Scroll to:
Recurrence of primary sclerosing cholangitis in the graft
https://doi.org/10.21886/2219-8075-2024-15-3-97-105
Abstract
Primary sclerosing cholangitis (PSC) is a disease characterized by inflammation, fibrosis and obliteration of both intra- and extrahepatic bile ducts, accompanied by cholestasis, with further outcome in biliary cirrhosis of the liver, cholangiocarcinoma. The pathogenesis of the disease is poorly understood, but, according to various sources, it involves genetic factors, innate and adaptive immunity mechanisms, the toxic effects of hydrophobic bile acids and, possibly, intestinal dysbiosis. The strong association with inflammatory bowel disease is associated with a significantly increased risk of colorectal cancer, which, along with cholangiocarcinoma, represents the most significant diagnostic challenge in the long-term management of PSC. The diagnosis of PSC is established based on the identification of typical cholangiographic lesions of the bile ducts and the exclusion of secondary causes of sclerosing cholangitis. Complex pathophysiology, heterogeneity of clinical features and the rare nature of the disease have led to the lack of effective therapy to date; there are no treatment algorithms, but a course of ursodeoxycholic acid in doses of 17–23 mg/kg/day can be prescribed for up to a year in order to monitor the dynamics of the decrease in levels serum alkaline phosphatase. A number of drugs are under investigation, including FXR (farnesoid X receptor) agonists with choleretic and antimicrobial properties. Clinically significant stenoses can be successfully treated with interventional endoscopy, but liver transplantation (LT) is currently the only curative treatment with a high survival rate. According to various literature data, 20–25% of patients develop disease relapse in the graft. Our case report of recurrent PSC in a patient 5 years after orthotopic LT provides an overview of management options from a practical, patient-centered perspective.
Keywords
For citations:
Pak E.S., Petrova T.M., Korobka R.V., Ushakov A.A., Kucherenko O.B., Katsiyaev V.Yu., Bukhtin O.V. Recurrence of primary sclerosing cholangitis in the graft. Medical Herald of the South of Russia. 2024;15(3):97-105. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-3-97-105
Introduction
The incidence rate of primary sclerosing cholangitis (PSC) is 16 per 100,000 people, the male-to-female ratio is 2:1, and the average age of patients is 25–40 years [1–3]. The pathogenesis of PSC involves genetic factors (nine loci associated with PSC development have been identified), activated lymphocytes in inflammatory bowel disease (IBD), increased permeability of the intestinal mucosa, decreased formation of secondary bile acids, and disturbances in the intestinal microbiome [3]. The most common symptoms are itching, fever, yellowing of the skin, and weight loss. Laboratory tests for PSC include increased levels of alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), hyperbilirubinemia, and a moderate increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [1]. Antibodies specific to PSC are not detected [4]. When diagnosing PSC, it is necessary to exclude causes that can lead to secondary sclerosis of the bile ducts (BD) (consequences of surgical interventions, as well as toxic, infectious, and other factors).
Methods of diagnosing PSC:
1) Expert transcutaneous ultrasound examination (TUS) allows establishing the localization of the BD block, which determines the choice of further methods of examination of patients to identify the cause of obstructive jaundice. TUS is an accessible, safe, and highly informative method for assessing the level of biliary obstruction. Its disadvantages include the subjectivity of the results, which depend on the experience of the operator, the class of equipment, and the preparation of the patient [5].
2) Magnetic resonance cholangiography (MR CG) is the main method for diagnosing PSC; it has a diagnostic value comparable to retrograde cholangiopancreatography (ERCP), while being a safer method for diagnosing PSC. MR CG visualizes BD strictures alternating with normal or dilated ducts ("beads") [1, 6, 7]. In international recommendations, preference is given to the results of MR CG [5].
3) ERCP is used when it is impossible to perform MR CG due to contraindications, to clarify the diagnosis in case of questionable MR CG results, or for therapeutic purposes, to expand narrowed BD and place stents [1][7].
4) Spiral computed tomography of the abdominal cavity with contrast agent is not the main method for diagnosing PSC, but is used for differential diagnosis of BD tumor lesions [1].
5) Histological examination is used when PSC with damage to small intrahepatic BD is suspected, as well as to detect autoimmune hepatitis (overlap syndrome). PSC is characterized by periductal fibrosis ("onion peel") and BD obliteration [1].
