Scroll to:
Dynamics of contamination by Vibrio cholerae of water bodies of the Rostov-on-Don environment and characteristics of biological properties of isolated strains
https://doi.org/10.21886/2219-8075-2025-16-1-81-88
Abstract
Objective: retrospective analysis of the results of monitoring studies of environmental water bodies in Rostov-on-Don for the presence of V. cholerae for the period from 1989 to 2023 at stationary sampling points assigned to the Rostov-on-Don Anti-Plague Institute of Rospotrebnadzor, identification of trends and features of the dynamics of isolation and biological properties isolated strains. Materials and methods: in the period from 1989 to 2023, 4362 samples from aquatic ecosystems were examined. The organization and conduct of research was carried out in accordance with regulatory and methodological documents (SanPiN 3.3686-21, MUK 4.2.3745- 22, MUK 4.2.3746-22). Sequencing of isolated strains was performed on the MiSeq (Illumina) platform. Results: during the analyzed period, V. cholerae strains were found in all studied reservoirs of the Rostov-on-Don: in the Don, Temernik and Dead Donets rivers, mainly in July and August. Of the 122 isolated strains, 8 are epidemiologically significant (toxigenic strains) and 114 are nontoxigenic. The largest number of strains was isolated in 2022 — 21 strains. V. cholerae strains O1 (119 strains) and O139 (3) of serogroups, biovar classic and El Tor (Ogawa, Inaba and Gikoshima serovars) were found. Conclusions: epidemiological risks of the introduction of the cholerae pathogen from endemic countries, as well as from neighboring countries, in cases of complications of the epidemic of this infection, remain. In the surface reservoirs of the Rostov-on-Don, there are favorable conditions for the circulation of V. cholerae and the risk of spreading infection by water, in case of contamination by the cholerae pathogen of aquatic ecosystems.
Keywords
For citations:
Ezhova M.I., Mogilenko V.S., Degtyareva O.N., Menshikova E.A., Duvanova O.V., Yeghiazaryan L.A., Evteev A.V., Podoynitsina O.A., Vodopianov S.O., Shipko E.S., Savina I.V., Kruglikov V.D. Dynamics of contamination by Vibrio cholerae of water bodies of the Rostov-on-Don environment and characteristics of biological properties of isolated strains. Medical Herald of the South of Russia. 2025;16(1):81-88. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-81-88
Introduction
The unfavorable epidemiological situation with cholera in the world determines the risks of infection importation into the territory of any subject of the Russian Federation (RF), with the probability of its spread through waterborne transmission. Contamination of environmental water bodies (EWBs), namely surface water bodies and wastewater, with cholera vibrios indicates their epidemiological danger [1][2]. As part of the epidemiological surveillance of cholera in the RF territory1, monitoring investigations of EWBs are carried out in order to promptly detect epidemiologically significant (toxigenic) strains of cholera vibrios of serogroups O1 and O139 that are atypical for the non-endemic territory of Russia. In addition, during the assigned monitoring, epidemiologically insignificant (non-toxigenic) strains are also identified. Analysis of the results of monitoring investigations conducted in the territories of different RF subjects in different time periods makes it possible to judge the epidemiological situation for cholera and draw predictions about its development and the potential epidemiological risk of the spread of infection by water in the case of toxigenic strain import [1][3–7]. Concurrently, dynamic monitoring with an expansion of time boundaries and the use of modern analysis methods for an in-depth investigation of the isolated cultures of cholera vibrios are of particular importance.
In this regard, it was of interest to trace the dynamics of the isolation of cholera vibrio cultures over a period of more than thirty years and to characterize the biological properties of Vibrio cholerae strains O1 and O139 isolated from EWBs of Rostov-on-Don city, a territory classified as type I in terms of epidemic manifestations of cholera2.
The aim of the study was a retrospective analysis of the results of monitoring investigations of EWBs of Rostov-on-Don for the presence of cholera vibrios over the period from 1989 to 2023 at stationary sampling points assigned to the Rostov-on-Don Anti-Plague Institute of Rospotrebnadzor, and identifying trends and features of the dynamics of isolation and biological properties of isolated strains.
