTZ
20. Xue Q, Qin MZ, Jia J, Liu JP, Wang Y. Association between frailty and the cardio-ankle vascular index. Clin Interv Aging. 2019;14:735-742. https://doi.org/10.2147/CIA.S195109
21.
22.
About the Authors
V. A. SafronenkoРоссия
Victoria A. Safronenko, Cand. Sci. (Med.), Associate Professor, Department of Internal Medicine No. 1
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. I. Chesnikova
Россия
Anna I. Chesnikova, Dr. Sci. (Med.), Professor, Head of the Department of Internal Medicine No. 1
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Safronenko V.A., Chesnikova A.I. Features of central aortic pressure at patients with arterial hypertension aged 80 years and older, taking into account the presence of chronic heart failure and senile asthenia syndrome. Medical Herald of the South of Russia. 2025;16(1):28-38. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-28-38
JATS XML







































