Scroll to:
Features of structural and functional remodeling of the heart in patients with chronic heart failure and senile asthenia syndrome
https://doi.org/10.21886/2219-8075-2025-16-2-83-91
Abstract
Objective: to evaluate the features of the structural and functional parameters of the heart in patients with chronic heart failure (CHF) and senile asthenia syndrome (SSA). Materials and methods: the study involved 161 respondents with CHF on the background of arterial hypertension (AH) (82 women and 79 men) aged 80 to 91 years. Taking into account the presence of CSA, patients were divided into 2 groups: group 1 — patients with CHF and CSA (n = 84), group 2 — patients with CHF without CSA (n = 77). Screening and diagnosis of CSA were performed using the "Age is not a hindrance" questionnaire and a comprehensive geriatric assessment. To determine the structural and functional parameters of the heart, transthoracic echocardiography (EchoCG) and Doppler EchoCG studies were performed. Results: in patients with hypertension and CHF with the development of CSA, compared with patients with hypertension and CHF without CSA, statistically significantly higher values of indexed indicators of anterior-posterior LP size (ILP), LP volume (IOLP), left ventricular myocardial mass (LVMI) (p <0.05) were observed, as well as a higher percentage of the development of concentric LV remodeling (CRLH) and a smaller one — concentric LV hypertrophy (CRLH), which is associated with an increased risk of adverse cardiovascular events. Analysis of LV systolic function revealed significantly higher LV end systolic size index (ICSR), LV end diastolic size index (ICDR), LV end diastolic volume index (ICDO), LV end systolic volume index (ICSO), lower LV minute volume index (MO), this reflects a decrease in LV myocardial contractility. In addition, patients with CSA have a lower percentage of LV ejection fraction (EF): 44% versus 59.2% (p=0.002). Accordingly, among patients with CSA, a lower percentage of heart failure with preserved LV ejection fraction (LVEF) was detected (p = 0.028). There was a statistically significant decrease in DT, IVRT, and e' indices, as well as an increase in the E/e' ratio, which indicates a more pronounced progression of LV diastolic dysfunction in "fragile" patients with hypertension and CHF. Conclusion: in the presence of CSA, patients with hypertension and CHF aged 80 years and older showed more significant changes in the structural and functional parameters of the heart, indicating a more pronounced violation of systolic and diastolic function.
Keywords
For citations:
Safronenko V.A., Chesnikova A.I., Safronenko A.V. Features of structural and functional remodeling of the heart in patients with chronic heart failure and senile asthenia syndrome. Medical Herald of the South of Russia. 2025;16(2):83-91. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-2-83-91
Introduction
It is well known that, in recent years, the demographic situation both worldwide and in the Russian Federation has been characterized by an increase in life expectancy. However, the growing proportion of elderly and senile patients is generally accompanied by an increase in comorbid pathology [1]. For instance, 65% of individuals aged 65–84 years old have comorbid conditions, and in patients older than 84 years old, the likelihood increases to 82%. Notably, arterial hypertension (AH) constitutes a component of comorbidity in 66% of elderly patients [2]. According to the national guidelines for the diagnosis and treatment of chronic heart failure (CHF, 2024), AH is the underlying cause of CHF in 88% of cases [3][4].
In turn, according to the EPOCH-CHF study, the prevalence of CHF in the country has increased over the past twenty years from 6.1% to 8.2% [5]. Moreover, according to several authors, the incidence of senile asthenia syndrome (SAS) also rises with age. This syndrome is accompanied by a decline in physiological reserves and leads to the progression of preexisting diseases [6][7]. The presence of SAS increases the risk of hospitalization by 1.2–1.8 times and the risk of death by 1.8–2.3 times, making it a prognostically unfavorable factor [8].
In this regard, experts from the Russian Association of Gerontologists and Geriatricians believe that the presence of SAS in elderly patients with cardiovascular diseases requires a more thorough assessment of laboratory and instrumental diagnostic findings. This is essential for determining the strategy and tactics of managing this category of patients, as well as for preventing the development of cardiovascular complications [8].
At present, special attention is being paid to studies focusing on the structural and functional parameters of the heart in elderly patients with AH and CHF, particularly in relation to the presence of SAS.
The aim of the study was to assess the characteristics of the structural and functional parameters of the heart in patients with CHF, depending on the presence or absence of SAS.
Materials and Methods
In a case–control study, 161 respondents with CHF secondary to AH (stages IIA–IIB and functional classes II–IV) aged 80 to 91 years old (82 women and 79 men) were enrolled. All participants were divided into two groups according to the presence of SAS: Group I – patients with CHF and SAS (n = 84), and Group II – patients with CHF without SAS (n = 77).
