Scroll to:
Pathomorphological changes in pulmonary vessels at different terms of lethal outcomes of patients with COVID-19
https://doi.org/10.21886/2219-8075-2021-12-2-54-61
Abstract
Objective: to study the pathomorphological changes in the vessels of the lungs at different times of death in patients with COVID-19. Material and methods: autopsy protocols of 40 deaths from COVID-19 with histological, histochemical examination, and photoregistration. To determine the degree of pulmonary vascular lesions, the authors developed and applied a semi-quantitative assessment scale for the sign based on counting the affected lung vessels in 10 fields of view, expressed as a percentage: no sign – (0%), weak sign + (1-25%), moderate sign ++ (26-50%), expressed sign +++ (51-75%), highly expressed sign ++++ (76-100%). Results: during the first 14 days of the disease, stasis and swelling of individual endotheliocytes were noted in the vessels of the microvasculature. After 15-21 days of the development of COVID-19, signs of alterations in endothelial cells and microthrombosis were observed in microvessels. After 22-28 days of the disease, the phenomena of repair and hyperplasia of endothelial cells were detected. At different times of the development of the disease in the lung tissue, the phenomena of acute respiratory distress syndrome, interstitial pneumonia, and focal pneumofibrosis were observed. Conclusions: the authors believe that COVID-19 is associated with a progressive microvascular endotheliopathy in the lungs which is characterized by swelling, alteration, and later, by hyperplasia, and regeneration of endothelial cells in combination with microthrombosis. Destructive changes in the walls of the microvessels of the lungs have superficial character without the destruction of the reticular frame and basal membranes. Endotheliopathy, microthrombus formation of microvessels of the lungs, and interstitial pneumonia create a vicious circle of severe respiratory failure, which must be taken into account in the clinic to correct the treatment of patients with COVID-19.
For citations:
Todorov S.S., Deribas V.Yu., Kazmin A.S., Reznikova G.L., Makarenko Yu.M., Polesovoy F.V., Todorov (jr) S.S. Pathomorphological changes in pulmonary vessels at different terms of lethal outcomes of patients with COVID-19. Medical Herald of the South of Russia. 2021;12(2):54-61. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-2-54-61
Introduction
Despite existing publications studying the role of the SARS-CoV-2 virus, which causes acute respiratory distress syndrome, the development of hemorheological disorders in organs and tissues, as well as the dynamics of pathological changes in pulmonary vessels in COVID-19 disease, remain poorly understood. The presented few works are typically based on analyzing the results of separate autopsies of COVID-19 patients [1][2][3][4][5][6][7][8].
The alteration of lung vessels is an important link in the patho- and thanatogenesis of patients who died of COVID-19. In this connection, the pathomorphological characteristic of the damages to various-size lung vessels in various periods of COVID-19 development takes on special significance due to blood clot organization, pneumorrhagia, and progressing inflammatory condition in the lungs. Without question, in developing and progressing respiratory difficulty, acute respiratory distress syndrome, and pneumonia, the damage to the air-hematic barrier represented by vascular and alveolar structures has significance. The development of acute and further chronic interstitial inflammation in the lungs, vascular disorders in COVID-19 may be a basis for disease progression with developing severe respiratory and tissue hypoxia [9][10].
So, the pathomorphological changes in pulmonary vessels in COVID-19 are of special interest due to the possibilities of timely and competent correction of the pathological process development.
The study objective is to study pathomorphological changes in pulmonary vessels in different terms of lethal outcomes of COVID-19 patients.
Materials and methods
The study material was autopsy records of 40 dead patients, which had been performed at two pathoanatomical institutions: at Rostov Regional Pathoanatomical Office (30 autopsies) and the Pathoanatomical Department of Rostov Clinical Hospital of the Southern District Medical Centre of the Federal Medical and Biological Agency of Russia (10 autopsies). In all clinical cases, patients were admitted to COVID contagious isolation monohospitals with confirmed PCR analysis for SARS-CoV-2.
In accordance with the Federal Law of 21 November 2011 No. 323-FZ On Fundamentals of Healthcare of the Citizens in the Russian Federation, Article 67, §4, performing pathoanatomical autopsies and conducting morphological studies are an integral part of the diagnostic process for assessing the underlying disease, assigning the cause of death and other conditions. The expressed will of the dead or his/her relatives for conducting such studies is not required.
