Scroll to:
Predictors of unfavorable progression and prognosis in patients with heart failure with preserved left ventricular ejection fraction
https://doi.org/10.21886/2219-8075-2024-15-4-38-48
Abstract
Heart failure with preserved ejection fraction (HFpEF) is the most common form of heart failure (HF) worldwide and is characterized by a severe course, poor prognosis, and limited effective treatments. To date, there are no reliable prognostic algorithms to identify high-risk patients, and prognostic significance has been determined only for generally accepted clinical and standard resting echocardiographic parameters. The discovery of independent predictors of poor prognosis/severe course of HFpEF is important for determining individual treatment tactics for such patients.The article provides a review of studies devoted to determining clinical, biochemical and hemodynamic predictors of unfavorable progression and prognosis of heart failure with preserved ejection fraction (HFpEF). Significance of assessing of these predictors for determining prognosis and choosing optimal treatment for patients with HFpEF is shown. Directions for further research were identified: identifying phenotypes of HFpEF, developing personalized therapy, construction of prognostic models to identify high-risk patients who require more careful monitoring and/or more intensive drug treatment.
Keywords
For citations:
Sobolevskaya M.S., Gvozdeva A.D., Svirida O.N., Filatova A.Y. Predictors of unfavorable progression and prognosis in patients with heart failure with preserved left ventricular ejection fraction. Medical Herald of the South of Russia. 2024;15(4):38-48. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-4-38-48
Introduction
The prevalence of chronic heart failure with preserved ejection fraction (HFpEF) demonstrates an annual increase; currently, more than half of all patients with heart failure (HF) have a preserved left ventricular (LV) ejection fraction (EF) [1][2]. HFpEF is characterized by a high frequency of hospitalizations and mortality: according to follow-up studies, every second patient with HFpEF is readmitted to the hospital within the first six months after discharge, while the 5-year mortality rate among patients with HFpEF discharged from the hospital reaches 65% [1]. In contrast to HF with reduced LV EF, treatment methods improving the prognosis in HFpEF patients are currently limited. This is largely stipulated by the population heterogeneity of patients, as well as the complexity of HFpEF diagnostics that has affected the results of the main clinical trials devoted to the treatment of this condition [3]. Nowadays, investigations on the identification of specific HFpEF phenotypes and the development of personalized therapy for them, as well as the improvement of HFpEF diagnostic algorithms, are underway.
Furthermore, an important task in the treatment of HFpEF is the development of reliable prognostic models, which would make it possible not only to identify high-risk patients in advance but also to select the optimal methods of treating and further monitoring of patients. Besides, identifying factors, which promote an unfavorable prognosis, can help develop new targeted methods for treatment of HFpEF patients.
The aim of this review was to assess the role of the main available clinical, hemodynamic, and biochemical markers of unfavorable prognosis in HFpEF patients. A search was conducted for original studies and systematic reviews devoted to assessing the prognosis of HFpEF published from January 1, 2000 to May 1, 2024. The search was performed in the PubMed/MEDLINE (for English-language publications) and RINTS (for studies in Russian) databases using the keywords "heart failure", "preserved ejection fraction", "left ventricle", "predictors", and "prognostic significance".
Clinical predictors of unfavorable prognosis and progression of HFpEF
According to follow-up studies, older age, and comorbidities are risk factors for an unfavorable prognosis in HFpEF patients. Polymorbidity is a hallmark of patients with HFpEF. With aging, there is an increase in the number of comorbidities such as anemia, chronic kidney disease, chronic obstructive pulmonary disease, and diabetes mellitus, which complicate treatment and may promote unfavorable outcomes [4].
Arterial hypertension was proven to be one of the major risk factors for the development of HF and is associated with an unfavorable prognosis. According to the Framingham study, 91% of participants with HF suffered from arterial hypertension [5]. Current risk prediction models show that elevated systolic blood pressure and elevated diastolic blood pressure are associated with the incidence of end points in HFpEF [6]. In addition, elevated pulse pressure is also a risk factor for an unfavorable prognosis [7].
Left ventricular hypertrophy (LVH) is a common complication of hypertension and a powerful risk factor for the development of HF. Studies by Ovchinnikov et al. conducted among asymptomatic patients with LVH showed that HFpEF developed in 72% of patients over 8 years of follow-up period [8]. LVH promotes the development and progression of left ventricular diastolic dysfunction by slowing myocardial relaxation, increasing cardiomyocyte stiffness, and excessive collagen deposition in the myocardial interstitial space. In addition, LVH increases the risk of developing cardiac rhythm disorders, such as atrial fibrillation (AF) and ventricular arrhythmias [9]. A number of studies showed that the severity of LVH correlated with an unfavorable prognosis in HFpEF patients. In particular, in the large PARAGON-HF study, LVH was an independent predictor of hospitalizations for HF and cardiovascular mortality in patients with HFpEF [10].
Obesity along with underweight is a significant factor affecting the prognosis of patients with HFpEF. A number of studies have revealed a U-shaped relationship between the body mass index and the combined endpoint (mortality and hospitalization for HF), where patients with a body mass index of less than 23.5 kg/m² and more than 35 kg/m² were at the highest risk group [11][12]. Patients with obesity and metabolic syndrome have an increased risk of cardiovascular events and hospitalizations for HF [13]. Low levels of physical activity are also associated with adverse outcomes. The investigation showed that adherence to the minimum recommended level of physical activity at least twice a week resulted in a 19% reduction in the risk of an adverse outcome compared to patients with HFpEF who did not adhere to the recommendations [14].
Type 2 diabetes mellitus is an independent risk factor for the development of HFpEF and significantly worsens the course of HFpEF in patients who already have this diagnosis [15]. Patients with HFpEF and type 2 diabetes mellitus have a lower quality of life, increased hospitalization frequency, and a high risk of cardiovascular complications [16]. According to a large registry, patients with HF and type 2 diabetes mellitus, hospitalized for HF decompensation, had a 28% lower three-year survival rate than patients without diabetes [17].