Possible PSC complications are the formation of BD gallstones, bacterial cholangitis, hepatic abscess, biliary cirrhosis, cholangiocarcinoma, and BD strictures.
In 60–80% of cases, PSC is combined with IBD: in 80% of cases – with nonspecific ulcerative colitis (UC), in 10% of cases – with Crohn's disease, and in 10% of cases – with indeterminate colitis [2, 8]. The debut of PSC occurs on average 10 years after the debut of IBD [3]. In IBD occurring against the background of PSC, the most typical problem is pancolitis with mild clinical symptoms [2][3]. Regular monitoring of biochemical parameters in patients with IBD is necessary; if the level of ALP and GGT increases, additional examination should be performed to confirm or exclude PSC. Patients with PSC may have asymptomatic or low-symptom IBD; therefore, such patients should undergo fibrocolonoscopy (FCS) with polyfocal biopsy. Patients with PSC and IBD have an increased risk of developing colorectal cancer and cholangiocarcinoma [7][9].
In PSC treatment, ursodeoxycholic acid (UDCA) preparations are used to normalize biochemical blood parameters [7], but UDCA does not affect the course of the disease itself [1][10][11]. Azathioprine and glucocorticosteroids also do not affect the PSC course but can be used when PSC is combined with autoimmune hepatitis [12]. Obeticholic acid has potential efficacy in PSC treatment; however, it is not currently registered in the Russian Federation [12][13]. Cholestyramine (first-line drug), rifampicin (second-line drug), naltrexone (third-line drug), and sertraline (fourth-line drug) can also be used in PSC treatment in order to relieve skin itching [12][13]; antibacterial therapy is prescribed in case of bacterial cholangitis development [12], enzyme replacement therapy (ERT) – in case of deficiency of fat-soluble vitamins. The ERT efficacy is assessed by the normalization of vitamin status, weight gain, and improvement of clinical indicators [14].
The only method of radical PSC treatment is liver transplantation (LT), [1][4][15][16], which provides a high curability of irreversible hepatic diseases (including PSC) [16]. The survival rate after one year after LT is 90% and after 5 years – 70% [1][4][12], which is comparable with the overall survival rates after LT (80–90%). [17]
In 20–25% of cases, after 5–10 years, relapse of PSC in the transplant occurs, which must be differentiated from ischemic cholangiopathy, cicatricial strictures of the anastomosis, and chronic transplant rejection [2][4][18-20].
The following Mayo criteria can be used to diagnose recurrent PSC: confirmed PSC diagnosis before LT; strictures of the intra- and extrahepatic BD on cholangiograms that occurred later than 90 days after transplantation; fibrous cholangitis, obliterating ductal lesions with or without ductopenia, cirrhosis based on the results of histological examination of the transplant biopsy; absence of hepatic artery thrombosis or stenosis, ductopenic rejection, strictures of the biliary tract anastomosis or strictures that occurred less than 90 days after LT, and donor-recipient incompatibility [18–21].
It should be noted that the risk of PSC recurrence is higher in cases of de novo IBD, history of acute cellular rejection, and in the presence of the HLA-DRB1*07 allele in the donor [20]. It should also be mentioned that cytomegalovirus (CMV) infection and the use of antilymphocyte antibodies to treat transplant rejection increase the risk of PSC recurrence.
PSC recurrence increases the risk of transplant loss and reduces patient survival rate [21]. There is no effective prevention of PSC recurrence. However, some studies suggest performing colectomy in patients with PSC and IBD before LT to reduce the risk of PSC recurrence [3][10][18].
Clinical case
In April 2006, patient G. first reported complaints of abdominal pain, nausea, chills, and loose stools for two weeks. He then sought medical attention at the local surgical department, where he was given a preliminary diagnosis of acute pancreatitis and acute intestinal infection. The therapy provided did not produce any significant positive effect, so the patient was transferred to the department of infectious diseases of the Semashko Central City Hospital in Rostov-on-Don. Bacteriological examination of feces for the coliform bacteria group was negative. The serological blood test for yersiniosis was negative. Complete blood count (CBC) was as follows: Hb – 122 g/L, erythrocytes – 4.4·1012, leukocytes – 9.9·109, and ESR – 8 mm/h. Urine diastase equaled to 64 units. The following treatment was provided: enzyme preparations and UDCA. The diagnosis was "irritable bowel syndrome"; FCS was recommended, according to the results of which UC and pancolitis were verified. The following therapy was prescribed: prednisolone, 5-aminosalicylic acid (sulfasalazine), omeprazole, phospholipids, probiotics, metronidazole, and drotaverine, with positive dynamics.
