Scroll to:
Angiotensinogen – a marker of non-developing pregnancy
https://doi.org/10.21886/2219-8075-2025-16-2-37-42
Abstract
Objective: to investigate the effects of angiotensinogen on the endometrium in missed abortion. Materials and methods: a prospective cohort that included 212 women, 160 with missed abortion and 52 with progressing pregnancies at 6–12 weeks. In all participants, the average serum concentration of angiotensinogen was measured. Additionally, a morphological examination of the endometrium was performed in patients with missed abortion. Results: women with missed abortion had significantly lower angiotensinogen levels compared to those with progressing pregnancies. Based on the results of a nonparametric analysis, there was a direct correlation between low angiotensinogen levels and spiral artery thrombosis in missed abortion patients at 11–12 weeks (r=0,37, p<0,01). Conclusion: these findings suggest that a decrease in angiotensinogen levels is associated with impaired angiogenesis in the endometrium and can lead to spiral artery thrombosis during on the endometrium in missed abortion.
For citations:
Orazmuradov A.A., Suleymanova Zh.Zh., Kuzmina E.A., Kostin I.N., Haddad Kh., Mukovnikova E.V. Angiotensinogen – a marker of non-developing pregnancy. Medical Herald of the South of Russia. 2025;16(2):37-42. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-2-37-42
Introduction
According to Rosstat, the total fertility rate in Russia decreased to 1.41 in 2023, indicating a continuing trend toward depopulation. For natural population growth, this indicator should reach at least 2.1. Key objectives for improving the demographic situation include reducing reproductive losses, strengthening the reproductive health of the population, and identifying additional reserves for increasing birth rates [1].
In most cases, routine examinations for non-developing pregnancy (NDP) fail to identify the primary etiology of early reproductive loss1. Approximately 15% of all clinically diagnosed pregnancies end in spontaneous miscarriage, with NDP accounting for more than 70% of spontaneous pregnancy losses occurring before 12 weeks of gestation2. Despite such high figures, the pathogenesis of NDP remains largely unknown3. The most frequently cited causes of miscarriage include structural chromosomal abnormalities of the conceptus, uterine malformations, hyperhomocysteinemia, and antiphospholipid syndrome [2]. Adequate angiogenesis within the chorionic villi plays a crucial role in maintaining early pregnancy [2]. Development of the fetoplacental complex during most of the first trimester occurs in a low-oxygen environment; placental circulation gradually establishes after the eighth week of gestation and becomes substantial only after 12 weeks [2].
The delicate balance between coagulation and fibrinolysis is critical during early pregnancy [3]. Plasminogen activator inhibitor-1 (PAI-1) and angiotensin-converting enzyme (ACE) play pivotal roles in the regulation of the fibrinolytic process. Multiple studies have reported an association between genetic polymorphisms in the PAI-1 and ACE genes and recurrent pregnancy loss [3].
Emerging evidence suggests that abnormal levels of angiotensinogen (AGT) substantially increase the incidence of reproductive loss and pregnancy complications [2–4]. By binding to the type I AGT receptor, AGT activates growth factors and intracellular signaling pathways that promote proliferation, angiogenesis, fibrosis, and trophoblast invasion [4]. In addition, AGT, acting through the Mas receptor, induces vasodilation, thereby preventing thrombus formation in the spiral arteries [3][4].
Thus, the detection of AGT in serum may serve as a novel approach for the early diagnosis of NDP [5][6].
The aim of the study was to evaluate the effects of AGT on the endometrium in cases of NDP.
Materials and Methods
A prospective cohort study was conducted involving 160 women with NDP and 52 women with viable pregnancies at gestational ages of up to 12 weeks. All participants with NDP were divided into three groups according to gestational age: Group I – 6–8 weeks (n = 54), Group II – 9–10 weeks (n = 54), and Group III – 11–12 weeks (n = 52). Women with viable pregnancies up to 12 weeks served as the control group (n = 52). Mean serum concentrations of AGT were measured in all participants. In the NDP groups, a morphological examination of the endometrium was additionally performed. Using statistical analysis, correlations between AGT concentration and structural changes in the endometrium were evaluated in patients with NDP.