Materials and methods
Within the period from 1989 to 2023, 4362 samples from the city’s EWBs were analyzed, of which 3981 (91.3%) were samples from surface water bodies, and 381 (8.7%) were samples from wastewater. The studies were organized and conducted in accordance with regulatory and methodological documents3. Water samples from surface water bodies, covering the main epidemiologically important water bodies of the city (the Don, Temernik, and Mertvy Donets Rivers), as well as from wastewater after treatment and disinfection at the city’s treatment facilities before discharge into the Don River, were collected weekly from May to September over the period from 1989 to 2021 and from April to October in 2022 and 2023. Sampling was carried out at stationary sampling points assigned to the Rostov-on-Don Federal State Healthcare Institution, the Anti-Plague Institute of Rospotrebnadzor. During the referred period, the number of stationary points fluctuated from six to nine. Moreover, studies were conducted at additional sampling points based on epidemiological indications.
Isolation and identification of cholera vibrios were performed using bacteriological, serological, and molecular biological research methods. Sequencing of isolated strains was performed on the MiSeq platform (Illumina) to obtain 250 bp reads using the Nextera DNA Flex Library Prep Reagent Kit (Illumina). Genome assembly of the studied strains was performed using SPAdes (v.3.11.1) [8]. Genetic determinants were identified in whole-genome sequences using the BioEdit 7.2.5 (http://www.mbio.ncsu.edu/bioedit) and BLASTN 2.2.29 (http://blast.ncbi.nlm.nih.gov) software.
Statistical processing of the research data was performed using the computer software STATISTIKA (StatSoftRussia) and parametric methods. The research results were considered significant at the probability of an error-free forecast P> 0.95 or p < 0.05.
Results
In total, 122 strains of V. cholerae serogroups O1 and O139 were isolated and identified from water bodies in Rostov-on-Don during the period under study. Thus, for the period from 1989 to 1998, only one (0.8%) strain of V. cholerae O1 was isolated. From 1999 to 2008 inclusive, strains were isolated annually with increasing quantitative dynamics, namely 41 strains (33.6%). From 2009 to 2018, with the exception of three years (2011, 2012, and 2017), strains were isolated annually. During this period, a decrease in the number of isolated strains was observed, namely 28 (23.0%). From 2019 to the present, the isolation of V. cholerae O1 has resumed again with an annual increase in the number of detected strains. As a result, 52 strains (42.6%) were isolated during the specified period. The trend line demonstrates a stable tendency toward an increase in the number of isolated strains of V. cholerae serogroups O1 and O139 from water bodies and from wastewater in Rostov-on-Don during the analyzed period (Fig. 1).
On average, 4.0 ± 0.8 strains were isolated during the year.
Most strains were found in surface water bodies. Thus, 54 strains (44.0%) were isolated from the Don River, 50 strains (41.0%) were isolated from the Temernik River, and 13 strains (11.0%) were isolated from the Mertvyi Donets River channel. Five strains (4.0%) were isolated from wastewater. According to average multiannual data, the largest strain number was detected in samples from water bodies in July and August (35 strains, that is 28.7%, and 44 strains, that is 36.1%, respectively) (Fig. 2).
The largest number of V. cholerae O1/O139 strains was detected in water samples from stationary points No. 4 (Don River, Right Bank near the Western Bypass railway and road bridge) and No. 6 (Temernik River, Botanical Garden, near the bridge, wastewater discharge) (Fig. 3).
Regarding the pheno- and genotypic characteristics of the isolated cultures, it should be noted that the performed identification attested to their belonging to the V. cholerae species by taxonomic characteristics and the features of biological properties. Thus, out of 122 isolated strains of cholera vibrio, 119 (97.5%) were classified as V. cholerae O1, and three (2.5%) as serogroup O139. Since 2000, strains of serogroup O139 have not been detected in water bodies of Rostov-on-Don. One hundred eighteen strains belonged to the El Tor biovar, and one strain, isolated in 1999 from the Don River, belonged to the classic biovar. Of the 118 strains of V. cholerae O1 El Tor, 96 strains (80.7%) belonged to serovar Ogawa, 21 strains (17.8%) to serovar Inaba, and two (1.7%) to serovar Gikoshima. From 2009 to 2023, strains of serovar Gikoshima were absent among the isolated strains of V. cholerae O1 (Table).