In October 2006, the patient sought consultation with a gastroenterologist at the State Autonomous Institution of the Rostov Region Regional Clinical Diagnostic Center with complaints of periodic pain in the right hypochondrium, bloating, rumbling, flatulence, and no blood in the stool. Additional examination was prescribed to exclude PSC, and the diagnosis was not confirmed based on the results of the additional examination. Subsequently, he noted remission for nine years.
In October 2015, the patient came to the State Autonomous Institution of the Rostov Region, the Regional Clinical Diagnostic Center, complaining of frequent stools up to nine times a day without impurities, painful urge to defecate, and itchy skin. Biochemical blood assay (BBA) from October 15, 2015: total bilirubin – 53.6 μmol/L, direct bilirubin – 43.2 μmol/L, AST – 203 U/L, ALT – 309 U/L, ALP – 1290 U/L, GGT – 639 U/L. FCS showed moderately active UC and dolichosigma. MR CG defined the following: the left hepatic duct was of uneven diameter, locally dilated to 7.2 mm. The right one was 3.7 mm. The middle third of the common bile duct was unevenly narrowed due to multiple annular strictures up to 2.5 mm in diameter, with uneven supra- and poststenotic dilation up to 7.5 mm. An unevenness of the lumen of the common hepatic duct was also identified, with small stenotic stenosis alternating with areas of the normal duct. The conclusion was as follows: changes in the extrahepatic and left hepatic ducts might correspond to cholangitis. Final diagnosis: UC, recurrent course, pancolitis, PSC. The following treatment was prescribed: 5-aminosalicylic acid (sulfasalazine) – 3 g/day, azathioprine – 100 mg, prednisolone – 30 mg, UDCA – 1000 mg/day. BBA from October 24, 2015: total bilirubin – 20.2 μmol/L, direct bilirubin – 8.9 μmol/L, AST – 51 U/L, ALT – 150 U/L, ALP – 683 U/L, GGT – 276 U/L. The patient was discharged with positive dynamics under the supervision of a physician in the home area.
In October 2017, during indirect hepatic elastometry, signs of FIB-4 were detected according to the Metavir scale.
In February 2018, the patient consulted a gastroenterologist at the State Budgetary Institution of the Rostov Region Regional Clinical Hospital with complaints of itchy skin and darkening of the skin. At the time of examination, he was receiving sulfasalazine – 2 g/day, azathioprine – 150 mg, prednisolone – 30 mg/day, and ursosan – 1,500 mg/day. Additional examination was prescribed to determine the indications for LT. Spiral CT of the abdomen showed signs of sclerosing cholangitis and hepatomegaly (179 mm) (Fig. 1). CA-19.9 test: 184 U/mL. In February 2018, the patient was put on the waiting list for LT.
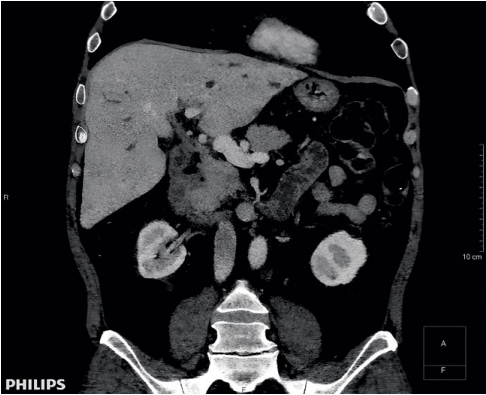
Рисунок 1. Компьютерная томограмма органов брюшной полости (февраль 2018г.)
Figure 1. Computed tomography of abdominal organs (February 2018)
In April 2018, due to the deterioration of his condition, the patient was hospitalized in the State Autonomous Institution of the Rostov Region, the Regional Clinical Diagnostic Center; periodic increases in temperature to febrile levels were noted. Based on the results of blood cultures for sterility, growth of S. pneumoniae was detected, and the following treatment was prescribed: ceftriaxone 2.0 + 0.9% NaCl solution 100.0 IV drip two times a day, metronidazole 5 mg/mL 100.0 IV drip two times a day, ciprofloxacin 2 mg/mL 100.0 IV drip two times a day, sulfasalazine 500 mg orally three times a day, fluconazole 50 mg one tablet orally one time a day, ademetionine 1200 mg + 0.9% NaCl solution 200.0 IV drip one time a day, meglumine sodium succinate 400.0 IV drip one time a day.