One of the common complications of HFpEF is AF. Epidemiological studies reveal that two-thirds of patients with HFpEF develop AF sooner or later. Moreover, the occurrence of AF promotes the worsening of HF symptoms, decreased quality of life, and an increased risk of hospitalization for HF and mortality compared to patients with sinus rhythm. Xie et al. showed that catheter ablation of AF in patients with HFpEF was associated with an improved prognosis [18].
The severity of HF symptoms, as well as a higher functional class of HF according to NYHA classification, determines to a large extent the prognosis for patients [19]. Dalos et al. showed that patients with HFpEF of functional classes III–IV by NYHA classification significantly more often reached a combined endpoint, which included hospitalizations for HF and mortality from cardiovascular diseases, compared to patients with functional class II. It is worth noting that in this study, the diagnosis of HFpEF was revealed on the basis of invasively measured pulmonary artery wedge pressure, while ischemic heart disease, which often accompanies HFpEF, was excluded angiographically in all patients [20]. Besides, recent previous hospitalization for HF decompensation was also a strong predictor of unfavorable prognosis. It was established that HFpEF patients with recent hospitalization due to HF exacerbation and more severe symptoms had a high risk of subsequent rehospitalizations and death [21].
Biochemical predictors of unfavorable prognosis and progression of HFpEF
Biomarker determination plays an important role in both the diagnosis and risk stratification of patients with HF. Although the main component of risk prediction scales in HF are natriuretic peptides (NPs), the number of markers reflecting certain pathophysiological mechanisms involved in HFpEF continues to grow.
Biologically active B-type natriuretic peptide (BNP) and its inactive N-terminal fragment (NT-proBNP) are synthesized in the ventricular myocardium in response to cardiomyocyte stretching and/or pressure overload. The main physiological effects of BNP are natriuresis, vasodilation, and suppressions of the renin-angiotensin-aldosterone system along with the sympathetic nervous system [22]. Determination of the NP level is widely used for diagnosis and prognosis assessment in patients with HF including HFpEF. In the PARAGON-HF and I-PRESERVE trials, NT-proBNP was a reliable predictor of cardiovascular mortality and hospitalization for HF [23][24]. Jhund et al. also analyzed the I-PRESERVE trial and showed that an increase in NT-proBNP concentrations over time was associated with an increased risk of cardiovascular death or hospitalization for HF, while a decrease in its level was associated with a tendency to a risk reduction [25].
However, it is well known that NPs reflect only one of many important links in HF pathogenesis. Interpretation of the results based on measuring the concentration of these peptides is often difficult due to the dependence of the NP concentration on kidney function, body mass index, thyroid status, and age and gender of patients [26]. Moreover, with a comparable risk of adverse outcomes, the NP level in patients with HFpEF may be several times lower than in patients with heart failure with reduced ejection fraction (HFrEF) [27]. These facts determine the relevance of the search for new modern markers for assessing the prognosis and efficacy of therapy for HFpEF patients.
Determination of cardiac troponins is the gold standard for the diagnosis of myocardial injury. Although the prognostic value of cardiac troponins in HFpEF has been studied to a lesser extent than in HFrEF, researchers revealed that in patients with HFpEF, elevated levels of high-sensitivity troponin I (hs-troponin) correlated with a higher risk of in-hospital mortality, longer hospital stay, and risk of HF readmission [28]. Furthermore, a secondary analysis of 34,233 patients admitted to hospital for HFpEF decompensation demonstrated an increased risk of 30-day mortality, 30-day readmission, and mortality within 1 year after discharge in case of elevated hs-troponin I concentration [29]. The combination of NT-proBNP and hs-troponin T has been shown to add additional and independent prognostic information on HF. In particular, in HFrEF, a robust biomarker-based risk model based on the combination of NT-proBNP and hs-troponin T was developed, proceeding from the results of the EMPEROR-Reduced trial [30]. The prognostic significance of the combination of NT-proBNP and hs-troponin T was also confirmed using data from the EMPEROR-Preserved trial, which included patients with HFpEF [31].
Chronic systemic inflammation plays a key role in the development and progression of HFpEF. In particular, when examining inflammatory markers such as high-sensitivity C-reactive protein (hsCRP), interleukin-6, tumor necrosis factor-α, etc., researchers found their higher levels in HFpEF compared to HFrEF [32]. Moreover, a number of markers were associated with the severity of the progression and unfavorable prognosis of HFpEF. Several studies revealed a relationship between elevated hsCRP levels and the risk of adverse outcomes in HFpEF, and specifically after adjustment for NP levels [33][34]. More recent studies have found a higher prognostic value of CRP levels in HFpEF compared with HFrEF for both all-cause and cardiovascular mortality [35].
Growth differentiation factor-15 (GDF-15), an inflammatory marker, is expressed in various cell types in response to tissue injury, ischemia, and stress. Investigations have shown that an elevated blood GDF-15 level was an independent risk factor for cardiovascular events, all-cause mortality, HF rehospitalization, and a combined endpoint (all-cause mortality and first HF hospitalization) in patients with HFpEF, and its prognostic value could be higher than that of NT-proBNP [36][37].
Endothelial dysfunction is one of the key links in the pathogenesis of HFpEF. For instance, von Willebrand factor is a glycoprotein secreted by vascular endothelial cells and megakaryocytes, which is considered a marker of endothelial cell injury and dysfunction. In the study by Kleber et al., von Willebrand factor served as an independent risk factor for all-cause mortality in patients with HFpEF after adjustment for age, gender, body mass index, NT-proBNP level, and renal function [38].
Trimethylamine N-oxide (TMAO) is one of the important metabolites of intestinal flora, and its metabolism is closely related to the occurrence of cardiovascular diseases. An elevated TMAO level can accelerate the progression of HF by provoking oxidative stress and inflammation, promoting myocardial fibrosis, affecting mitochondrial energy metabolism and other processes [39]. Dong et al. found that plasma TMAO levels in patients with HFpEF were significantly higher than those in the control group without HF [40]. Experimental studies on mouse models of HFpEF have shown an association of circulating TMAO with the severity of myocardial fibrosis and LV diastolic dysfunction [41]. Nevertheless, data on the prognostic value of TMAO in HFpEF are contradictory. Schuett et al. did not find a correlation between the blood TMAO level and prognosis in HFpEF [42]. However, in a number of studies, an increase in the concentration of TMAO in the blood serum was associated with the risk of adverse outcomes, including mortality and hospitalization for HF [43][44].