The study was conducted at the clinical base of the Department of Obstetrics and Gynecology with a course in Perinatology, Medical Institute, Peoples’ Friendship University of Russia (RUDN University), between April and December 2023, in the gynecological department of V.M. Buyanov Municipal Clinical Hospital (Chief Physician – PhD A.V. Salikov; Head of Department – PhD O.A. Demina).
The inclusion criteria for patient selection were as follows: spontaneous conception; in the main three groups – diagnosis of non-developing intrauterine pregnancy confirmed by two consecutive ultrasound examinations; in the control group – diagnosis of progressing intrauterine pregnancy.
The exclusion criteria were as follows: multiple pregnancy, gestational age exceeding 12 weeks, and pregnancy achieved through assisted reproductive technologies.
Between 6 and 12 weeks of gestation, ultrasonographic examination was performed using an Alpinion E-Cube 15 system (ALPINION Company, South Korea) equipped with a single-crystal transducer. Ultrasound assessment included measurement of the gestational sac diameter, evaluation of yolk sac shape and size, and detection of embryonic cardiac activity with heart rate recording. Gestational age was determined based on crown–rump length.
To determine the mean serum AGT concentrations, mass spectrometry and chromatography techniques were used.
Endometrial specimens obtained surgically were fixed in a 10% formalin solution for 24 hours. Tissue fixation was performed using isopropyl alcohol, followed by preparation of histological sections with a thickness of 4–6 mm. The biopsies were subsequently stained with hematoxylin and eosin.
The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki of the World Medical Association (1964, revised in 2013) and was approved by the Ethics Committee of the RUDN University. Written informed consent was obtained from each participant prior to enrollment.
Statistical analysis was performed using IBM SPSS Statistics, version 26 for Windows (IBM Corporation, Somers, NY, USA). The Shapiro–Wilk test was applied to assess the normality of data distribution. Categorical variables are presented as absolute and relative frequencies, whereas continuous variables with normally distributed data are expressed as the mean (M) ± standard deviation. For non-normally distributed data, the median (Me) and interquartile range (Q1–Q3) were reported. Fisher’s exact test was used to evaluate differences between categorical variables, and the Mann–Whitney test was applied for continuous variables. The differences were considered statistically significant at p<0.05.
Correlation analysis was performed using Spearman’s nonparametric method (R). The strength of association was interpreted as strong for absolute correlation coefficients (r) ≥ 0.7, moderate for r values between 0.3 and 0.69, and weak for r < 0.3. Statistical significance was set at p < 0.05.
Results
In the groups of women with NDP, the frequency of two pregnancy termination methods was analyzed: (1) ultrasound-guided vacuum aspiration of the gestational sac, and (2) sequential administration of 200 mg mifepristone followed 24 hours later by 800 µg misoprostol (Table 1).
Table 1 demonstrates that in the cohort of patients with NDP, the medical method of pregnancy termination predominated, accounting for 64.4%.
To determine plasma AGT concentrations in women from the study groups, a metabolomic analysis was performed. Statistically significant differences in AGT concentrations were identified between patients with NDP and those in the control group (Table 2).
As shown by the presented data, the mean serum AGT concentration in patients with NDP was significantly lower compared with women with ongoing pregnancies: 0.057 ± 0.047 ng/mL versus 0.405 ± 0.094 ng/mL in the control group (p < 0.05).
Morphological examination revealed that the structure of the endometrium in patients with NDP could be classified into two histological types. The first morphological pattern was characterized by leukocytic infiltration of the stroma, fibrinoid necrosis, and thrombosis of the spiral arteries (Fig. 1). The second type showed no thrombosis in the lumen of the spiral arteries; however, stromal fibrosis, vascular wall hyalinosis, and edematous–dystrophic changes were observed (Fig. 2).
Thus, the primary distinguishing feature between the two histological patterns was the presence or absence of spiral artery thrombosis.
We analyzed the share of cases with spiral artery thrombosis among patients with NDP (Table 3).