In June 2018, orthotopic cadaveric LT was performed for liver cirrhosis resulting from PSC. Histological examination of the liver showed the following: acute sclerosing cholangitis, cholangiolitis, chronic cholestatic hepatitis resulting in micronodular cirrhosis, purulent pericholecystitis, and chronic cholecystitis (Figs. 2, 3).
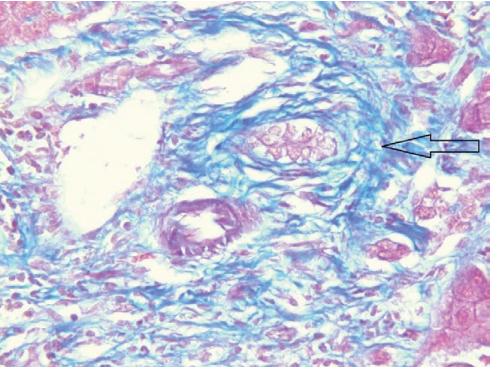
Рисунок 2. Удалённая печень, окраска по Массону, мелкий желчный проток с перидуктальным фиброзом (увеличение ×400)
Figure 2. Removed liver, Masson staining, small bile duct with periductal fibrosis (×400 magnification)
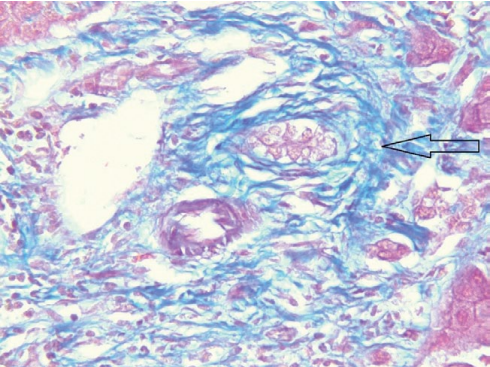
Рисунок 3. Удалённая печень, окраска гематоксилин и эозин, мелкий желчный проток (увеличение ×400)
Figure 3. Removed liver, hematoxylin and eosin staining, small bile duct (×400 magnification)
After transplantation, the following drugs were prescribed: tacrolimus – 9 mg/day, methylprednisolone – 16 mg/day, nystatin – one tablet four times a day, sulfamethoxazole + trimethoprim – 120 mg four times a day, valganciclovir – 900 mg/day, 5-aminosalicylic acid (sulfasalazine) – 3 g/day, and acetylsalicylic acid – 100 mg. Two months after LT, CMV infection was detected, a response was obtained while taking valganciclovir. Tacrolimus concentration was 6.7 ng/mL.
In December 2018, the patient was hospitalized in the gastroenterology department (GED) of the State Budgetary Institution of the Rostov Region Regional Clinical Hospital with complaints of loose stool, discomfort in the left hypochondrium, subfebrile fever, and the appearance of annular erythema. CBC was as follows: Hb – 114 g/L, erythrocytes – 3.64·1012, leukocytes – 6.1·109, platelets – 254·109. BBA was as follows: total bilirubin – 12.3 μmol/L, direct bilirubin – 2.5 μmol/L, ALT – 45 U/L, AST – 17 U/L, ALP – 73 U/L, GGT – 124 U/L, total protein – 64 g/L, urea – 8.5 mmol/L, and creatinine – 114.2 μmol/L. Coagulogram: APTT – 32.1 sec, INR – 1.0, PTI – 102.8%. Tacrolimus concentration was 6.1 ng/mL, control analysis after seven days showed the value of 9.6 ng/mL. IgM to the capsid antigen of the Epstein-Barr virus (EBV) were detected. Enhanced MRI of the abdomen using bolus tracking showed the condition after LT. The liver transplant was located normally, the contours were clear and smooth, and the dimensions were as follows: 177 mm craniocaudal. The structure of the liver parenchyma was homogeneous. The portal vein was 16 mm in diameter before the anastomosis, 14 mm at the level of the anastomosis, and 16 mm after the anastomosis. The splenic artery was 10 mm in diameter, the superior mesenteric vein was 13 mm. The hepatic artery had an average diameter of 6 mm along its entire length. The intrahepatic BD and common bile duct were not dilated. The duodenum was brought to the porta hepatis; in the area of the choledochoduodenoanastomosis, no visible pathological changes were identified. The following diagnosis was made: "Liver transplant dysfunction". Condition after orthotopic LT (OLT) from a cadaveric donor for liver cirrhosis as a result of high-activity, steroid-dependent PSC, F4 according to the Metavir scale, MELD 19. Complications: chronic EBV infection, reactivation. High-activity cholestasis syndrome, recurrent cholangitis. The following treatment was performed: tacrolimus 9 mg orally, methylprednisolone 4 mg orally, valganciclovir 900 mg orally, UDCA 250 mg, two capsules orally three times a day, microsphere enzymes 25,000 IU orally three times a day, acetylsalicylic acid 100 mg orally, 5-aminosalicylic acid (sulfasalazine) 500 mg orally three times a day, metronidazole 100.0 IV drip, and ciprofloxacin 100.0 IV drip. The patient was discharged with improvement; recommendations were given to him.