The following conditions were considered exclusion criteria: acute cerebrovascular accident or transient ischemic attack within the previous 6 months, history of coronary artery disease, hemodynamically significant heart defects, implanted pacemaker, severe liver pathology (elevation of transaminase levels ≥ five times the upper normal limit) or kidney disease (glomerular filtration rate ≤ 30 mL/min), and malignant neoplasms.
The study included patients with a previously established diagnosis of AH and CHF.
Screening for SAS (also referred to as frailty) was performed using the “Age Is Not a Barrier” questionnaire, the Short Physical Performance Battery (SPPB), handgrip dynamometry, and the Mini-Cog test. A score of ≥ 5 points on the “Age Is Not a Barrier” questionnaire, ≤ 7 points on the SPPB, and/or < 3 points on the Mini-Cog test was considered highly suggestive of SAS. To confirm the diagnosis, a geriatrician performed a comprehensive geriatric assessment [9].
All patients underwent transthoracic echocardiography and Doppler echocardiography using a MyLab70 system (Esaote, Italy). The examination protocol included assessment of the following linear and volumetric parameters of the left ventricle (LV): anteroposterior diameter of the left atrium (LA, mm), indexed LA anteroposterior diameter (ILA, mm/m²), LA volume (LAV, mL), indexed LA volume (LAVI, mL/m²), interventricular septal thickness (IVS, mm), posterior wall thickness of the LV (PWTLV, mm), relative wall thickness index (RWT), LV myocardial mass (LVM, g), LV mass index (LVMI, g/m²), LV end-diastolic diameter (EDD, mm), indexed LV end-diastolic diameter (iEDD, mm/m²), LV end-diastolic volume (EDV, mL), indexed LV end-diastolic volume (iEDV, mL/m²), LV end-systolic diameter (ESD, mm), indexed LV end-systolic diameter (iESD, mm/m²), LV end-systolic volume (ESV, mL), indexed LV end-systolic volume (iESV, mL/m²); left ventricular ejection fraction (LVEF, %) determined by the Simpson method, stroke volume (SV, mL), stroke index (SI, mL/m²), cardiac output (CO, mL/min), cardiac index (CI, L/min/m²), fractional shortening (FS, %), and myocardial stress (MS, g/cm²).
Diastolic function was assessed using the ratio of peak early diastolic transmitral flow velocity (E, m/s) to peak late diastolic transmitral flow velocity (A, m/s), the ratio of E (m/s) to early diastolic mitral annular velocity (e′, m/s), as well as the isovolumic relaxation time (IVRT, ms) and deceleration time of early LV filling (DT, ms).
Statistical analysis of the obtained data was performed using STATISTICA 12.0 (StatSoft Inc., USA), SPSS 21.0, and MedCalc (version 9.3.5.0). The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to assess the normality of distribution. For normally distributed quantitative variables, Student’s t-test was applied. In cases of non-normal distribution, the Yates-corrected χ² test was used for qualitative variables, and the Mann–Whitney U test was applied for quantitative variables. Comparisons among three or more groups were performed using ANOVA and the Kruskal–Wallis test. Results were considered statistically significant at p < 0.05.
Results
The prevalence of risk factors and comorbidities among study participants is presented in Figure 1. It was found that patients with AH and CHF who had SAS, compared with those without SAS, demonstrated a significantly higher prevalence of chronic kidney disease (by 26.4%, p < 0.001) and atrial fibrillation (by 25.3%, p < 0.001). At the same time, obesity was significantly less frequent in Group I (by 13.5%, p = 0.032).
The duration of AH history in the studied groups was 22.0 ± 1.7 years, with no significant intergroup differences in this parameter (p > 0.05). All patients in both groups had stage III hypertension and a very high cardiovascular risk.
No statistically significant differences (p > 0.05) were found between the groups in terms of CHF duration, which averaged 8.4 ± 3.6 years, or in terms of CHF stage distribution. However, patients with AH, CHF, and SAS were significantly more likely to have NYHA functional class III CHF (61.9% vs. 45.5%, p = 0.041), whereas patients without SAS were significantly more likely to have NYHA functional class II CHF (41.6% vs. 28.6%, p = 0.041).
It should be noted that “frail” patients with AH and CHF, compared with “robust” patients, had a statistically significantly higher Rating Scale of Clinical State score (RSCS) (by 28.6%, p < 0.001) and a lower 6-minute walk test (6MWT) distance (238.5 m [ 181.3–310.8] vs. 365.0 m [ 261.5–405.5], p < 0.001).
All study participants received treatment for AH and CHF in accordance with current guidelines, with no significant differences in pharmacotherapy between the groups (p > 0.05) (Figure 2) [10][11].