Most V. cholerae O1 strains, namely 102 strains (83.6%), were resistant to cholera diagnostic phages. Eighteen strains (14.7%) were sensitive to the El Tor phage, and two (1.7%) were sensitive to the C phage. Only 22 (18.6%) of the 118 V. cholerae O1 El Tor strains were assigned to a specific phage type. The most common phage types were 15 (9 strains) and 16 (5 strains).
The isolated strains of V. cholerae O1 differed in their epidemic significance. Among them, eight (6.7%) were epidemiologically significant (toxigenic ctxAB+ tcpA+). These strains were isolated from EWBs in 1999, 2000, 2001, 2003, 2011, 2014, and 2023 (Table, Fig. 4).
The conducted sequencing and results of bioinformatic analysis of toxigenic strains showed that strains with the genetic characteristic ctxAB+ differed in the structure of the B subunit of cholera toxin. Thus, the genome of the V. cholerae classical strain (1999) contained the B1 subunit of the classical type, and the V. cholerae El Tor strain (2000) contained the ctxB3 allele of the El Tor type. At the same time, in the genomes of V. cholerae El Tor strains (2001, 2003, 2014, 2023), a replacement of the ctxB3 allele with ctxB1 was detected.

Рисунок 1. Динамика выделения холерных вибрионов О1, О139 серогрупп из поверхностных водоёмов и сточных вод на территории г. Ростова-на-Дону в период 1989–2023 гг.
Figure 1. Dynamics of the release of V. cholerae O1, O139 serogroups from surface reservoirs and wastewater in the territory of Rostov-on-Don in the period 1989–2023.
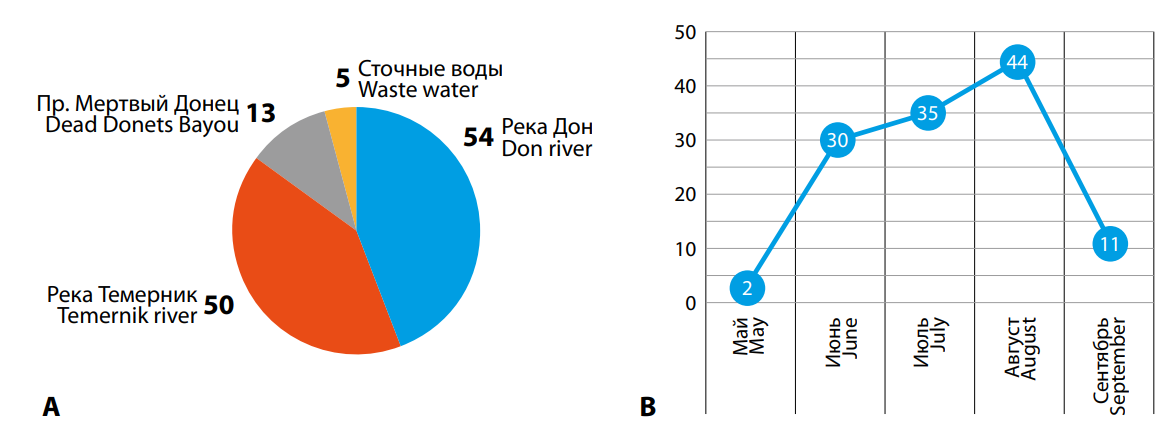
Рисунок 2. Количественное распределение изолированных штаммов (абс.) V. cholerae O1/О139 по водным объектам г. Ростова-на-Дону (А) и сезонность (Б) выделения штаммов за период 1989–2023 гг.
Figure 2. Quantitative distribution of isolated strains (abs.) of V. cholerae O1/O139 in the water bodies of Rostov-on-Don (A) and seasonality (B) of strain isolation for the period 1989–2023.