Osteopontin is a protein involved in signaling between cardiomyocytes and extracellular matrix components, regulation of angiogenesis and tissue repair; it also promotes the transformation of fibroblasts to myofibroblasts and the synthesis of extracellular matrix proteins. Increased expression of osteopontin is associated with the progression of fibrosis and an increased risk of developing HF [45]. In a study by Tromp et al., osteopontin demonstrated prognostic value for all-cause mortality and the risk of HF rehospitalization within 18 months in HFpEF but not in HFrEF [32]. In a multivariable prognostic model of HFpEF, which included demographic, clinical, and biochemical parameters, plasma osteopontin concentration was an independent predictor of all-cause mortality [46]
Assessment of markers of myocardial fibrosis and remodeling is important for insight into HFpEF pathophysiology. Experimental and clinical studies attest to the association of galectin-3 with HF development and its participation in various processes involved in the pathogenesis of HFpEF including myofibroblast proliferation, fibrogenesis, inflammation, and cardiac and vascular remodeling [47]. In the study by Wu et al., the galectin-3 level in both plasma and myocardium correlated with the severity of diastolic dysfunction [48]. To date, the prognostic value of galectin-3 has been demonstrated in several studies involving patients with HFpEF. In the PRIDE trial, the plasma galectin-3 level was correlated with increased filling pressure (higher E/e' ratio) [49]. Furthermore, the highest blood galectin-3 levels were associated with a higher risk of 4-year mortality regardless of LV size and function [50].
The ST2 receptor, a member of the interleukin-1 receptor family, is produced by fibroblasts and cardiomyocytes in response to cardiomyocyte stretch and/or pressure overload. A high level of soluble ST2 was associated with myocardial fibrosis, hypertrophy, and adverse remodeling of the heart [51]. Measurement of soluble ST2 could be useful in risk stratification of patients with HFrEF. For instance, in the PARADIGM-HF trial, the initial level of soluble ST2 proved to be an independent predictor of HF hospitalization, cardiovascular death, and their combination [52]. However, the prognostic value of soluble ST2 in HFpEF is controversial: studies available to date have been mostly retrospective in nature and have differed significantly in inclusion criteria [53].
Hemodynamic predictors of unfavorable prognosis and progression of HFpEF
The main hemodynamic disturbance in HFpEF is increased LV filling pressure due to diastolic dysfunction [54]. Increased LV filling pressure is the main cause of cardiac dyspnea and low exercise tolerance in patients with HFpEF. It was revealed that the prognosis in patients with HFpEF depended on the severity of diastolic dysfunction and the level of LV filling pressure at rest [55][56]. However, in HFpEF, the most common impairment of diastolic function is isolated relaxation delay, in which LV filling pressure at rest is usually normal, echocardiographic signs of increased filling pressure are absent, and brain natriuretic hormone level is slightly elevated or even within normal limits [57]. For instance, a study by Obokata et al. showed that in 44% of patients with HFpEF, LV filling pressure, namely pulmonary capillary wedge pressure assessed by right heart catheterization, was less than 15 mm Hg at rest, while it increased significantly under load above 25 mm Hg [58]. Therefore, in many patients with HFpEF, the exercise tolerance and the severity of the disease can be assessed only through a diastolic stress test, which makes it possible to assess the reserve capacities of the body including primarily diastolic, systolic, chronotropic, and left atrial reserves; the preservation of these functions ensures normal exercise tolerance. According to European guidelines for the diagnosis of HFpEF, performing a diastolic stress test is the most important component of the diagnostic algorithm for HFpEF [59].
A number of studies have demonstrated the high prognostic value of the diastolic stress test. Holland et al. found that an increase in filling pressure during exercise was associated with an unfavorable prognosis in HFpEF; the worst prognosis was revealed in individuals with myocardial ischemia during exercise [60]. A study by Shim et al. showed that in patients with increased pulmonary artery pressure during exercise, the E/e′ ratio > 15, indicating increased filling pressure, was an independent predictor of unfavorable prognosis at a load of 50 Watts [61]. In an invasive study, Dorfs et al. examined 355 patients with suspected HFpEF and showed that LV filling pressure both at rest and at the height of exercise load predicted prognosis with high accuracy [56].
In HFpEF, the main reason for premature termination of exercise is an increase in LV filling pressure and the lack of appropriate increase in relaxation rate related to a decrease in diastolic reserve. Moreover, many patients manifest not only impaired diastolic reserve but also decreased systolic reserve, when the LV is unable to increase its contractility to the proper degree under load. Studies have shown that impaired systolic reserve promotes reduced exercise tolerance, decreased LV suction effect and cardiac output, as well as increased LV filling pressure [62, 63]. Kosmala et al. found that impairment of both diastolic and systolic reserves were independent predictors of unfavorable prognosis and enhanced the prognostic value of clinical parameters and NT-proBNP [64].
It should be noted that despite the weakening of the diastolic reserve, in patients with asymptomatic diastolic dysfunction, normal LV filling is maintained by increasing the contractility of the left atrium (LA) [64]. However, in patients with HFpEF, the LA contractile reserve is also weakened, and the necessary increment in LV filling during exercise is achieved only due to an increase in the average value of LA pressure. In the early stages of HFpEF, the average value of LA pressure increases only during exercise but later it remains elevated even at rest due to increased LA stiffness [65]. Functional impairments of the LA have been reported to be the earliest pathophysiological disorders in the transition from an asymptomatic course of the disease to HFpEF [66]. An increase in the size of the LA is a reliable ultrasound indicator reflecting dysfunction and increased pressure in the LA cavity, and serves as a predictor of an unfavorable prognosis in HF, including HFpEF [67]. However, an assessment of volumetric indicators alone is often insufficient to identify LA dysfunction. Meanwhile, analysis of LA deformation is a reliable method for identifying LA dysfunction, and a decrease in its reservoir function has prognostic value in HFpEF [68].