In February 2020, the patient was re-hospitalized in the GED of the State Budgetary Institution of the Rostov Region Regional Clinical Hospital with complaints of loose stool, discomfort in the right and left hypochondrium, and a rise in temperature to subfebrile numbers. CBC was as follows: Hb – 128 g/L, erythrocytes – 4.05·1012, leukocytes – 10.5·109, platelets – 235·109. BBA was as follows: total bilirubin – 18.5 μmol/L, direct bilirubin – 3.9 μmol/L, ALT – 95 U/L, AST – 42 U/L, total protein – 64 g/L, urea – 10.7 mmol/L, creatinine – 131.5 μmol/L, GGT – 421 U/L, APT – 73 U/L. Coagulogram: APTT – 27.8 sec., INR – 0.9, PTI – 114%. Tacrolimus concentration was 6.6 ng/mL. MRI of the abdomen: dilation of the intrahepatic BD; at the anastomosis site, the common bile duct was unevenly narrowed to 2 mm. During treatment, there was a decrease in symptoms and a decrease in liver enzyme levels (ALT 23 U/L, AST 16 U/L, GGT 146 U/L, ALP 33 U/L). The following diagnosis was made: stricture of the terminal section of the common bile duct, recurrent cholangitis. The patient was transferred to the surgical department, where ERCP was performed: intrahepatic ducts were up to 7 mm, homogeneous. The common bile duct was 8–9 mm, tortuous, at the border of the middle and distal thirds conically narrowed to 1.5 mm over a length of 5 mm; at the border of the common hepatic duct and the middle third, it was conically narrowed to 1.5 mm over a length of 5 mm. Endoscopic retrograde papillosphincterotomy and balloon dilation of the common bile duct were performed. The control MRI of the abdomen dated June 11, 2020 showed dilation of the intrahepatic BD up to 4–5 mm, filling defects (air?) in the lumen of the common hepatic duct, the common bile duct diameter was up to 8 mm; at the anastomosis site, the common bile duct was unevenly narrowed to 2 mm.
In March 2021, the patient was hospitalized in the endovideosurgery department of the Regional Clinical Hospital due to the deterioration of his condition after a coronavirus infection. BBA was as follows: total bilirubin – 106.7 μmol/L, direct bilirubin – 60.8 μmol/L, ALT – 137 U/L, AST – 187 U/L, GGT – 256 U/L, APT – 170 U/L. ERCP: a short narrowing of up to 2 mm was determined at the border of the middle and upper third of the common bile duct, balloon bougienage of stenosis was performed, there was no narrowing on the control cholangiogram. Control BBA: total bilirubin – 13.9 μmol/L, direct bilirubin – 3.9 μmol/L, ALT – 23 U/L, AST – 12 U/L, GGT – 87 U/L, ALP – 72 U/L.