According to the Morisky–Green questionnaire (8-item Morisky Medication Adherence Scale, MMAS-8), patients in both study groups demonstrated moderate adherence to therapy (Group I – 6.4 ± 1.2 points, Group II – 6.4 ± 1.1 points), with no significant differences between the groups (p > 0.05).
Comparative analysis of echocardiographic findings showed that “frail” patients with AH and CHF, compared with “robust” patients with AH and CHF, had significantly higher values of both linear (by 12.3%, p = 0.018) and volumetric (by 16.6%, p = 0.018) LA parameters, as well as their indexed values (by 30%, p = 0.007 and by 28.4%, p = 0.006, respectively), which are associated with an increased risk of adverse cardiovascular events (Table 1).
Furthermore, the linear and volumetric LV parameters, as well as their indexed values, also differed significantly between the study groups. In Group I patients, these parameters were significantly higher compared with those in Group II: iEDD – by 17.7% (p = 0.047), iESD – by 23.3% (p = 0.041), EDV – by 19.2% (p < 0.01), iEDV – by 28% (p < 0.01), ESV – by 31.7% (p < 0.01), and iESV – by 41% (p < 0.01). These findings are most likely related to hemodynamic overload with subsequent cardiac remodeling in patients not only with AH and CHF, but also in the presence of SAS.
Analysis of LV morphometric parameters revealed the presence of LV hypertrophy in all patients included in the study. Despite the absence of significant differences in LV wall thickness (IVS and PWTLV) between the groups (p > 0.05), LVH was statistically significantly more pronounced in patients with AH and CHF in the presence of SAS, as evidenced by a 22.2% higher LV mass index (LVMI) (p = 0.001).
Based on RWT and LVMI values, the types of LV remodeling were determined in the study participants. As shown in Figure 3, patients with AH, CHF, and SAS had a significantly higher prevalence of concentric LV remodeling and a lower prevalence of concentric LV hypertrophy (CLVH) compared with patients with AH and CHF without SAS (p < 0.05). The prevalence of eccentric LV hypertrophy (ELVH) did not differ significantly between the groups (p > 0.05).
Special attention was given to LV systolic function. Patients in Group I had a significantly lower LVEF (44.0% [ 42.4; 47.32]), indicating the presence of LV systolic dysfunction, compared with patients in Group II (59.2% [ 57.79; 60.54]) (p = 0.002), which corresponded to heart failure with preserved ejection fraction (HFpEF).
Analysis of CHF phenotypes according to LVEF revealed significant intergroup differences. HFpEF was identified in 38.1% of patients in Group I and 76.6% of patients in Group II (p = 0.028), heart failure with mildly reduced ejection fraction (HFmrEF) in 45.2% and 14.3% (p = 0.003), and heart failure with reduced ejection fraction (HFrEF) in 16.7% and 9.1% of patients, respectively (p = 0.031).
No significant differences were observed between the groups in LV functional activity as assessed by SV, SI, CI, FS, and MS (p > 0.05). However, patients in Group I had a significantly lower CO (5.4 mL/min [ 4.8; 5.8]) compared with those in Group II (6.6 mL/min [ 5.9; 7.3]) (p = 0.008).
Particular attention was paid to the assessment of LV diastolic function in the study population. It is well known that in the presence of permanent atrial fibrillation, evaluation of LV diastolic function is not feasible [12]. Therefore, for the assessment of transmitral flow (E/A ratio), patients with permanent atrial fibrillation were excluded from the study. The results showed that patients in both Group I and Group II had type II LV diastolic dysfunction (pseudonormal LV filling pattern, 0.8 < E/A < 2) and comparable values for early diastolic LV filling velocity (E wave), late diastolic filling velocity during LA systole (A wave), and the E/A ratio (p > 0.05).
However, when evaluating DT and IVRT, patients with AH, CHF, and SAS, compared with those with AH and CHF without SAS, demonstrated a significant reduction in LV early filling deceleration time (<150 ms) (by 24%, p = 0.002) and isovolumic relaxation time (<80 ms) (by 35%, p = 0.020), indicating elevated LV filling pressures and more pronounced diastolic dysfunction in the presence of SAS (Table 2).
In addition, assessment of the mitral annular early diastolic velocity (e′), which is known to be an indicator of LV diastolic relaxation and compliance, revealed significant differences. “Frail” patients with AH and CHF had a statistically significantly lower lateral mitral annular e′ velocity (by 26.1%, p = 0.026) and a higher E/e′ ratio (by 33.7%, p = 0.018) compared with “robust” patients with AH and CHF.