Chronic elevation of LA pressure leads to pathological remodeling of the pulmonary vascular bed, development of pulmonary hypertension, and often right ventricular (RV) dysfunction, as a consequence [69]. Certain studies demonstrate a close relationship between pulmonary hypertension and prognosis in HFpEF. In particular, it has been revealed that higher pulmonary artery systolic pressure correlated with an increased number of rehospitalizations in patients with HFpEF [70][71]. In addition, increased systolic pressure in the RV, as well as decreased systolic function of the RV, were associated with a higher risk of mortality in HFpEF [72][73]. It was not by chance that researchers have isolated a separate phenotype of HFpEF with mixed post-/precapillary pulmonary hypertension, characterized by a more pronounced impairment of exercise tolerance and a worse prognosis [74].
Besides, some patients with HFpEF have limitations of pulmonary vascular reserve, which manifests itself as an inability to reduce pulmonary vascular resistance upon exercise [62]. Impaired pulmonary vascular reserve has also been found to be associated with adverse clinical outcomes [75].
RV function is one of the main determinants for prognosis in patients with HFpEF and pulmonary hypertension. A number of studies have described the contribution of RV systolic function dynamics to the prognosis for patients with HFpEF. The results of a meta-analysis by Gorter et al. demonstrated that a decrease in the value of the tricuspid annular plane systolic excursion (TAPSE) by 5 mm was associated with a 38% increase in the hospitalization risk for HF and a 26% increase in the death risk in patients with HFpEF [76]. In the study by Melenovsky et al., RV dysfunction in patients with HFpEF was associated with a 2.2-fold increase in the risk of death from all causes after adjusting for the level of systolic pulmonary artery pressure (SPAP) [77]. Right ventricular arterial coupling, estimated as the ratio of TAPSE to SPAP, according to echocardiography, was also a powerful predictor of survival in patients with HFpEF. A TAPSE/SPAP value of <0.35 mm/mm Hg was associated with a ten-fold increase in the death risk in patients with HF [78].
RV dysfunction in HFpEF is not mediated solely by the high afterload stipulated by pulmonary hypertension [79]. Many patients with near-normal resting pulmonary artery pressures also have RV dysfunction. Patients with HFpEF and preserved resting RV function have impaired right ventricular reserve attesting that the pathophysiology of HFpEF is not limited to disordered LV diastolic function [64]. RV dysfunction is associated with right heart remodeling. Increased RV diameter, area, and wall thickness have been shown to predict an unfavorable outcome in HFpEF [80].
The main cause of premature termination of exercise in HFpEF patients is an increase in LV filling pressure and the lack of an appropriate increment in the relaxation rate (decreased diastolic reserve). Concurrently, many patients may also have other disorders, each of which contributes to low exercise tolerance: insufficient increase in the contractility of both ventricles (decreased systolic reserve) and heart rate (decreased chronotropic reserve), as well as dysfunction of the left atrium (decreased atrial reserve), etc. Patients with HFpEF may differ in the degree of depletion of these reserves; the prognostic value of these differences is unclear.
We conducted a study involving 348 patients with stable HFpEF of NYHA functional class II–III; the median follow-up period was 5.4 [ 3.5; 7.0] years. The results of the analysis made it possible to identify that the prognosis of HFpEF did not depend on the value of LV filling pressure (E/e’ ratio) and right ventricular contractility (TAPSE index) at rest but depended on the degree of changes in these indicators during physical exertion (diastolic stress test), that is, on the state of diastolic and right ventricular reserves, respectively (Fig. 1) [81].
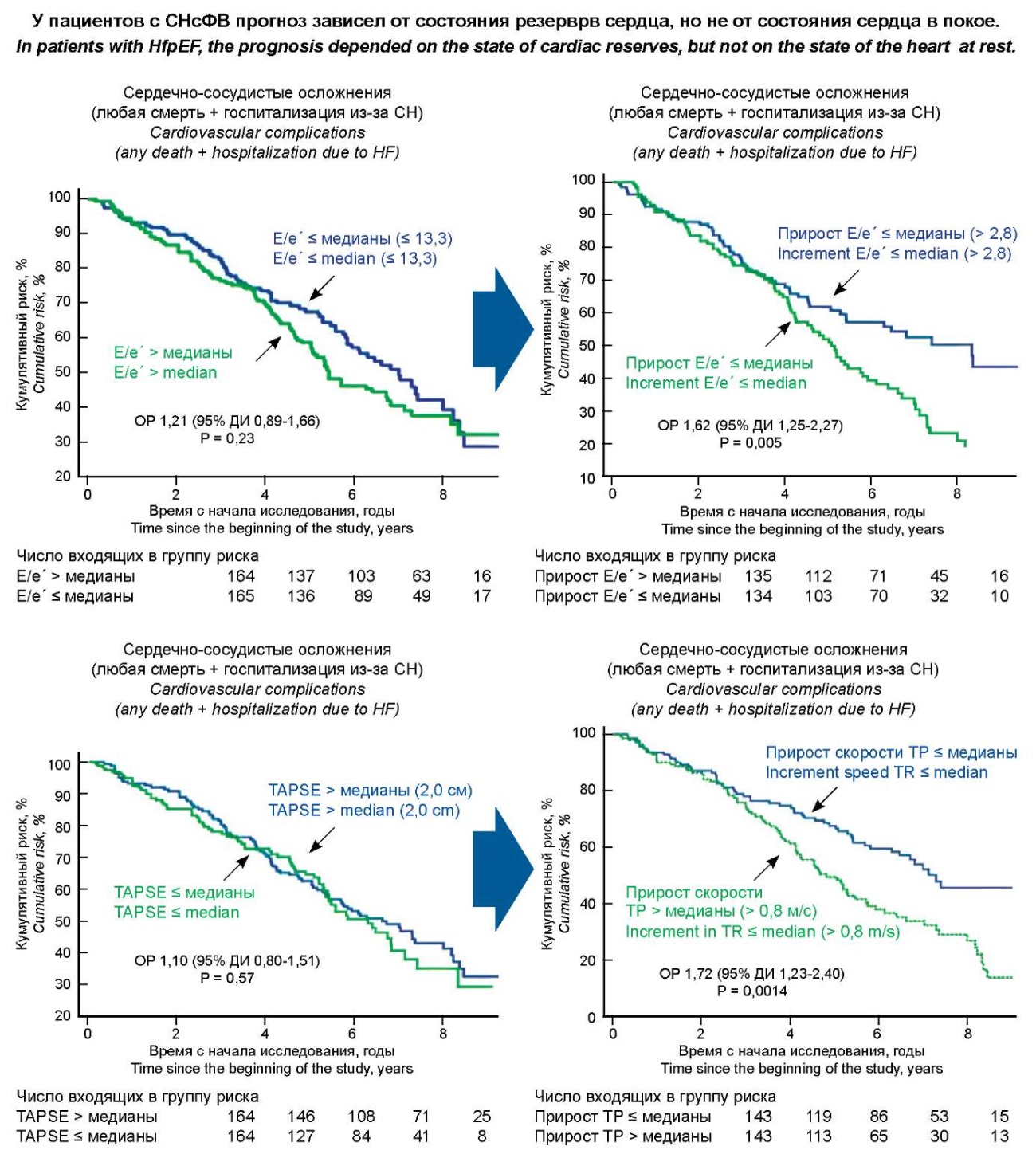
Рисунок 1. Влияния диастолического и правожелудочкового резервов сердца на прогноз СНсФВ.