MRI of the abdomen from October 11, 2021: signs of biliary hypertension, block at the level of the porta hepatis and the lower third of the common bile duct, signs of a cyst in the right lobe of the transplant. MR CG from September 12, 2022: uneven expansion of the intrahepatic BD, with more expansion in the left lobe (up to 6.8 mm); lobar ducts had a diameter of 3 mm, the common hepatic duct had a diameter of up to 4 mm, the common bile duct had a diameter of up to 5.3 mm; in the lower third, the common bile duct was unevenly narrowed to 2 mm; MR signals from the lumen were uneven. The walls of the common hepatic duct and common bile duct were thickened, the internal contour was uneven. Liver antibodies from October 21, 2022: ANA, AMA 2, AT to gp 210, sp 100, SLA/LP, LKM-1, LC-1 were not detected.
On May 4, 2023, a liver biopsy was performed, and the following conclusion was received: eight portal tracts were noted in the delivered liver trephine biopsy specimens, three of them were with F1 fibrosis and lymphocytic infiltration with the formation of large clusters; plasma cells and segmented leukocytes were absent; 3–5 small BD triads were defined, some of them were with an obliterated lumen; the remaining five portal tracts were relatively intact, with moderate edema and focal lymphocytic infiltration. Hepatocytes were with severe granular dystrophy, as well as intracellular cholestasis; central veins had wall edema; necrobiotic changes were seen in zone 3 hepatocytes in the surrounding parenchyma; sinusoids were edematous with rare lymphoid elements and erythrocytes. Conclusion: no signs of cellular and humoral rejection were detected 0R, AMR0. The morphological picture was more typical for relapse of sclerosing cholangitis in the transplant; signs of moderate transplant ischemia were also detected (Figs. 4, 5).
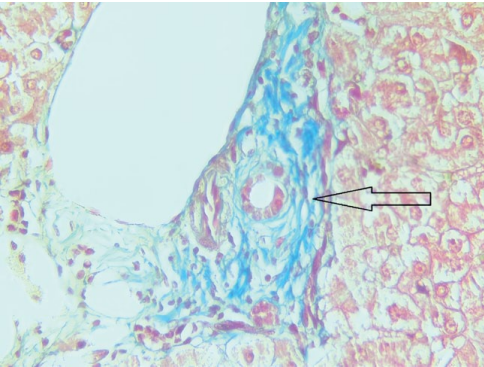
Рисунок 4. Биоптат трансплантата печени, окраска по Массону, мелкий желчный проток с перидуктальным фиброзом (увеличение ×400)
Figure 4. Liver transplant biopsy, Masson staining, small bile duct with periductal fibrosis (×400 magnification)
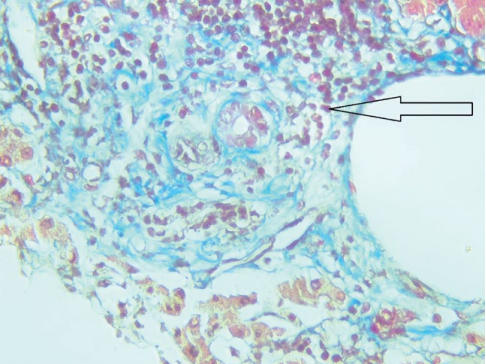
Рисунок 5. Биоптат трансплантата печени, окраска по Массону, портальный тракт с лимфоидной инфильтрацией и перидуктальным фиброзом (увеличение ×400)
Figure 5. Liver transplant biopsy, Masson staining, portal tract with lymphoid infiltration and periductal fibrosis (×400 magnification)
Spiral CT of the abdomen with intravenous contrast from August 3, 2023 showed the following: intrahepatic BD on the right were not dilated (up to 2 mm), left lobar and segmental ducts had a diameter of up to 3–4 mm, common hepatic duct – a diameter of up to 4 mm, common bile duct – a diameter of up to 7 mm. At the level of the major duodenal papilla, a diverticulum measuring 8.5×12.5×18 mm was identified; in the lumen, liquid content and air with a horizontal level were defined.
MR CG from September 11, 2023 showed the following: dilation of the intrahepatic BD up to 6 mm, right lobar duct was 4.4 mm, left lobar duct – 3.8 mm, the common hepatic duct had a diameter of up to 6 mm; the common bile duct was determined along its entire length with an uneven diameter of up to 3–5 mm, the internal contour was uneven in the anastomosis area (Fig. 6).