As shown in the presented data, the incidence of spiral artery thrombosis among women with NDP was twice as high at 11–12 weeks of gestation (p = 0.01).
To assess the association between spiral artery thrombosis and serum AGT levels in patients with NDP, a correlation analysis was performed using Spearman’s nonparametric method (Table 4).
Analysis of Table 4 revealed a direct correlation between serum AGT concentration and the presence of spiral artery clots in patients with NDP at 11–12 weeks of gestation (R = 0.37, p < 0.01).
Таблица / Table 1
Частота использования методов прерывания неразвивающейся беременности
Frequency of use of methods for terminating a non-developing pregnancy
|
Группа, нед. Group, weeks |
Медикаментозный Medicamental |
Хирургический Surgical |
||
|
Абс. Abs. |
% |
Абс. Abs. |
% |
|
|
6–8 (n = 54) |
35 |
64,8 |
19 |
35,2 |
|
9–10 (n = 54) |
35 |
64,8 |
19 |
35,2 |
|
11–12 (n = 52) |
31 |
59,6 |
21 |
40,4 |
|
Всего Total (n = 160) |
103 |
64,4 |
57 |
35,6 |
Таблица / Table 2
Средние значения концентраций AGT в плазме крови, нг/мл
Average values of AGT concentrations in blood plasma, ng/ml
|
Группа, нед. Group, weeks |
M |
Std |
95% ДИ/CI
|
|
6-8 (n = 54) |
0,067* |
0,067 |
0,086 |
|
9-10 (n = 54) |
0,055* |
0,044 |
0,068 |
|
11-12 (n = 52) |
0,051* |
0,036 |
0,064 |
|
Всего Total (n=160) |
0,057* |
0,047 |
0,061 |
|
Контроль Control (n = 47) |
0,405 |
0,094 |
0,429 |
Примечание: * — различия показателей статистически значимы (p <0,05).
Note: * — differences in indicators are statistically significant (p <0.05).

Рисунок 1. Срез эндометрия: лейкоцитарная инфильтрация стромы, фибриноидный некроз, тромбоз спиральных артерий. Окраска гематоксилин-эозином, увеличение 250×.
Figure 1. Endometrial section: leukocyte infiltration of the stroma, fibrinoid necrosis, thrombosis of the spiral arteries. Hematoxylin-eosin staining, magnification 250×.
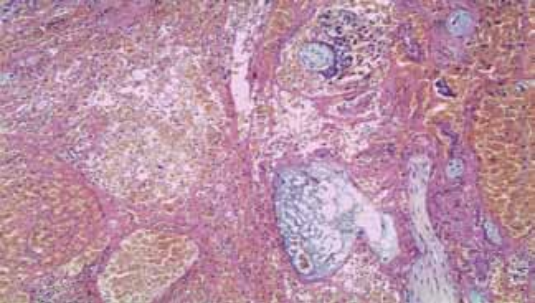
Рисунок 2. Фрагмент эндометрия с фиброзом стромы, гиалинозом стенок спиральных артерий и отечно-дистрофическими изменениями. Окраска гематоксилин-эозином, увеличение 250×.
Figure 2. A fragment of the endometrium with stromal fibrosis, hyalinosis of the walls of the spiral arteries and edematous-dystrophic changes. Hematoxylin-eosin staining, magnification 250×.
Таблица / Table 3
Тромбоз спиральных артерий у пациенток с неразвивающейся беременностью
Thrombosis of the spiral arteries in patients with non-developing pregnancy
|
Группа НБ, нед. Group missed abortion, weeks |
Наличие тромбоза (М1) The presence of thrombosis (М1) |
Отсутствие тромбоза (М2) Absence of thrombosis (M2) |
||
|
Абс. / Abs. |
% |
Абс. / Abs. |
% |
|
|
6–8 (n = 54) |
28 |
51,9 |
26 |
48,1 |
|
9–10 (n = 54) |
30 |
55,6 |
24 |
44,4 |
|
11–12 (n = 52) |
35 |
67,3* |
17 |
32,7* |
|
Всего Total (n=160) |
93 |
58,1 |
67 |
41,9 |
Примечание: * — различия показателей статистически значимы (p <0,05).