Figure 1. The influence of diastolic and right ventricular reserves of the heart on the prognosis of HFpEF.
Conclusion
Various clinical, hemodynamic, and biochemical predictors of unfavorable prognosis and progression of HFpEF were considered in this review. Currently, the possibilities of effective treatment of HFpEF are limited, and the prognostic value has been determined only for the generally accepted clinical and standard echocardiographic parameters at rest. Recent advances in early diagnosis and approaches to the treatment of patients with HFpEF may improve the prognosis and course of the disease in these patients. Identification of independent predictors of unfavorable prognosis/severe progression of HFpEF will enable the development of a prognostic algorithm that can be used to identify patients who require more careful monitoring and/or more intensive drug treatment.
References
1. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun jj, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. Erratum in: Circulation. 2022;145(18):e1033. Erratum in: Circulation. 2022;146(13):e185. Erratum in: Circulation. 2023;147(14):e674. https://doi.org/10.1161/CIR.0000000000001063
2. Tsao Cw, Aday Aw, Almarzooq ZI, Alonso A, Beaton AZ, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145(8):e153-e639. Erratum in: Circulation. 2022;146(10):e141. https://doi.org/10.1161/CIR.0000000000001052.
3. Ageev F.T., Ovchinnikov A.G. Treatment of patients with heart failure and preserved ejection fraction: reliance on clinical phenotypes. Kardiologiia. 2022;62(7):44-53. (In Russ.) https://doi.org/10.18087/cardio.2022.7.n2058
4. Iorio A, Senni M, Barbati G, Greene Sj, Poli S, et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail. 2018;20(9):1257-1266. https://doi.org/10.1002/ejhf.1202
5. Kenchaiah S, Vasan RS. Heart Failure in women--Insights from the Framingham Heart Study. Cardiovasc Drugs Ther. 2015;29(4):377-390. https://doi.org/10.1007/s10557-015-6599-0
6. Huang R, wu R, Lin Y, Zhong x, Ye x, et al. Time-averaged cumulative blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: analysis from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. J Hypertens. 2022;40(10):1918-1926. https://doi.org/10.1097/HjH.0000000000003177
7. Suzuki K, Claggett B, Minamisawa M, Nochioka K, Mitchell GF, et al. Pulse Pressure, Prognosis, and Influence of Sacubitril/Valsartan in Heart Failure with Preserved Ejection Fraction. Hypertension. 2021;77(2):546-556. https://doi.org/10.1161/HYPERTENSIONAHA.120.16277
8. Ovchinnikov A, Belyavskiy E, Potekhina A, Ageev F. Asymptomatic Leſt Ventricular Hypertrophy Is a Potent Risk Factor for the Development of HFpEF but Not HFrEF: Results of a Retrospective Cohort Study. J Clin Med. 2022;11(13):3885. https://doi.org/10.3390/jcm11133885
9. Garg P, Assadi H, jones R, Chan wB, Metherall P, et al. Leſt ventricular fibrosis and hypertrophy are associated with mortality in heart failure with preserved ejection fraction. Sci Rep. 2021;11(1):617. https://doi.org/10.1038/s41598-020-79729-6
10. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, et al. Echocardiographic Features of Patients with Heart Failure and Preserved Leſt Ventricular Ejection Fraction. J Am Coll Cardiol. 2019;74(23):2858-2873. https://doi.org/10.1016/j.jacc.2019.09.063
11. Haass M, Kitzman Dw, Anand IS, Miller A, Zile MR, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4(3):324-331. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959890
12. Pandey A, Berry jD, Drazner MH, Fang jC, Tang wHw, Grodin jL. Body Mass Index, Natriuretic Peptides, and Risk of Adverse Outcomes in Patients with Heart Failure and Preserved Ejection Fraction: Analysis From the TOPCAT Trial. J Am Heart Assoc. 2018;7(21):e009664. https://doi.org/10.1161/jAHA.118.009664
13. Voulgari C, Moyssakis I, Papazafiropoulou A, Perrea D, Kyriaki D, et al. The impact of metabolic syndrome on leſt ventricular myocardial performance. Diabetes Metab Res Rev. 2010;26(2):121-127. https://doi.org/10.1002/dmrr.1063
14. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69(9):1129-1142. https://doi.org/10.1016/j.jacc.2016.11.081
15. MacDonald MR, Petrie MC, Varyani F, Ostergren j, Michelson EL, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377-1385. https://doi.org/10.1093/eurheartj/ehn153
16. Kristensen SL, Mogensen UM, jhund PS, Petrie MC, Preiss D, et al. Clinical and Echocardiographic Characteristics and Cardiovascular Outcomes According to Diabetes Status in Patients with Heart Failure and Preserved Ejection Fraction: A Report From the I-Preserve Trial (Irbesartan in Heart Failure with Preserved Ejection Fraction). Circulation. 2017;135(8):724-735. https://doi.org/10.1161/CIRCULATIONAHA.116.024593
17. Bjorck LM, Lanitis M, Lappas G, Novak M, Rosengren A. Mortality Trends 1987 to 2004 in 404,480 Hospitalized Heart Failure Patients with and without Diabetes. Circulation. 2012;125:AP208. https://doi.org/10.1161/circ.125.suppl_10.AP208
18. xie Z, qi B, wang Z, Li F, Chen C, et al. Ablation for atrial fibrillation improves the outcomes in patients with heart failure with preserved ejection fraction. Europace. 2023;26(1):euad363. https://doi.org/10.1093/europace/euad363