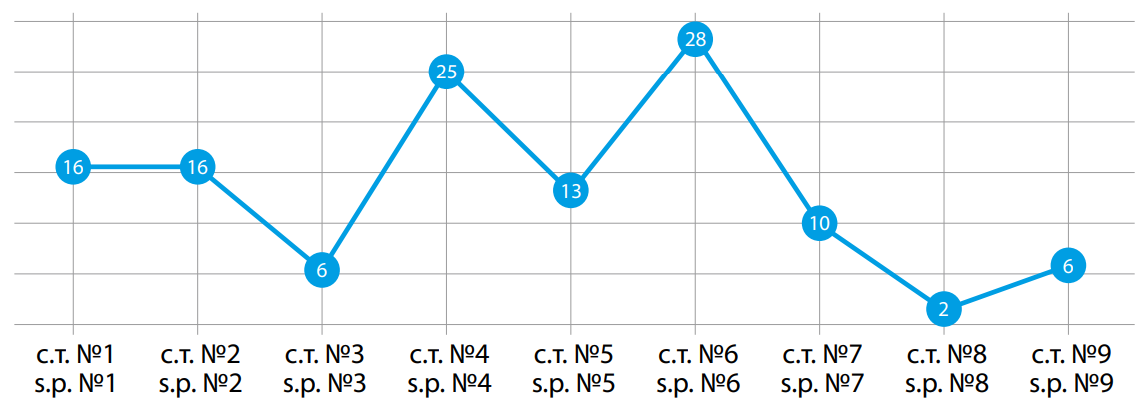
Рисунок 3. Количественное распределение выделенных культур холерных вибрионов О1, О139 серогрупп (абс.) по стационарным точкам в период с 1989–2023 гг. в г. Ростове-на-Дону. с. т. — стационарная точка.
Figure 3. Quantitative distribution of isolated cultures of V. cholerae O1,O139 serogroups (abs.) by stationary points in the period from 1989–2023 in Rostov-on-Don. s.p. — stationary point.
Таблица / Table
Характеристика биологических свойств культур холерных вибрионов, выделенных из водных объектов окружающей среды на территории г. Ростова-на-Дону с 1989 по 2023 гг.
Characteristics of the biological properties of V. cholerae cultures isolated from environmental water bodies on the territory of Rostov-on-Don from 1989 to 2023.
|
Год выделения |
Всего культур V. cholerae |
Из них V. cholerae О1 |
Из них V. cholerae О139 |
Из них эпидемически значимые (токсигенные) |
||||
|
Биовар |
Серовар |
|||||||
|
Clas-sical |
El Tor |
Огава |
Инаба |
Гикошима |
||||
|
1989 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1990 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1991 |
1 |
- |
1 |
1 |
- |
- |
- |
- |
|
1992 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1993 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1994 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1995 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1996 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1997 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1998 |
- |
- |
- |
- |
- |
- |
- |
- |
|
1999 |
6 (1*) |
1* |
2 |
3 (1*) |
- |
- |
3 |
1 |
|
2000 |
1* |
- |
1* |
- |
1* |
- |
- |
1 |
|
2001 |
6 (3*) |
- |
6 (3*) |
6 (3*) |
- |
- |
- |
3 |
|
2002 |
4 |
- |
4 |
2 |
1 |
1 |
- |
- |
|
2003 |
1* |
- |
1* |
1* |
- |
- |
- |
1 |
|
2004 |
2 |
- |
2 |
2 |
- |
- |
- |
- |
|
2005 |
4 |
- |
4 |
3 |
1 |
- |
- |
- |
|
2006 |
5 |
- |
5 |
- |
5 |
- |
- |
- |
|
2007 |
6 |
- |
6 |
4 |
2 |
- |
- |
- |
|
2008 |
6 |
- |
6 |
5 |
- |
1 |
- |
- |
|
2009 |
6 |
- |
6 |
4 |
2 |
- |
- |
- |
|
2010 |
14 |
- |
14 |
11 |
3 |
- |
- |
- |
|
2011 |
- |
- |
- |
- |
- |
- |
- |
- |
|
2012 |
- |
- |
- |
- |
- |
- |
- |
- |
|
2013 |
3 |
- |
3 |
2 |
1 |
- |
- |
- |
|
2014 |
1* |
- |
1* |
- |
1* |
- |
- |
1 |
|
2015 |
1 |
- |
1 |
1 |
- |
- |
- |
- |
|
2016 |
2 |
- |
2 |
2 |
- |
- |
- |
- |
|
2017 |
- |
- |
- |
- |
- |
- |
- |
- |
|
2018 |
1 |
- |
1 |
1 |
- |
- |
- |
- |
|
2019 |
1 |
- |
1 |
1 |
- |
- |
- |
- |
|
2020 |
9 |
- |
9 |
8 |
1 |
- |
- |
- |
|
2021 |
10 |
- |
10 |
10 |
- |
- |
- |
- |
|
2022 |
21 |
- |
21 |
21 |
- |
- |
- |
- |
|
2023 |
11 (1*) |
- |
11 (1*) |
8 (1*) |
3 |
- |
- |
1 |
|
Всего |
122 (8*) |
1* |
118 (7*) |
96 (6*) 95 - El Tor, 1 - classical
|
21 (2*) |
2 |
3 |
8 |
Примечание: * — эпидемически значимый (токсигенный) штамм ctxАВ+tcpА+.