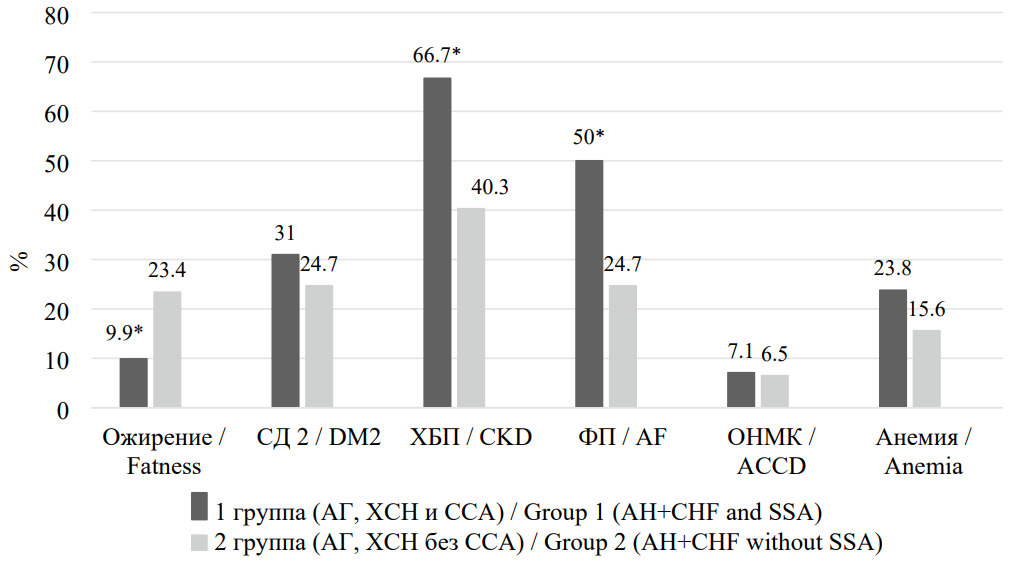
Рисунок 1. Распространённость факторов риска и сопутствующей патологии у пациентов, включенных в исследование
Figure 1. Prevalence of risk factors and concomitant pathology in patients included in the study
Примечание: * — р < 0,05 при сравнении со II группой. СД 2 — сахарный диабет 2-го типа; ХБП — хроническая болезнь почек; ФП — фибрилляция предсердий; ОНМК — острое нарушение мозгового кровообращения.
Note: * — p < 0.05 when compared with group II.DM2 — type 2 diabetes mellitus; CKD — chronic kidney disease; AF — atrial fibrillation; ACCD — acute cerebrovascular accident.
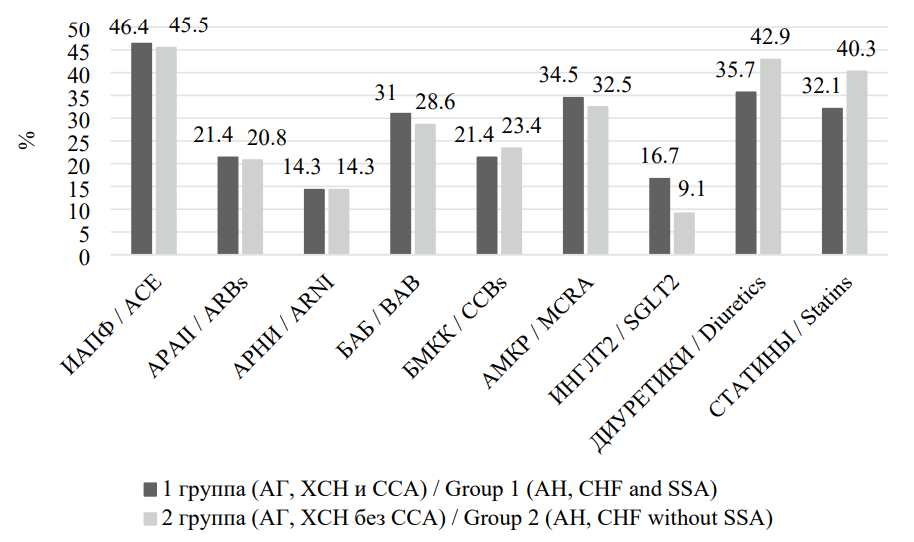
Рисунок 2. Частота назначения различных классов препаратов у пациентов, включенных в исследование
Figure 2. The frequency of prescribing different classes of drugs in patients included in the study
Примечание: ИАПФ — ингибиторы ангиотензинпревращающего фермента; АРАII — антагонисты рецепторов ангиотензина II типа; АРНИ — ангиотензиновых рецепторов и неприлизина ингибитор; ББ — бета-блокаторы; БМКК — блокаторы медленных кальциевых каналов; АМКР — антагонисты минералокортикоидных рецепторов; ИНГЛТ-2 — ингибиторы натрий-глюкозного котранспортера 2-го типа.