19. Ahmed A, Aronow wS, Fleg jL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved leſt ventricular function. Am Heart J. 2006;151(2):444-450. https://doi.org/10.1016/j.ahj.2005.03.066
20. Dalos D, Mascherbauer j, Zotter-Tufaro C, Duca F, Kammerlander AA, et al. Functional Status, Pulmonary Artery Pressure, and Clinical Outcomes in Heart Failure with Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68(2):189-199. https://doi.org/10.1016/j.jacc.2016.04.052
21. Vaduganathan M, Claggett BL, Desai AS, Anker SD, Perrone SV, et al. Prior Heart Failure Hospitalization, Clinical Outcomes, and Response to Sacubitril/Valsartan Compared with Valsartan in HFpEF. J Am Coll Cardiol. 2020;75(3):245-254. https://doi.org/10.1016/j.jacc.2019.11.003
22. O'Meara E, de Denus S, Rouleau jL, Desai A. Circulating bio-markers in patients with heart failure and preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):350-358. https://doi.org/10.1007/s11897-013-0160-x
23. Solomon SD, McMurray jjV, Anand IS, Ge j, Lam CSP, et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381(17):1609-1620. https://doi.org/10.1056/NEjMoa1908655
24. Anand IS, Rector TS, Cleland jG, Kuskowski M, McKelvie RS, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4(5):569-577. https://doi.org/10.1161/CIRCHEARTFAILURE.111.962654
25. jhund PS, Anand IS, Komajda M, Claggett BL, McKelvie RS, et al. Changes in N-terminal pro-B-type natriuretic peptide levels and outcomes in heart failure with preserved ejection fraction: an analysis of the I-Preserve study. Eur J Heart Fail. 2015;17(8):809-817. https://doi.org/10.1002/ejhf.274
26. Luchner A, Behrens G, Stritzke j, Markus M, Stark K, et al. Long-term pattern of brain natriuretic peptide and N-terminal pro brain natriuretic peptide and its determinants in the general population: contribution of age, gender, and cardiac and extra-cardiac factors. Eur J Heart Fail. 2013;15(8):859-867. https://doi.org/10.1093/eurjhf/hſt048
27. Shah Sj, Kitzman Dw, Borlaug BA, van Heerebeek L, Zile MR, et al. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134(1):73-90. https://doi.org/10.1161/CIRCULATIONAHA.116.021884
28. Okuyama R, Ishii j, Takahashi H, Kawai H, Muramatsu T, et al. Combination of high-sensitivity troponin I and N-terminal pro-B-type natriuretic peptide predicts future hospital admission for heart failure in high-risk hypertensive patients with preserved leſt ventricular ejection fraction. Heart Vessels. 2017;32(7):880-892. https://doi.org/10.1007/s00380-017-0948-9
29. Pandey A, Golwala H, Sheng S, DeVore AD, Hernandez AF, et al. Factors Associated with and Prognostic Implications of Cardiac Troponin Elevation in Decompensated Heart Failure with Preserved Ejection Fraction: Findings From the American Heart Association Get with The Guidelines-Heart Failure Program. JAMA Cardiol. 2017;2(2):136-145. https://doi.org/10.1001/jamacardio.2016.4726
30. Pocock Sj, Ferreira jP, Gregson j, Anker SD, Butler j, et al. Novel biomarker-driven prognostic models to predict morbidity and mortality in chronic heart failure: the EMPEROR-Reduced trial. Eur Heart J. 2021;42(43):4455-4464. https://doi.org/10.1093/eurheartj/ehab579
31. Pocock Sj, Ferreira jP, Packer M, Zannad F, Filippatos G, et al. Biomarker-driven prognostic models in chronic heart failure with preserved ejection fraction: the EMPEROR-Preserved trial. Eur J Heart Fail. 2022;24(10):1869-1878. https://doi.org/10.1002/ejhf.2607
32. Tromp j, Khan MA, Klip IT, Meyer S, de Boer RA, et al. Biomarker Profiles in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J Am Heart Assoc. 2017;6(4):e003989. https://doi.org/10.1161/jAHA.116.003989
33. Gottdiener jS, Arnold AM, Aurigemma GP, Polak jF, Tracy RP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628-1637. https://doi.org/10.1016/s0735-1097(00)00582-9
34. Tromp j, westenbrink BD, Ouwerkerk w, van Veldhuisen Dj, Samani Nj, et al. Identifying Pathophysiological Mechanisms in Heart Failure with Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72(10):1081-1090. https://doi.org/10.1016/j.jacc.2018.06.050
35. Lakhani I, wong MV, Hung jKF, Gong M, waleed KB, et al. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail Rev. 2021;26(5):1141-1150. https://doi.org/10.1007/s10741-020-09927-x
36. Izumiya Y, Hanatani S, Kimura Y, Takashio S, Yamamoto E, et al. Growth differentiation factor-15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol. 2014;30(3):338-344. https://doi.org/10.1016/j.cjca.2013.12.010
37. Yin D, Yan x, Bai x, Tian A, Gao Y, Li j. Prognostic value of Growth differentiation factors 15 in Acute heart failure patients with preserved ejection fraction. ESC Heart Fail. 2023;10(2):1025-1034. https://doi.org/10.1002/ehf2.14271
38. Kleber ME, Koller L, Goliasch G, Sulzgruber P, Scharnagl H, et al. Von willebrand factor improves risk prediction in addition to N-terminal pro-B-type natriuretic peptide in patients referred to coronary angiography and signs and symptoms of heart failure and preserved ejection fraction. Circ Heart Fail. 2015;8(1):25-32. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001478