Note: * — epidemically significant (toxigenic) strain ctxAB+ tcpA+.
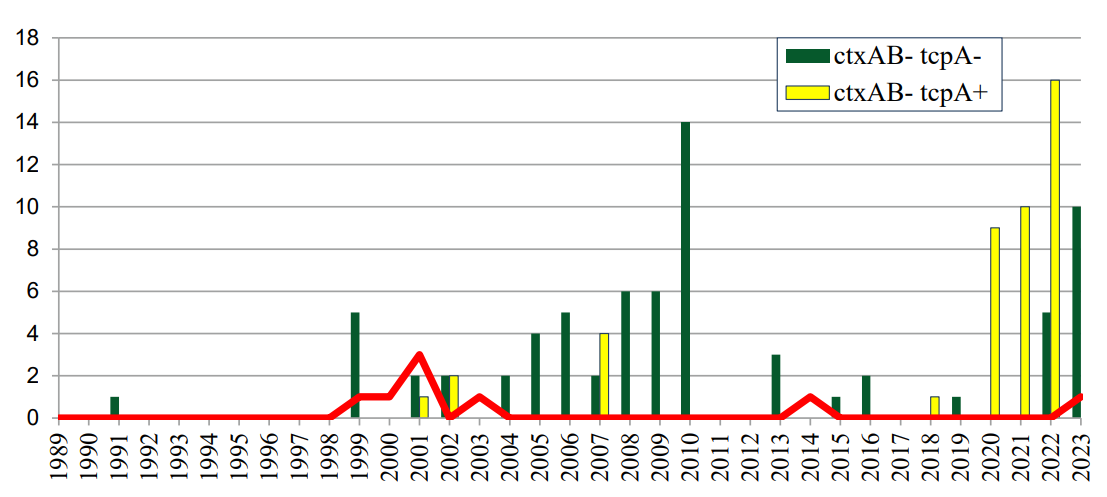
Рисунок 4. Генетическая характеристика штаммов, выделенных из водных ООС на территории г. Ростова-на-Дону за период 1989–2023 гг.
Figure 4. Genetic characteristics of isolated strains from aquatic environmental protection systems on the territory of Rostov-on-Don for the period 1989–2023.
Discussion
Analysis of the dynamics of isolation of strains of cholera vibrios serogroups O1 and O139 in Rostov-on-Don during the period under study has shown a stable increase in the number of isolated strains since the end of the 1980s, which indicates increasing contamination of water bodies and growing risks of cholera spread by water in the case of carrying the infection. Summer seasonality, typical for these microorganisms, was noted in the detection of strains. Strains of cholera vibrios were detected in all epidemiologically significant water bodies of the city and in water samples from all stationary sampling points. It is worth emphasizing that the trend observed in the period 1989–2018 [7] and associated with the quantitative distribution of strains across the water bodies where they were isolated remained stable. An analysis of the distribution of cholera vibrio strains by stationary sampling points revealed a virtually identical number of isolated strains from stationary points located along the Temernik and Don Rivers. Strains of V. cholerae O139 have not been detected over the past 23 years, which apparently indicates a decrease in its persistent potential.
When analyzing the distribution of V. cholerae strains of serogroup O1 isolated from water bodies in Rostov-on-Don, it was found that the majority of them, namely 117 strains, were isolated from surface water bodies, and their detection was recorded almost annually throughout the studied period, with an increasing trend. Five strains of V. cholerae serogroup O1 were isolated from wastewater within the period from 1989 to 2008, but in subsequent years, strains of cholera vibrios were not detected in these water objects (Table).
Among the eight toxigenic strains, genetically unchanged ones were identified including V. cholerae classical (1999) and V. cholera O1 El Tor strain (2000) containing the ctxB3 allele, which was designated as “prototypic” according to Monakhova et al. (2020); the remaining toxigenic strains of V. cholera O1 El Tor were representatives of the “first genovariants” differing from the prototype ones only by replacing the ctxB3 allele with ctxB1. Despite the fact that the majority of toxigenic strains of cholera vibrios currently circulating in the world belong to the “Haitian” or “post-Haitian” B7 group [1][9][10], the toxigenic strain isolated in 2023 from the water of the Temernik River also belonged to the “first genovariants”. The detection of toxigenic strains in water objects against the background of an epidemiologically favorable situation regarding cholera can reflect an unspecified import from Asian regions, where such clones are registered.