The autopsy was conducted in morgues equipped for performing autopsies of the dead with infectious diseases and using personal protective equipment by personnel following the Russian recommendations. The team of anatomic pathologists with high experience in managing infectious diseases took part in autopsies in both hospitals.
The autopsies were performed by the method of incomplete evisceration. Fragments of organs and tissues were cut and fixed in 10% buffered formalin solution during 48–72 hours for subsequent routine histodiagnosis. Histological and histochemical stages of the study included staining microslides by hematoxylin and eosin, pikrofuxin by Van Gieson's method, and silver methenamine for assessing collagenous and argyrophilic fibers of pulmonary vessels, interstitial tissue. Photographic registration was performed using a Leica DM 1000 microscope with an ICC50 E camera with a built-in platform for visualization (Germany).
Pathoanatomical diagnoses were set on the basis of autopsy results of dead patients with due regard to clinical features, laboratory data, results of macroscopic and microscopic studies. To determine the damage level of pulmonary vessels, the authors developed and used the scale of semiquantitative assessment of a sign, which took into account the quantity of affected pulmonary vessels in 10 fields of view, expressed in percent: no sign – (0%), mild sign + (1–25%), moderate sign ++ (26–50%), pronounced sign +++ (51–75%), strongly-pronounced sign ++++ (76–100%).
Results
Among the dead with COVID-19, there were 24 males, 16 females; the mean age of patients was 68 years (range of 53–86 years). According to clinical data, the timelines for developing diseases were the following: 3–7 days (3 observations, 7.5%), 8–14 days (9 observations, 22.5%), 15–21 days (14 observations, 35%), 22–28 days (14 observations, 35%).
When admitting, all patients had conducted computer tomography of the lungs, which revealed the signs of interstitial viral pneumonia (ground-glass symptoms).
In all cases, in clinical and pathoanatomical diagnoses, new coronavirus infection (COVID-19) was the prior disease. Among the associated diseases in patients, aortic, cardiac coronary artery advanced arteriosclerosis (50%), hypertensive disease (10%), diabetes mellitus type 2 (25%), and fatness of degrees 1–2 (15%) were noted.
The immediate cause of death was acute respiratory distress syndrome (45%), thrombosis of the pulmonary artery branches (30%), and multiple organ dysfunction (25%).
The resulting data of pathoanatomical changes in the lungs in COVID-19 were assessed with due regard to the terms of lethal outcomes of patients at COVID hospitals and damages of various-diameter vessels.
Among the pathologic signs of pulmonary vessel damages, the following were noted: stasis of erythrocytes, endotheliocyte alteration, thrombosis, neutrophilic infiltration of vessel walls, fibrinoid changes, endothelial hyperplasia, and endothelial regeneration.
In patients dead in 3–7 days after the disease manifestation, the signs of acute respiratory distress syndrome with the presence of hyaline membranes in the walls of the alveoli. The vessels of the microvasculature within the whole length were ectatic, with sharp plethora, stasis, sludge of erythrocytes (55%, +++) (Fig. 1).
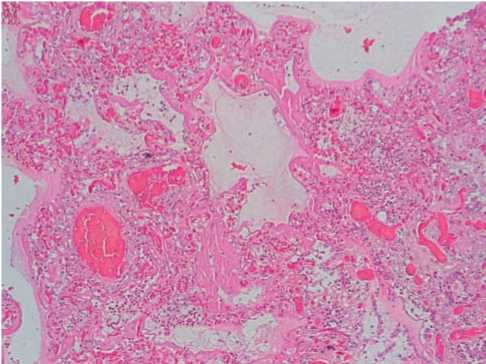
Fig. 1. Changes in the lungs in COVID-19: hyaline membranes of the alveoli walls, sharp plethora, stasis, sludge of erythrocytes of microvessels. Hematoxylin-eosin, x100.
In the capillaries, there were small lodgments of neutrophils and signs of single endotheliocytes (15%, +). The lumens of separate small branches of the pulmonary artery were obturated by mixed thrombi consisted in fibrin strains, lysed erythrocytes, and few neutrophils (30%, ++) (Fig. 2).