39. Naghipour S, Cox Aj, Peart jN, Du Toit EF, Headrick jP. Trimethylamine N-oxide: heart of the microbiota-CVD nexus? Nutr Res Rev. 2021;34(1):125-146. https://doi.org/10.1017/S0954422420000177
40. Dong Z, Zheng S, Shen Z, Luo Y, Hai x. Trimethylamine NOxide is Associated with Heart Failure Risk in Patients with Preserved Ejection Fraction. Lab Med. 2021;52(4):346-351. https://doi.org/10.1093/labmed/lmaa075
41. Salzano A, Israr MZ, Yazaki Y, Heaney LM, Kanagala P, et al. Combined use of trimethylamine N-oxide with BNP for risk stratification in heart failure with preserved ejection fraction: findings from the DIAMONDHFpEF study. Eur J Prev Cardiol. 2020;27(19):2159-2162. https://doi.org/10.1177/2047487319870355
42. Schuett K, Kleber ME, Scharnagl H, Lorkowski S, März w, et al. Trimethylamine-N-oxide and Heart Failure with Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70(25):3202-3204. https://doi.org/10.1016/j.jacc.2017.10.064
43. Salzano A, Israr MZ, Yazaki Y, Heaney LM, Kanagala P, et al. Combined use of trimethylamine N-oxide with BNP for risk stratification in heart failure with preserved ejection fraction: findings from the DIAMONDHFpEF study. Eur J Prev Cardiol. 2020;27(19):2159-2162. https://doi.org/10.1177/2047487319870355
44. Kinugasa Y, Nakamura K, Kamitani H, Hirai M, Yanagihara K, et al. Trimethylamine N-oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction. ESC Heart Fail. 2021;8(3):2103-2110. https://doi.org/10.1002/ehf2.13290
45. Abdelaziz Mohamed I, Gadeau AP, Hasan A, Abdulrahman N, Mraiche F. Osteopontin: A Promising Therapeutic Target in Cardiac Fibrosis. Cells. 2019;8(12):1558. https://doi.org/10.3390/cells8121558
46. Rosenberg M, Zugck C, Nelles M, juenger C, Frank D, et al. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ Heart Fail. 2008;1(1):43-49. https://doi.org/10.1161/CIRCHEARTFAILURE.107.746172
47. Yu L, Ruifrok wP, Meissner M, Bos EM, van Goor H, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fi-brogenesis. Circ Heart Fail. 2013;6(1):107-117. https://doi.org/10.1161/CIRCHEARTFAILURE.112.971168
48. wu CK, Su MY, Lee jK, Chiang FT, Hwang jj, et al. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci Rep. 2015;5:17007. https://doi.org/10.1038/srep17007
49. van Kimmenade RR, januzzi jL jr, Ellinor PT, Sharma UC, Bakker jA, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48(6):1217-1224. https://doi.org/10.1016/j.jacc.2006.03.061
50. Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, januzzi jL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12(8):826-832. https://doi.org/10.1093/eurjhf/hfq091
51. Vianello E, Dozio E, Tacchini L, Frati L, Corsi Romanelli MM. ST2/IL-33 signaling in cardiac fibrosis. Int J Biochem Cell Biol. 2019;116:105619. https://doi.org/10.1016/j.biocel.2019.105619
52. O'Meara E, Prescott MF, Claggett B, Rouleau jL, Chiang LM, et al. Independent Prognostic Value of Serum Soluble ST2 Measurements in Patients with Heart Failure and a Reduced Ejection Fraction in the PARADIGM-HF Trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018;11(5):e004446. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004446
53. Shah KB, Kop wj, Christenson RH, Diercks DB, Henderson S, et al. Prognostic utility of ST2 in patients with acute dyspnea and preserved leſt ventricular ejection fraction. Clin Chem. 2011;57(6):874-882. https://doi.org/10.1373/clinchem.2010.159277
54. Zile MR, Baicu CF, Gaasch wH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the leſt ventricle. N Engl J Med. 2004;350(19):1953-1959. https://doi.org/10.1056/NEjMoa032566
55. Adamson PB, Abraham wT, Bourge RC, Costanzo MR, Hasan A, et al. wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935-944. https://doi.org/10.1161/CIRCHEARTFAILURE.113.001229
56. Dorfs S, Zeh w, Hochholzer w, jander N, Kienzle RP, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35(44):3103-3112. https://doi.org/10.1093/eurheartj/ehu315
57. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588-595. https://doi.org/10.1161/CIRCHEARTFAILURE.109.930701
58. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135(9):825-838. https://doi.org/10.1161/CIRCULATIONAHA.116.024822
59. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297-3317. Erratum in: Eur Heart J. 2021;42(13):1274. https://doi.org/10.1093/eurheartj/ehz641
60. Holland Dj, Prasad SB, Marwick TH. Prognostic implications of leſt ventricular filling pressure with exercise. Circ Cardiovasc Imaging. 2010;3(2):149-156. https://doi.org/10.1161/CIRCIMAGING.109.908152
61. Shim CY, Kim SA, Choi D, Yang wI, Kim jM, et al. Clinical outcomes of exercise-induced pulmonary hypertension in subjects with preserved leſt ventricular ejection fraction: implication of an increase in leſt ventricular filling pressure during exercise. Heart. 2011;97(17):1417-1424. https://doi.org/10.1136/hrt.2010.220467
62. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37(43):3293-3302. https://doi.org/10.1093/eurheartj/ehw241
63. Tan YT, wenzelburger F, Lee E, Heatlie G, Leyva F, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54(1):36-46. https://doi.org/10.1016/j.jacc.2009.03.037
64. Kosmala w, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of Abnormal Leſt Ventricular Functional Reserve with Outcome in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc Imaging. 2018;11(12):1737-1746. https://doi.org/10.1016/j.jcmg.2017.07.028
65. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507-515. https://doi.org/10.1038/nrcardio.2014.83
66. Ovchinnikov AG, Potekhina A, Belyavskiy E, Gvozdeva A, Ageev F. Leſt atrial dysfunction as the major driver of heart failure with preserved ejection fraction syndrome. J Clin Ultrasound. 2022;50(8):1073-1083. https://doi.org/10.1002/jcu.23318
67. Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, et al. Potential Usefulness and Clinical Relevance of Adding Leſt Atrial Strain to Leſt Atrial Volume Index in the Detection of Leſt Ventricular Diastolic Dysfunction. JACC Cardiovasc Imaging. 2018;11(10):1405-1415. https://doi.org/10.1016/j.jcmg.2017.07.029
68. Obokata M, Reddy YNV, Borlaug BA. Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245-257. https://doi.org/10.1016/j.jcmg.2018.12.034
69. Santos AB, Roca Gq, Claggett B, Sweitzer NK, Shah Sj, et al. Prognostic Relevance of Leſt Atrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2016;9(4):e002763. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002763
70. Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7(2):367-377. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000823
71. Hidalgo C, Granzier H. Tuning the molecular giant titin through phosphorylation: role in health and disease. Trends Cardiovasc Med. 2013;23(5):165-171. https://doi.org/10.1016/j.tcm.2012.10.005
72. Kosmala w, jellis CL, Marwick TH. Exercise limitation associated with asymptomatic leſt ventricular impairment: analogy with stage B heart failure. J Am Coll Cardiol. 2015;65(3):257-266. https://doi.org/10.1016/j.jacc.2014.10.044
73. Lam CS, Roger VL, Rodeheffer Rj, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119-1126. https://doi.org/10.1016/j.jacc.2008.11.051
74. Mohammed SF, Hussain I, AbouEzzeddine OF, TakahamaH, Kwon SH, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130(25):2310-2320. Erratum in: Circulation. 2015;131(17):e424. doi: 10.1161/CIR.0000000000000202. Abou Ezzeddine, Omar F [corrected to AbouEzzeddine, Omar F]. https://doi.org/10.1161/CIRCULATIONAHA.113.008461
75. Ovchinnikov A, Potekhina A, Belyavskiy E, Ageev F. Heart Failure with Preserved Ejection Fraction and Pulmonary Hypertension: Focus on Phosphodiesterase Inhibitors. Pharmaceuticals (Basel). 2022;15(8):1024. https://doi.org/10.3390/ph15081024
76. Huang w, Oliveira RKF, Lei H, Systrom DM, waxman AB. Pulmonary Vascular Resistance During Exercise Predicts Long-Term Outcomes in Heart Failure with Preserved Ejection Fraction. J Card Fail. 2018;24(3):169-176. https://doi.org/10.1016/j.cardfail.2017.11.003
77. Gorter TM, Hoendermis ES, van Veldhuisen Dj, Voors AA, Lam CS, et al. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2016;18(12):1472-1487. https://doi.org/10.1002/ejhf.630
78. Melenovsky V, Hwang Sj, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452-3462. https://doi.org/10.1093/eurheartj/ehu193
79. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1373-81. https://doi.org/10.1152/ajpheart.00157.2013
80. Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7(2):288-299. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000854
81. Ovchinnikov A, Filatova A, Sobolevskaya M, Potekhina A, Svirida O, et al. The prognostic role of cardiac hemodynamic reserves in heart failure with preserved ejection fraction. Journal of Hypertension. 2024;42(Suppl 1):p e242. https://doi.org/10.1097/01.hjh.0001021964.41256.b4
About the Authors
M. S. SobolevskayaRussian Federation
Maria S. Sobolevskaya, Laboratory Assistant-Researcher, Department of Outpatient Treatment and Diagnostic Technologies, A.L. Myasnikov Institute of Clinical Cardiology
Moscow
Competing Interests:
Authors declares no conflict of interest.
A. D. Gvozdeva
Russian Federation
Anna D. Gvozdeva, Cand. Sci. (Med.), functional diagnostics doctor
Moscow
Competing Interests:
Authors declares no conflict of interest.
O. N. Svirida
Russian Federation
Olga N. Svirida, Cand. Sci. (Med.), junior researcher, Laboratory of Myocardial Fibrosis and Heart Failure with Preserved Ejection Fraction of the A.L. Myasnikov Institute of Clinical Cardiology, Researcher of the Department of Outpatient Treatment and Diagnostic Technologies of the A.L. Myasnikov Institute of Clinical Cardiology
Moscow
Competing Interests:
Authors declares no conflict of interest.
A. Y. Filatova
Russian Federation
Anastasiia Y. Filatova, Cand. Sci. (Med.), researcher of Laboratory of Myocardial Fibrosis and Heart Failure with Preserved Ejection Fraction of the A.L. Myasnikov Institute of Clinical Cardiology, researcher of Laboratory of Cell Immunology of the ac. V.N. Smirnov Institute of Experimental Cardiology
Moscow
Competing Interests:
Authors declares no conflict of interest.
Review
For citations:
Sobolevskaya M.S., Gvozdeva A.D., Svirida O.N., Filatova A.Y. Predictors of unfavorable progression and prognosis in patients with heart failure with preserved left ventricular ejection fraction. Medical Herald of the South of Russia. 2024;15(4):38-48. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-4-38-48







