Note: ACE — Аngiotensin Converting Enzyme inhibitors; ARBs — Angiotensin II Receptor Blockers; ARNI — Angiotensin receptor-neprilysin inhibitor; BAB — beta blockers; CCBs — slow calcium channel blockers; MCRA — mineralocorticoid receptor antagonists; SGLT2 - Sodium glucose cotransporter-2 inhibitors.
Таблица / Table 1
Линейные и объемные показатели левых отделов сердца у пациентов, включенных в исследование
Linear and volumetric parameters of the left heart in patients included in the study
|
Показатель / Index |
I группа АГ+ХСН+ССА (n=84) / Group 1 AH+ CHF + SSA (n=84) |
II группа АГ+ХСН без ССА (n=77) / Group 2 AH+ CHF without SSA (n=77) |
р |
|
ИЛП, мм/м2 / LAI mm/m2 |
30,3 [ 26,85;32,28] |
23,3 [ 22,2;24,45] |
0,007
|
|
ИОЛП, мл/м2 / LAVI ml/m2 |
47,0 [ 45,38;50,53] |
36,6 [ 34,7;37,95] |
0,006
|
|
ИКДР, мм/м2 / LVDDI mm/m2 |
29,3 [ 27,3;30,98] |
24,9 [ 23,9;25,7] |
0,047
|
|
ИКСР, мм/м2 / LVSDI mm/m2 |
20,1 [ 19,2;21,4] |
16,3 [ 15,7;17,1] |
0,041
|
|
ИКДО, мл/м2 / LV EDVI ml/m2 |
95,7 [ 86,85;100,63] |
68,9 [ 65,3;72,7] |
<0,001
|
|
ИКСО, мл/м2 / LV ESVI ml/m2 |
47,6 [ 42,3;50,48] |
28,1 [ 27;28,95] |
<0,001
|
|
МЖП, мм / IVSd mm |
12,9 [ 12,7;13,1] |
12,4 [ 11,9;12,7] |
0,728
|
|
ЗСЛЖ, мм / PWLVd mm |
12,9 [ 12,5;13,3] |
12,1 [ 11,9;12,5] |
0,758
|
|
ИОТ / RWTI |
0,57 [ 0,50;0,61] |
0,52 [ 0,50;0,58] |
0,452
|
|
ИММЛЖ, г/м2 / LVMI g/m2 |
212,0 [ 184,63;223,48] |
164,9 [ 158,2;173,3] |
0,001 |
Примечание: ИЛП — индекс передне-заднего размера левого предсердия, ИОЛП — индекс объёма левого предсердия, ИКДР ЛЖ — индекс конечного диастолического размера левого желудочка, ИКДО ЛЖ — индекс конечного диастолического объёма левого желудочка, ИКСР ЛЖ — индекс конечного систолического размера левого желудочка, ИКСО ЛЖ — индекс конечного систолического объёма левого желудочка; МЖП — толщина межжелудочковой перегородки левого желудочка, ЗСЛЖ — толщина задней стенки левого желудочка, ИОТ — индекс относительной толщины стенок левого желудочка, ИММЛЖ — индекс массы миокарда ЛЖ.
Note: LAI — index of antero-posterior size of the left atrium, LAVI — left atrium volume indexed, LVDDI — left ventricular diastolic dimension index, LVSdI — left ventricular sistolic dimension index, LV EDVI — indexed left ventricular-end-diastolic volume, LV ESVI — left ventricular-end-systolic volume; IVSd — interventricular septal thickness, PWLVd — thickness of the posterior wall of the left ventricle, RWTI — index of relative wall thickness of the left ventricle, LVMI — left ventricular mass index.
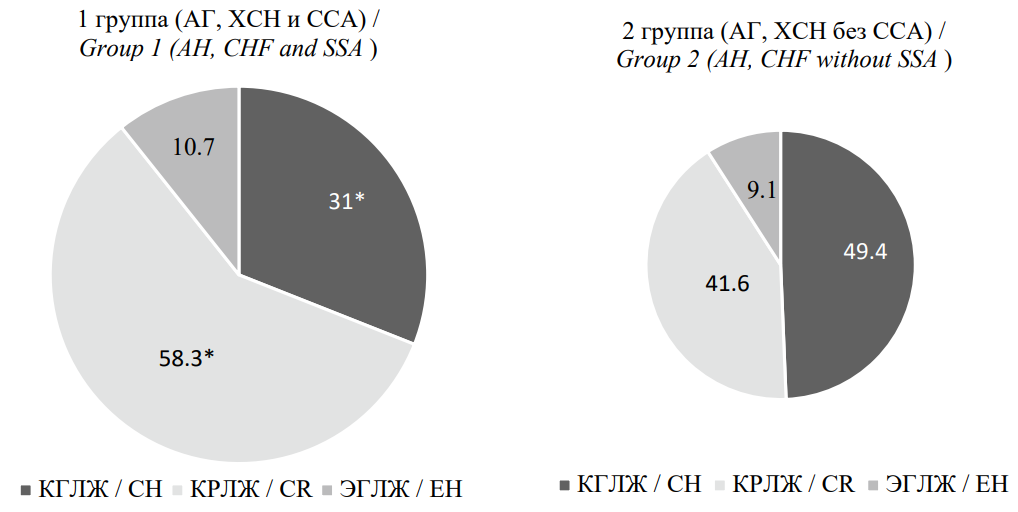
Рисунок 3. Типы ремоделирования левого желудочка у пациентов, включённых в исследование
Figure 3. Types of left ventricular remodeling in patients included in the study
Примечание: * — р < 0,05 при сравнении со II группой. КГЛЖ — концентрическая гипертрофия левого желудочка; КРЛЖ — концентрическое ремоделирование левого желудочка; ЭГЛЖ — эксцентрическая гипертрофия левого желудочка.