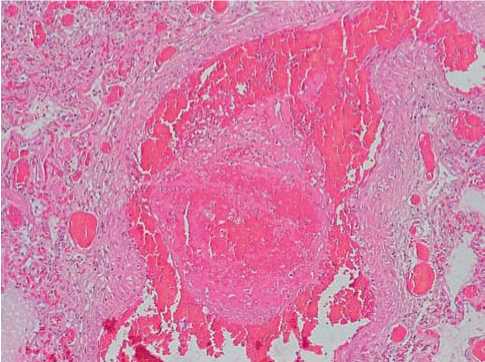
Fig. 2. In the lumen of a small branch of the pulmonary artery in COVID-19 – an obturating mixed thrombus. Sharp plethora, stasis, perivascular hemorrhage. Hematoxylin-eosin, x100.
In the lumens of the alveoli, around the capillaries and small arteries, in the interstitial tissue, there were free-laying erythrocytes, surrounded by a fine network of fibrin strains.
In the lungs of patients dead in 8–14 days from the onset of the disease, the signs of acute respiratory distress syndrome characterized by dense thick hyaline membranes in the walls of the alveoli with surrounded intra-alveolar infiltration with neutrophils with the signs of cell lysis were found (Fig. 3). In the vessels of the microvasculature, blood stasis and sharp plethora were noted (60%, +++).
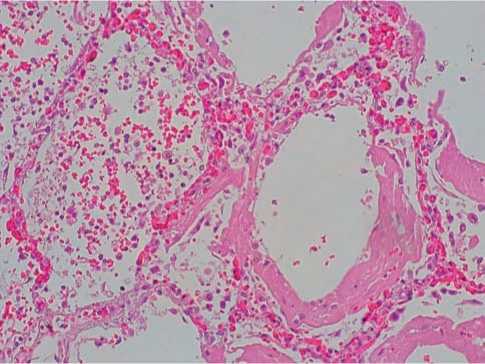
Fig. 3. Hyaline membranes of the alveolar walls, neutrophilic infiltration with cell lysis with COVID-19. Hematoxylin-eosin, x100.
In the lumens of few small and medium branches of the pulmonary artery, there were obturating mixed thrombi (40%, ++) represented by fibrin units surrounded by lysed erythrocytes. In the walls of separate small branches of the pulmonary artery, there were small lodgments of neutrophils, the alteration of individual groups of endotheliocytes (35%, ++) (Fig. 4).

Fig. 4. Obturating mixed blood thrombi in the lumen of small branches of the pulmonary artery, alteration of endothelial cells in COVID-19. Hematoxylin-eosin, x100.
In patients dead in 15–21 days from the onset of the disease, in the vessels of the microvasculature, a high pronounced blood stasis (75%, +++) and perivascular hemorrhage were noticed. Small branches of the pulmonary artery were dramatically ectatic and included mural mixed thrombi in the majority of fields of view (85%, ++++). In endotheliocytes of these vessels, along the large length, the phenomena of alteration were noticed (80%, ++++); in the walls of arteries, moderately pronounced neutrophilic infiltration (70%, +++) and fibrinoid changes (55%, +++) were noted (Fig. 5). The alveolar septa were destructed; the lumens of alveoli were filled with plenty of neutrophils (Fig. 6). The reticular frame of the walls of small arteries was saved and well-defined.
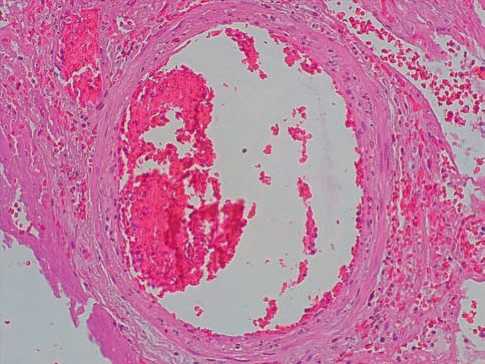
Fig. 5. COVID-19: mixed parietal thrombus in the lumen of a branch of the pulmonary artery. Alteration of endothelial cells, neutrophilic infiltration of the artery wall. Hematoxylin-eosin, x100.
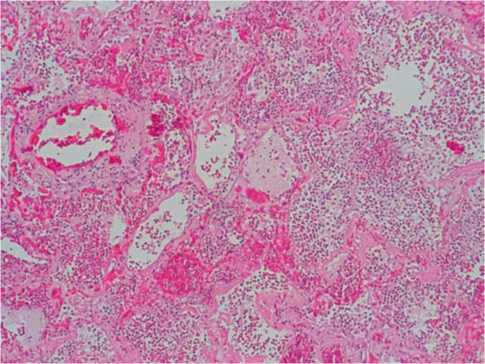
Fig. 6. COVID-19: in the lumen of the alveoli — pronounced neutrophilic infiltration with the destruction of the alveoli, hemorrhages. Hematoxylin-eosin, x100.