This fact should be considered as a real risk of aggravation of the cholera epidemic situation in Rostov-on-Don. Moreover, it requires a thorough epidemiological investigation to establish the source of contamination of EWBs and identify the risks of cholera spread caused by waterborne transmission of the pathogen. In addition, this determines the relevance of forecasting the probability of infection import in the medium and long term.
The vast majority of strains were identified as epidemiologically insignificant, namely non-toxigenic ctxAB-tcpA+ and ctxAB-tcpA‒. However, such strains can cause sporadic diseases or outbreaks with clinical manifestations of gastroenteritis due to the expression of genetic determinants of a number of pathogenicity factors. It should be noted that non-toxigenic strains, isolated from water bodies of Rostov-on-Don, have produced clonal complexes, representatives of which are capable of persistence and spread in water bodies. The genomes of these strains contained the VPI pathogenicity island with a cluster of TCP genes, which are a key adhesion factor of cholera vibrios [9]. The experience of multiannual detection indicates an increasing risk of the water factor being involved in the spread of infection, which requires measures to establish the source of contamination to reduce the risk of possible spread of cholera by water in the case of importation of a toxigenic strain and complications of the cholera epidemic situation in Rostov-on-Don.
Conclusions
A retrospective analysis of V. cholerae contamination dynamics in the main epidemiologically significant EWBs of Rostov-on-Don and the biological properties of strains isolated during monitoring from 1989 to 2023 showed the following:
- Strains of cholera vibrios of serogroup O1 are isolated from water bodies of the city almost every year. This indicates that surface water bodies and wastewater provide favorable conditions for the circulation of cholera vibrios, implying the risk of spreading the infection by water in the case of contamination of aquatic ecosystems.
- The prediction of the epidemiological situation for cholera indicates the continued epidemiological risks of importation of the cholera pathogen from currently endemic territories, as well as from neighboring countries in cases of activation of the epidemic process for the infection there.
- The conduct of monitoring investigations within the framework of epidemiological surveillance in the territory of Rostov-on-Don ensures the timely detection of the pathogen and the prompt implementation of anti-epidemic (preventive) measures to prevent the possible spread of infection through water as a transmission factor.
1. SanPiN 3.3686-21 “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases”.
2. MUK 4.2.3746-22 “Organization and implementation of laboratory diagnostics of cholera in laboratories of various levels”.
3. SanPiN 3.3686-21 “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases”.
References
1. Popova A.Yu., Noskov A.K., Ezhlova E.B., Kruglikov V.D., Monakhova E.V., et al. Epidemiological Situation on Cholera in the Russian Federation in 2023 and Forecast for 2024. Problems of Particularly Dangerous Infections. 2024;(1):76-88. (In Russ.) https://doi.org/10.21055/0370-1069-2024-1-76-88
2. Moskvitina E.A., Yanovich E.G., Kurilenko M.L., Kruglikov V.D., Noskov A.K. Environmental and Epidemiological Aspects of V. cholerae O1 Contamination of Water Bodies in the Republic of Kalmykia. Public Health and Life Environment – PH&LE. 2021;1(12):79-86. (In Russ.) https://doi.org/10.35627/2219-5238/2021-29-12-79-86
3. Mironova L.V., Bochalgin N.O., Gladkikh A.S., Feranchuk S.I., Ponomareva A.S., Balakhonov S.V. Phylogenetic Affinity and Genome Structure Features of ctxAB– tcpA+ Vibrio cholerae from the Surface Waterbodies in the Territory that is Non-Endemic as Regards Cholera. Problems of Particularly Dangerous Infections. 2020;(1):115-123. (In Russ.) https://doi.org/10.21055/0370-1069-2020-1-115-123