Note: * — p <0.05 when compared with group 2. CH — concentric hypertrophy left ventricular; CR — concentric remodeling left ventricular; EH — eccentric hypertrophy left ventricular.
Таблица / Table 2
Показатели количественной оценки диастолической функции ЛЖ у пациентов, включённых в исследование
Indicators of quantitative assessment of LV diastolic function in patients included in the study
|
Показатель / Index |
I группа АГ+ХСН+ССА (n=84) / Group 1 AH+ CHF + SSA (n=84) |
II группа АГ+ХСН без ССА (n=77) / Group 2 AH+ CHF without SSA (n=77) |
р |
|
DT, мс / DT, ms |
146,5 [ 143,63;150,86] |
192,7 [ 186,79;199,37] |
0,002
|
|
IVRT, мс / IVRT, ms |
68,8 [ 67,36;70,05] |
105,7 [ 100,57;109,9] |
0,020
|
|
Е/А / Е/А |
1,42 [ 1,38;1,47] (n=42) |
1,43 [ 1,38;1,45] (n=58) |
0,847
|
|
Е/е' / Е/е' |
13,9 [ 10,5;14,2] |
10,4 [ 9,64;11,3] |
0,018
|
Примечание: DT — время замедления скорости потока быстрого наполнения ЛЖ, IVRT — фаза изоволюмического расслабления, Е/А — отношение скоростей раннего и позднего диастолического наполнения ЛЖ через митральный клапан, Е/e′ — отношение максимальных скоростей раннего наполнения трансмитрального кровотока и движения фиброзного кольца митрального клапана.
Note: DT — the time of deceleration of the flow rate of rapid filling of the left ventricle, IVRT — isovolumic relaxation time, Е/А — the ratio of the maximum flow rate in the phase of early LV diastole to the maximum flow rate in the phase of late LV diastole through the mitral valve, Е/e′ — the ratio of the maximum flow velocity through the mitral valve in the phase of early LV diastole to early LV diastolic elongation.
Discussion
It is well known that in CHF, the myocardium undergoes a series of pathogenetic changes characterized by structural and functional remodeling. Compensatory activation of the sympathoadrenal and renin–angiotensin–aldosterone systems leads to increased collagen synthesis and the development of fibrosis, which is subsequently accompanied by increased stiffness and reduced elasticity of the myocardium. As the process progresses, the myocardial walls become thinner, the LV cavity enlarges, and its geometry changes from an elliptical to a spherical shape, ultimately resulting in the development of both systolic and diastolic LV dysfunction [13].
It should be taken into account that in elderly individuals without cardiovascular disease, structural and functional changes of the myocardium may still occur as part of the aging process [14].
In recent years, there has been an increase in the prevalence of SAS among elderly and senile patients with CHF, which leads to a more severe course of the disease and worsens the prognosis [15] – a finding that was also confirmed in our study.
“Frail” patients with CHF had significantly higher LAVI and LVMI values, which resulted in a higher prevalence of concentric LV remodeling. This type of remodeling is characterized by elevated peripheral vascular resistance and increased arterial stiffness – features also observed in this patient category and reported in our previously published studies [16]. Importantly, such structural alterations contribute to an increased risk of adverse cardiovascular outcomes in senile patients with AH, CHF, and SAS.
It should be noted that, according to several studies, LV mass (LVM) in elderly individuals tends to decrease slightly with age [17][18]. On the other hand, as vascular stiffness increases, myocardial afterload rises, leading to the development of LV hypertrophy (LVH) and, consequently, an increase in LVM [19]. In addition, aging is associated with an increase in LV wall thickness, with predominant development of concentric LV remodeling [19].
Rightward displacement of the aorta, along with the physiological shortening of the LV, results in a change in its geometry from an elliptical to a spherical shape [20]. The left atrium (LA) also undergoes age-related changes, including cavity dilation and wall hypertrophy [21].