The given morphological signs are indicative of preferred involvement of endothelium cells of the microvasculature vessels in the process of destruction with maintaining the structure of their basement membranes and argyrophilic fibers.
In patients dead in 22–28 days from the onset of the disease, in the majority of small branches of the pulmonary arteries, there were obturating mixed thrombi (70%, +++) consisting in lumpy fibrin and lysed erythrocytes. In the intima of vessels, local hyperplasia of the endothelium cells was noted with forming “pillowlike” thickenings (30%, ++). The endotheliocytes of individual small arteries formed structures of the “palisade” type (50%, ++). In some small vessels, there were fibrinoid changes of walls with forming obturating mixed thrombi (45%, ++) (Fig. 7).

Fig. 7. Focal hyperplasia of endothelial cells of the small branch of the pulmonary artery (arrow), obturating mixed thrombus. Hematoxylin-eosin, x100.
In the pulmonary tissue, along with acute inflammatory changes (hyaline membranes, intra-alveolar neutrophilic infiltration with cell lysis), proliferative processes (fibrous transformation with forming fibrous nodules of the tissue) were observed (Fig. 8).
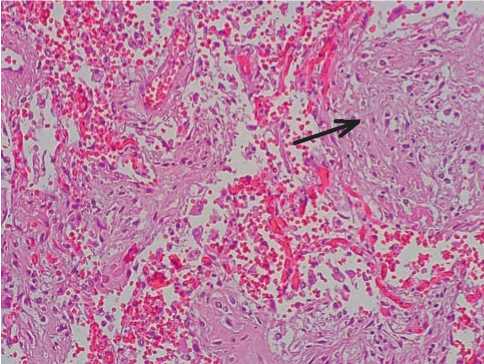
Fig. 8. Formation of fibrous nodules (arrow) in the lung tissue in COVID-19. Hematoxylin-eosin, x100.
The reticular frame of the walls of the microvasculature vessels was saved; there was an increase in argyrophilic fibers in the interalveolar septa.
The obtained results of the pulmonary vessel pathomorphological changes in COVID-19 are shown in Fig. 9.
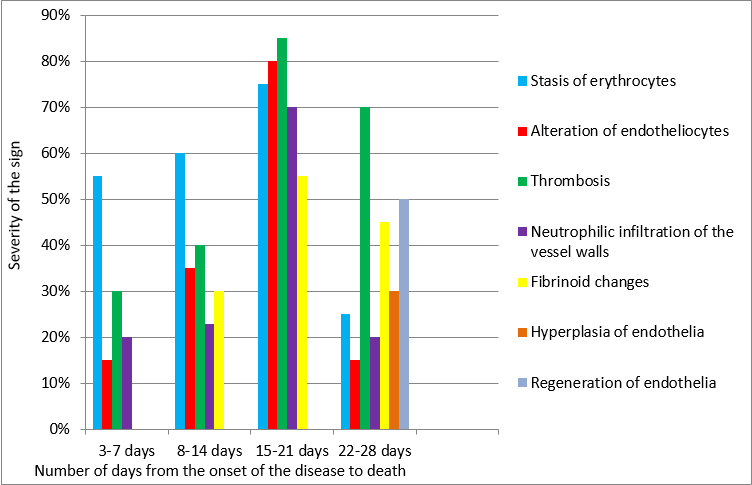
Fig. 9. Pathomorphological changes in the pulmonary vessels in COVID-19 at different times of the development of the disease (on the abscissa – the number of days from the onset of the disease to death; on the ordinate – the severity of the sign).
Discussion
The scientific platform of the study was the autopsy material of 40 dead with COVID-19 determined in vivo (PCR, computer tomography of the lungs) and by means of macro- and microscopic signs.
In the pathomorphological study of the lungs in COVID-19, particular emphasis was placed on the peculiarities of the blood vessel changes in different terms of lethal outcomes (3–7, 8–14, 15–21, 22–28 days from an early manifestation of the disease). The authors did not find any similar scientific works in modern literature.