Note: * — differences in indicators are statistically significant (p <0.05).
Таблица / Table 4
Непараметрическая корреляция Спирмена между тромбозом спиральных артерий и средней сывороточной концентрацией ангиотензиногена
Spearman's nonparametric correlation between spiral artery thrombosis and average serum angiotensinogen concentration
|
Группа НБ, нед. Group missed abortion, weeks |
R |
t (N-2) |
p-value |
|
6–8 |
0,14 |
1,1 |
0,3 |
|
9–10 |
0,18 |
1,42 |
0,15 |
|
11–12 |
0,37 |
3,05 |
< 0,01 |
Примечание: R — коэффициент корреляции Спирмена, t (N-2) — критерий Стьюдента, p-value — уровень значимости.
Note: R — Spearman's correlation coefficient, t (N-2) — Student's criterion, p–value — significance level.
Discussion
Despite a notable increase in research aimed at identifying biomarkers of NDP in recent years, the issue of predicting early pregnancy loss remains unresolved. To date, several serological, inflammatory, immunological, and oxidative stress markers have been identified, each demonstrating a certain degree of prognostic value [7–10].
The role of AGT in the pathogenesis of NDP during early pregnancy requires further investigation. Heidari et al. (2019) reported findings that appear to contrast with those obtained in the present study [11]. The authors demonstrated that AGT gene polymorphism was a genetic determinant of the risk of developing idiopathic NDP. However, they also found that increased serum AGT concentrations correlated directly and proportionally with the risk of NDP [11].
In our study, decreased AGT levels were associated with an increased risk of NDP, which is fully consistent with the findings of Xiong et al. (2021) [10]. The authors investigated the correlation between recurrent pregnancy loss and the prevalence of AGT gene polymorphism. The proposed pathogenic mechanism involves thrombosis of the spiral arteries, leading to hypoxia and impairing adequate trophoblast invasion [10].
Conclusion
The findings of this study suggest a direct association between decreased serum AGT levels and the risk of NDP. Reduced AGT concentrations appear to be linked to impaired angiogenesis within the endometrium, a process likely driven by the formation of clots within the lumina of spiral arteries in patients with NDP at 11–12 weeks of gestation.
1. Non-developing pregnancy. 3rd ed., revised and enlarged. Edited by V.E. Radzinsky. Moscow: GEOTAR-Media, 2019. 178 p. (in Russian).
2. History of undeveloped pregnancy: rehabilitation and preparation for the next gestation. Methodical recommendations of MARS (Interdisciplinary Association of Specialists in Reproductive Medicine). Version 2.0. Moscow: Editorial office of the journal StatusPraesens, 2021. 68 p. (in Russian).
3. Early pregnancy. From pregravidar preparation to healthy gestation / Edited by V.E. Radzinsky, A.A. Orazmuradova. 3rd ed., revised and enlarged. Moscow: Editorial office of the journal StatusPraesens, 2020. 798 p. (in Russian).