In general, global LV systolic function in healthy individuals does not change with age [22]; however, there is evidence of alterations in contraction biomechanics, specifically in the “twist–untwist” mechanism [23].
In the study by Xi (2023), the impact of SAS on the LV structure and function in patients with HFpEF was demonstrated. “Frail” patients were found to have increased LVMI, enlarged LAV, and increased LA conduit function [24].
The results of our study also showed that the presence of SAS in patients with CHF led to a more pronounced increase in iESD, iEDD, iEDV, and iESV, as well as lower CO values, reflecting greater hemodynamic load, cardiac remodeling, and reduced LV contractile function. The statistically significantly lower CO, calculated as the product of SV and HR, in “frail” patients was likely due to a significantly lower HR in patients with AH, CHF, and SAS compared with those with AH and CHF without SAS.
In addition, LV systolic dysfunction in patients with SAS was accompanied by a lower LVEF and a higher proportion of patients with HFmrEF.
It is well known that with the progression of fibrosis formation, myocardial stiffness increases and elasticity decreases, leading to the development of LV diastolic dysfunction with age [25].
The differences in LV diastolic function parameters identified in our study – namely, significant reductions in DT, IVRT, and e′, along with an increase in the E/e′ ratio – indicate more advanced progression of LV diastolic dysfunction in “frail” patients with AH and CHF.
Similar findings were reported by other researchers. For example, Kusunose (2018) and Xi (2023) demonstrated that “frail” patients with HFpEF developed LV diastolic dysfunction, which was significantly associated with an increased risk of adverse cardiovascular events [24][26].
Conclusion
The study demonstrated that SAS in patients aged 80 years and older with AH and CHF exerted an additional impact on the structural and functional parameters of the heart, characterized by more pronounced impairments in LV systolic and diastolic functions, which contributed to the progression of CHF.
References
1. Drapkina O.M., Kontsevaya A.V., Kalinina A.M., Avdeev S.N., Agaltsov M.V., et al. Comorbidity of patients with noncommunicable diseases in general practice. Eurasian guidelines. Cardiovascular Therapy and Prevention. 2024;23(3):3996. (In Russ.) https://doi.org/10.15829/1728-8800-2024-3996. EDN: AVZLPJ
2. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37-43. https://doi.org/10.1016/S0140-6736(12)60240-2
3. Fomin IV. Arterial hypertension in the Russian Federationthe last 10 years. What’s next? Heart. 2007;6(3):1-6. (In Russ.)
4. Sitnikova M.Yu., Lyasnikova E.A., Yurchenko A.V., Trukshina M.A., Libis R.A., et al. The results of the Russian hospital register of chronic heart failure in 3 regions of the Russian Federation. Kardiologija. 2015;55(10):5-13. (In Russ.) eLIBRARY ID: 24872760 EDN: UYHOGD
5. Polyakov D.S., Fomin I.V., Belenkov Yu.N., Mareev V.Yu., Ageev F.T., et al. Chronic heart failure in the Russian Federation: what has changed over 20 years of follow-up? Results of the EPOCH-CHF study. Kardiologiia. 2021;61(4):4-14. (In Russ.) https://doi.org/10.18087/cardio.2021.4.n1628
6. Davydov E.L., Tikhonova N.V., Glushanko V.S., Shulmin A.V., Zakharova A.S. Senile asthenia syndrome: features of diagnosis, treatment and rehabilitation. Siberian Medical Review. 2020;5:40-48. (In Russ.) https://doi.org/10.20333/2500136-2020-5-40-48
7. Khazova E.V., Smetanina E.D., Malkova M.I. Frailty in patients with cardiovascular diseases: issues of epidemiology, diagnosis, prognosis. Meditsinskii al'manakh. 2023;3(76):98- 106. (In Russ.) eLIBRARY ID: 54678736 EDN: YELHVQ
8. Tkacheva O.N., Kotovskaya Yu.V., Runihina N.K., Frolova E.V., Milto A.S., et al. Comprehensive geriatric assessment in elderly and senile patients with cardiovascular diseases. Expert opinion of the Russian Association of Gerontologists and Geriatricians. Kardiologiia. 2021;61(5):71-78. https://doi.org/10.18087/cardio.2021.5.n1349
9. Tkacheva O.N., Kotovskaya Yu.V., Runikhina N.K., Frolova E.V., Naumov A.V., et al. Clinical guidelines Frailty. Russian Journal of Geriatric Medicine. 2025;1(21):6-48. https://doi.org/10.37586/2686-8636-1-2025-6-48