The authors showed that, for 3–7 days from the onset of the COVID-19 disease, in the vessels of the microvasculature, acute circulatory injuries were paramount with developing stasis of erythrocytes and sharp plethora, which were the most pronounced by 15–21 days. In capillaries and small arteries, the signs of the alteration of individual endotheliocytes first emerged (3–7 days) with developing the destruction of cell groups on 15–21 days of COVID-19, which was accompanied by thrombosis and fibrinoid changes with neutrophilic infiltration of vessel walls. The reticular and collagenous frame of the walls of small arteries was saved, which indicated the superficial nature of vascular lesions.
Following 15–21 days of the COVID-19 development, high pronounced neutrophilic infiltration of the alveoli with the destruction of the alveolar septa was noted, which was probably caused by the alteration of the endothelia of vessels with increased vascular permeability and activating neutrophils.
Following 22–28 days of the COVID-19 development, in the walls of pulmonary vessels, reparatory phenomena, such as hyperplasia and regeneration of endotheliocytes, were observed with forming local thickenings of the intima with the presence of obturating fibrin mixed thrombi. In pulmonary tissue, the foci of interstitial fibrosis were observed with forming fibrous nodules.
Conclusion
The findings of this study agree with the results of other scientific works devoted to the role of coagulopathy and thrombosis of vessels in COVID-19. Disorders of blood coagulation are observed in the area of diffuse alveolar lesions and accompanied by developing severe hypoxemia [11][12][13][14][15].
The authors suppose that, in COVID-19, progressing endotheliopathy of microvessels in the lungs occurs, and it is characterized by swelling, alteration, and further hyperplasia and regeneration of endotheliocytes along with microthrombosis. Destructive changes of the walls of pulmonary microvessels bear a superficial nature of damage without destruction of the reticular frame and basement membranes.
The authors first noted that, by 15–21 days of the COVID-19 development, in the vessels of the microvasculature, small pulmonary arteries, alteration changes of endotheliocytes, microthrombosis of blood vessels were pronounced, which, along with neutrophilic infiltration of the walls of vessels and pulmonary tissue, facilitated the destruction of alveolar septa.
The authors suppose that the given terms of the COVID-19 development (15–21 days) are critical for forming a vicious circle of the development of pathological processes in the lungs (microthrombosis, pneumonia), which leads to severe hypoxemia and acute respiratory failure.
Reparative changes in the endothelial cells of microvessels in COVID-19, which may be essential to further development of obturating microangiopathy of the lungs, are of special interest. It is possible that such “pillowlike” thickenings of the intima due to hyperplasia of the endothelial cells may resemble the ones that occurred in the intima hyperplasia of arteries caused by other pathologic processes described in the literature [16]. These structural changes of the intima of pulmonary microvessels in COVID-19 may be, in the distant period, a ground for developing hyperplasia of the vessels of the pulmonary circulation and forming cor pulmonale with subsequent development of chronic forward heart failure. With this in mind, subsequently, it is necessary to continue studying immunohistochemical and molecular-biological peculiarities of the damage of pulmonary vessels in COVID-19.
So, endotheliopathy, microthrombosis of pulmonary microvessels, and interstitial pneumonia create a vicious circle of the development of severe respiratory failure, which is necessary to consider in clinical practice for correcting the treatment of patients with COVID-19.