References
1. Aleshkina O.S., Konovalov O.E. Opinion of Obstetricians and Gynecologists on Problems of Rehabilitation of Women with Early Reproductive Losses. I.P. Pavlov Russian Medical Biological Herald. 2024;32(1):17-24. (In Russ.) https://doi.org/10.17816/PAVLOVJ566777
2. Biyik I, Albayrak M, Keskin F. Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio in Missed Abortion. Rev Bras Ginecol Obstet. 2020;42(5):235-239. https://doi.org/10.1055/s-0040-1709693
3. Tamanna S, Morosin SK, Delforce SJ, van Helden DF, Lumbers ER, Pringle KG. Renin-angiotensin system (RAS) enzymes and placental trophoblast syncytialisation. Mol Cell Endocrinol. 2022;547:111609. https://doi.org/10.1016/j.mce.2022.111609
4. Hashem A, Sarsam SD. The Impact of Incidental Ultrasound Finding of Subchorionic and Retroplacental Hematoma in Early Pregnancy. J Obstet Gynaecol India. 2019;69(1):43-49. https://doi.org/10.1007/s13224-017-1072-6
5. Haddad Kh., Orazmuradov A.A., Suleymanova Zh.Zh., Demina O.A., Morozov S.G., Krylova Yu.V. Pathogenetic aspects of missed pregnancy. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie [Obstetrics and Gynecology: News, Opinions, Training]. 2023;11(Supplement):139–143. (In Russ.) https://doi.org/10.33029/2303-9698-2023-11-suppl-139-143
6. Utrobin M.V., Yuryev S.Yu. Immunological and genetic changes as predictors in the loss of pregnancy in the formation of retrochorial hematoma in the first trimester. Acta Biomedica Scientifica. 2018;3(5):9-15. (In Russ.) https://doi.org/10.29413/ABS.2018-3.5.1
7. Lima J, Cambridge G, Vilas-Boas A, Martins C, Borrego LM, Leandro M. Serum markers of B-cell activation in pregnancy during late gestation, delivery, and the postpartum period. Am J Reprod Immunol. 2019;81(3):e13090. https://doi.org/10.1111/aji.13090
8. Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, et al. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis. 2017;267:49-60. https://doi.org/10.1016/j.atherosclerosis.2017.10.025
9. Petrov Yu.A., Blesmanovich A.E., Alyokhina A.G. Thyroid hypofunction and pregnancy. Modern problems of science and education. 2018;(5):4. (In Russ.) eLIBRARY ID: 36367792 EDN: YMRKBV
10. Xiong YM, Pan HT, Ding HG, He Y, Zhang J, et al. Proteomic and functional analysis of proteins related to embryonic development of decidua in patients with recurrent pregnancy loss†. Biol Reprod. 2021;105(5):1246-1256. https://doi.org/10.1093/biolre/ioab140
11. Heidari MM, Sheikholeslami M, Yavari M, Khatami M, Seyedhassani SM. The association of renin-angiotensinogen system genes polymorphisms and idiopathic recurrent pregnancy loss. Hum Fertil (Camb). 2019;22(3):164-170. https://doi.org/10.1080/14647273.2017.1388545
About the Authors
A. A. OrazmuradovRussian Federation
Agamurad A. Orazmuradov, Dr. Sci. (Med.), Prof., Depart. of Obstetrics and Gynecology with a course of perinatology, Medical Institute
Moscow
Competing Interests:
Authors declare no conflict of interest
Zh. Zh. Suleymanova
Russian Federation
Zhasmin Zh. Suleymanova, Depart. of Obstetrics and Gynecology with a course of perinatology, Medical Institute
Moscow
Competing Interests:
Authors declare no conflict of interest
E. A. Kuzmina
Russian Federation
Ekaterina A. Kuzmina, Depart. of Obstetrics and Gynecology with a course of perinatology, Medical Institute
Moscow
Competing Interests:
Authors declare no conflict of interest
I. N. Kostin
Russian Federation
Igor N. Kostin, Dr. Sci. (Med.), Prof., Depart. of Obstetrics and Gynecology with a course of perinatology, Medical Institute
Moscow
Competing Interests:
Authors declare no conflict of interest
Kh. Haddad
Russian Federation
Khalid Haddad, Cand. Sci. (Med.), Assistant, Depart. Of Obstetrics and Gynecology with a course of perinatology, Medical Institute
Moscow
Competing Interests:
Authors declare no conflict of interest
E. V. Mukovnikova
Russian Federation
Ekaterina V. Mukovnikova, Depart. of Obstetrics and Gynecology with a course of perinatology, Medical Institute
Moscow
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Orazmuradov A.A., Suleymanova Zh.Zh., Kuzmina E.A., Kostin I.N., Haddad Kh., Mukovnikova E.V. Angiotensinogen – a marker of non-developing pregnancy. Medical Herald of the South of Russia. 2025;16(2):37-42. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-2-37-42







