10. Kobalava Zh.D., Konradi A.O., Nedogoda S.V., Shlyakhto E.V., Arutyunov G.P., et al. Clinical practice guidelines for Hypertension in adults. Russian Journal of Cardiology. 2024;29(9):6117. (In Russ.) https://doi.org/10.15829/1560-4071-2024-6117 . EDN GUEWLU
11. Galyavich A.S., Tereshchenko S.N., Uskach T.M., Ageev F.T., Aronov D.M., et al. 2024 Clinical practice guidelines for Chronic heart failure. Russian Journal of Cardiology. 2024;29(11):6162. (In Russ.) https://doi.org/10.15829/1560-4071-2024-6162. EDN: WKIDLJ
12. Maslov A.P., Libis R.A. Diastolic dysfunction of the left ventricle in combination with CHF and permanent atrial fibrillation. Journal of Heart Failure. 2012;13(4):205-208. (In Russ.) eLIBRARY ID: 18379397 EDN: PMFCOV
13. Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985;57(1):84-95. https://doi.org/10.1161/01.res.57.1.84
14. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107(1):139-146. https://doi.org/10.1161/01.cir.0000048892.83521.58
15. Podobed I.V., Proshchaev K.I., Akhmedov T.A. Rukavishnikov A.S., Kovalenko O.JU. Geriatric aspects of the course of chronic heart failure. Modern Problems of Health Care and Medical Statistics. 2021;(1):303-325. (In Russ.) eLIBRARY ID: 46327505 EDN: FQAAHB
16. Safronenko V.A., Chesnikova A.I. Features of central aortic pressure at patients with arterial hypertension aged 80 years and older, taking into account the presence of chronic heart failure and senile asthenia syndrome. Medical Herald of the South of Russia. 2025;16(1):28-38. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-28-38
17. Khouri MG, Maurer MS, El-Khoury Rumbarger L. Assessment of age-related changes in left ventricular structure and function by freehand three-dimensional echocardiography. Am J Geriatr Cardiol. 2005;14(3):118-125. https://doi.org/10.1111/j.1076-7460.2005.03845.x
18. Nikitin NP, Loh PH, de Silva R, Witte KK, Lukaschuk EI, et al. Left ventricular morphology, global and longitudinal function in normal older individuals: a cardiac magnetic resonance study. Int J Cardiol. 2006;108(1):76-83. https://doi.org/10.1016/j.ijcard.2005.04.009
19. Ganau A, Saba PS, Roman MJ, de Simone G, Realdi G, Devereux RB. Ageing induces left ventricular concentric remodelling in normotensive subjects. J Hypertens. 1995;13(12 Pt 2):1818-1822. PMID: 8903659.
20. Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90(11):1231-1236. https://doi.org/10.1016/s0002-9149(02)02840-0
21. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490-497. https://doi.org/10.1161/01.cir.0000048894.99865.02
22. Younis LT, Melin JA, Robert AR, Detry JM. Influence of age and sex on left ventricular volumes and ejection fraction during upright exercise in normal subjects. Eur Heart J. 1990;11(10):916-924. https://doi.org/10.1093/oxfordjournals.eurheartj.a059613
23. Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006;19(9):1077-1084. https://doi.org/10.1016/j.echo.2006.04.011
24. Xi L, Xuemei Z, Ling Y, Changchun C, Zhuo H, et al. Correlation between frailty and cardiac structure and function in echocardiography in elderly patients with normal ejection fraction. Aging Clin Exp Res. 2023;35(4):775-784. https://doi.org/10.1007/s40520-023-02363-5
25. Burlew BS. Diastolic dysfunction in the elderly--the interstitial issue. Am J Geriatr Cardiol. 2004;13(1):29-38. https://doi.org/10.1111/j.1076-7460.2004.00059.x
26. Kusunose K, Okushi Y, Yamada H, Nishio S, Torii Y, et al. Prognostic Value of Frailty and Diastolic Dysfunction in Elderly Patients. Circ J. 2018;82(8):2103-2110. https://doi.org/10.1253/circj.CJ-18-0017
27.
About the Authors
V. A. SafronenkoRussian Federation
Victoria A. Safronenko, Cand. Sci. (Med.), Associate Professor, Department of Internal Medicine No. 1
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. I. Chesnikova
Russian Federation
Anna I. Chesnikova, Dr. Sci. (Med.), Professor, Head of the Department of Internal Medicine No. 1
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. V. Safronenko
Russian Federation
Andrej V. Safronenko, Dr. Sci. (Med.), Prof., Head of the Chair of Pharmacology and Clinical Pharmacology
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Safronenko V.A., Chesnikova A.I., Safronenko A.V. Features of structural and functional remodeling of the heart in patients with chronic heart failure and senile asthenia syndrome. Medical Herald of the South of Russia. 2025;16(2):83-91. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-2-83-91







