References
1. Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. // Lancet Infect Dis. – 2020. – V.20(10). – P. 1135-1140. DOI: 10.1016/S1473-3099(20)30434-5
2. Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. // Zhonghua Bing Li Xue Za Zhi. 2020. – V.49(5). – P. 411-417. (In Chinese). DOI: 10.3760/cma.j.cn112151-20200312-00193
3. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. // Histopathology. – 2020. – V.77(2). – P. 198-209. DOI: 10.1111/his.14134
4. Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. // Lancet Haematol. – 2020. – V.7(8). – P. e575-e582. DOI: 10.1016/S2352-3026(20)30216-7
5. Katneni UK, Alexaki A, Hunt RC, Schiller T, DiCuccio M, et al. Coagulopathy and Thrombosis as a Result of Severe COVID-19 Infection: A Microvascular Focus. // Thromb Haemost. – 2020. – V. 120(12). – P. 1668-1679. DOI: 10.1055/s-0040-1715841
6. Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. // EBioMedicine. – 2020. – V.58. – P.102925. DOI: 10.1016/j.ebiom.2020.102925
7. do Espírito Santo D.A., Lemos A.C.B., Miranda C.H. In vivo demonstration of microvascular thrombosis in severe COVID-19. // J Thromb Thrombolysis. – 2020. – V. 50(4). – P. 790-794. DOI: 10.1007/s11239-020-02245-x
8. Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. // Inflamm Res. – 2020. – V. 69(12). P.1181-1189. DOI: 10.1007/s00011-020-01401-6
9. Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. // Thromb Res. – 2020. – V.191. – P.145-147. DOI: 10.1016/j.thromres.2020.04.013
10. Zhou F., Yu T., Du R., Fan G., Liu Y., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. // Lancet. 2020. – V.395(10229). – P. 1054-1062. DOI: 10.1016/S01406736(20)30566-3
11. Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. // Br J Haematol. – 2020. V.189(5). – P.846-847. DOI: 10.1111/bjh.16727
12. Bray M.A., Sartain S.E., Gollamudi J., Rumbaut R.E. Microvascular thrombosis: experimental and clinical implications. // Transl Res. – 2020. – V. 225. – P. 105-130. DOI: 10.1016/j.trsl.2020.05.006
13. Rybakova M.G., Karev V.E., Kuznetsova I.A. Patologicheskaya anatomiya novoi koronavirusnoi infektsii COVID-19. Pervye vpechatleniya [Anatomical pathology of novel coronavirus (COVID-19) infection. First impressions]. Arkh Patol. 2020;82(5):5-15. (In Russ.). DOI: 10.17116/patol2020820515
14. Odilov A.A., Tsimbalist N.S., Volkov A.V., Babichenko I.I. Izmeneniya organov, vyyavlennye pri posmertnom issledovanii patsientov s COVID-19 [Organ changes found by postmortem examination in COVID-19 patients]. Arkh Patol. 2020;82(6):63-69. (In Russ.). DOI: 10.17116/patol20208206163
15. Samsonova M.V., Mikhaleva L.M., Zairatyants O.V., Varyasin V.V., Bykanova A.V., et al. Lung pathology of COVID-19 in Moscow. Archive of Pathology = Arkhiv patologii. 2020;82(4):32–40. (In Russ.). DOI: 10.17116/patol20208204132
16. Todorov S.S., Sidorov R.V., Talalaev E.P., Shlyk I.F. ¢e role of glycosaminoglycanes in the development of intimal hyperplasia in the shunting of coronary arteries. Medical Herald of the South of Russia. 2018;9(3):94-98. (In Russ.). DOI: 10.21886/2219-8075-2018-9-3-94-98
About the Authors
S. S. TodorovРоссия
Sergey S. Todorov, Dr. Sci. (Med.), Associate Professor of the Department of Operative Surgery, Clinical Anatomy and Pathological Anatomy, head of the morphological department of the clinic, pathologist of the highest category
Rostov-on-Don
V. Yu. Deribas
Россия
Viktoria Y. Deribas, head of the department, pathologist of the highest category, assistant of the department of pathological anatomy
Rostov-on-Don
A. S. Kazmin
Россия
Andrey S. Kazmin, Assistant of the Department of Pathological Anatomy, Pathologist of the Highest Category
Rostov-on-Don
G. L. Reznikova
Россия
Galina L. Reznikova, Cand. Sci. (Med.), Chief Physician, Pathologist of the Highest Category
Rostov-on-Don
Yu. M. Makarenko
Россия
Yuri M. Makarenko, Cand. Sci. (Med.), Head of the Department of Organizational and Methodological Work
Rostov-on-Don
F. V. Polesovoy
Россия
Philip V. Polesovoy, Head of the Pathology Department, Pathologist
Rostov-on-Don
S. S. Todorov (jr)
Россия
Sergey S. Todorov (Jr.), student of the Faculty of Treatment and Prevention
Rostov-on-Don
Review
For citations:
Todorov S.S., Deribas V.Yu., Kazmin A.S., Reznikova G.L., Makarenko Yu.M., Polesovoy F.V., Todorov (jr) S.S. Pathomorphological changes in pulmonary vessels at different terms of lethal outcomes of patients with COVID-19. Medical Herald of the South of Russia. 2021;12(2):54-61. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-2-54-61
JATS XML







