4. Kritsky A.A., Smirnova N.I., Kalyaeva T.B., Obrotkina N.F., Gracheva I.V., Katyshev A.D., Kutyrev V.V. Comparative Analysis of Molecular-Genetic Properties in Non-Toxigenic Vibrio cholerae O1 Strains Biovar El Tor, Isolated in Russia and on Cholera Endemic Territories. Problems of Particularly Dangerous Infections. 2021;(3):72-82. (In Russ.) https://doi.org/10.21055/0370-1069-2021-3-72-82
5. Agafonova E.Y., Smirnova N.I., Alkhova Z.V., Krasnov Y.M., Livanova L.F., et al. Non-toxigenic strains of Vibrio cholerae biovar El Tor, isolated in the territory of Russia: moleculargenetic peculiarities and pathogenic properties. Journal of microbiology, epidemiology and immunobiology. 2019;96(2):13- 24. (In Russ.) https://doi.org/10.36233/0372-9311-2019-2-13-24
6. Noskov A.K., Kruglikov V.D., Lopatin A.A., Chemisova O.S., Levchenko D.A., et al. Results of cholera monitoring in administrative territories of Russia from 2013 to 2019. Journal of microbiology, epidemiology and immunobiology. 2021;98(2):163-175. (In Russ.) https://doi.org/10.36233/0372-9311-56
7. Ezhova M.I., Levchenkо D.А., Arkhangelskaya I.V., Kruglikov V.D., Nepomnyashchaya N.B. Features of the Biological Properties of Vibrio cholerae Isolated during the Monitoring of Water Bodies in Rostov-on-Don from 1989 to 2018. Problems of Particularly Dangerous Infections. 2021;(1):148-151. (In Russ.) https://doi.org/10.21055/0370-1069-2021-1-148-151
8. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455-477. https://doi.org/10.1089/cmb.2012.0021
9. Monakhova E.V., Ghosh A., Mutreja A., Weill F., Ramamurthy T. Endemic Cholera in India and Imported Cholera in Russia: What is Common? Problems of Particularly Dangerous Infections. 2020;(3):17-26. (In Russ.) https://doi.org/10.21055/0370-1069-2020-3-17-26
10. Vodopyanov S.O., Vodopyanov A.S., Oleynikov I.P., Monakhova E.V. Elaboration of a Toxigenic Vibrio cholerae Typing Scheme Based on Bioinformatiсs Analysis Data. Public Health and Life Environment – PH&LE. 2022;(7):66-71. (In Russ.) https://doi.org/10.35627/2219-5238/2022-30-7-66-71
11.
About the Authors
M. I. EzhovaRussian Federation
Maria I. Ezhova, researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
V. S. Mogilenko
Russian Federation
Victoria S. Mogilenko, laboratory assistant at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
O. N. Degtyareva
Russian Federation
Olga N. Degtyareva, junior researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
E. A. Menshikova
Russian Federation
Elena A. Menshikova, Dr. Sci. (Biol.), Senior Researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
O. V. Duvanova
Russian Federation
Olga V. Duvanova, Dr. Sci. (Biol.), Senior Researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
L. A. Yeghiazaryan
Russian Federation
Liana A. Yeghiazaryan, junior researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. V. Evteev
Russian Federation
Artyom V. Evteev, junior researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
O. A. Podoynitsina
Russian Federation
Oksana A. Podoynitsina, Dr. Sci. (Biol.) researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
S. O. Vodopianov
Russian Federation
Sergey O. Vodopianov, Dr. Sci. (Med.) Chief Scientific Researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
E. S. Shipko
Russian Federation
Elena S. Shipko, junior researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
I. V. Savina
Russian Federation
Isabella V. Savina, junior researcher at the Department of Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
V. D. Kruglikov
Russian Federation
Vladimir D. Kruglikov, Dr. Sci. (Med.), Chief Researcher, Acting Head of the Departmentof Microbiology of Cholera
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Ezhova M.I., Mogilenko V.S., Degtyareva O.N., Menshikova E.A., Duvanova O.V., Yeghiazaryan L.A., Evteev A.V., Podoynitsina O.A., Vodopianov S.O., Shipko E.S., Savina I.V., Kruglikov V.D. Dynamics of contamination by Vibrio cholerae of water bodies of the Rostov-on-Don environment and characteristics of biological properties of isolated strains. Medical Herald of the South of Russia. 2025;16(1):81-88. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-81-88







