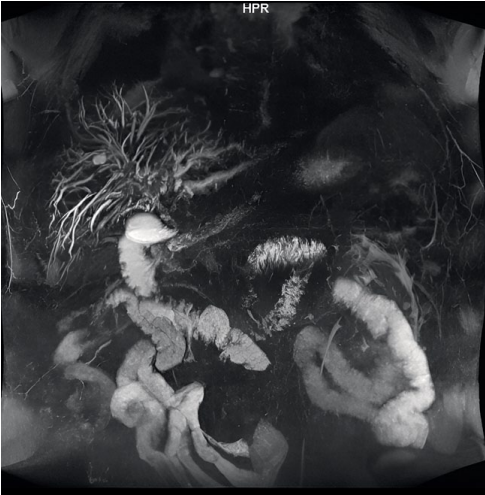
Рисунок 6. Магниторезонансная холангиография от 11.09.2023 г.
Figure 6. Magnetic resonance cholangiography from 09/11/2023
FCS from November 30, 2023 showed the following: the Bauhinian valve was labial in shape, closed tightly. The intestinal lumen was of normal shape. Intestinal walls were elastic. Tonus was segmentally reduced. Peristalsis was sluggish. Folds were expressed according to the intestinal sections. The mucosa was focally hyperemic, clean, vascular pattern was normal. Ulcers, neoplasms were not detected. Conclusion: chronic catarrhal colitis.
Antibodies to HLA were detected: A*11,25,26,43,66; B*15, DQA1*01; DQB1*05,06.
Immunoglobulins were defined: immunoglobulin G – 21.3 g/L, immunoglobulin M – 2.4 g/L, immunoglobulin A – 1.1 g/L.
Based on the results of additional examination, the diagnosis was "Relapse of primary sclerosing cholangitis in the transplant (de novo)"; and the patient was included in the waiting list for liver retransplantation. Currently, the patient is receiving the following therapy: tacrolimus – 2.5 mg/day, methylprednisolone – 8 mg/day, UDCA – 500 mg three times a day, acetylsalicylic acid – 100 mg/day, 5-aminosalicylic acid (sulfasalazine) – 500 mg two times a day, rabeprazole – 20 mg, microsphere enzymes – 25,000 IU three times a day.
Conclusion
PSC recurrence in a transplant (de novo) remains a pressing problem in transplantology and often leads to the loss of the transplanted organ. The absence of proven methods for preventing this condition requires constant laboratory and instrumental monitoring of patients with PSC after OLT. When this type of transplant damage is detected, the situation is aggravated by the progressive course of the disease, often the ineffectiveness of conservative therapy, and the need for surgical intervention in patients on immunosuppressive therapy. Herewith, the frequency of association of PSC with IBD, in particular with UC, forces doctors to be vigilant in monitoring patients for possible prevention of surgical complications characteristic of this group of diseases. CMV infection is another risk factor for the development of PSC recurrence in a liver transplant, which must be promptly identified and prevented. In the provided clinical case, patient G. had factors predisposing to the development of PSC relapse in the liver transplant: male gender and middle age, combined course of PSC with UC, and CMV infection in the early postoperative period. Despite all possible attempts to prevent the development of this condition (immunosuppressive therapy, constant intake of UDCA drugs, control of the UC course, treatment of CMV in a short time) and constant dispensary observation, PSC returned, progression of the disease was noted, requiring liver retransplantation, in connection with which the patient was put on the waiting list for LT.
References
1. Vinnitskaya E.V., Abdulkhakov S.R., Abdurakhmanov D.T., Alikhanov R.B., Bakulin I.G., et al. Important problems in the diagnosis and treatment of primary sclerosing cholangitis (based on the Russian consensus on diagnosis and treatment autoimmune hepatitis. Moscow, 2018). Terapevticheskii arkhiv. 2019;91(2):9-15. (In Russ.) https://doi.org/10.26442/00403660.2019.02.000075
2. Baykova O.A., Nikolaeva N.N., Grishchenko E.G., Nikolaeva L.V., Salamatova M.M., Makarkin A.A. A case of combining primary sclerosing cholangitis and Crohn’s disease. Medical & pharmaceutical journal "Pulse". 2020;22(4):13-21. (In Russ.) https://doi.org/10.26787/nydha-2686-6838-2020-22-4-13-21
3. Nikitin A.V., Volynets G.V. Sclerosing cholangitis and inflammatory bowel disease: which comes first? Rossiyskiy Vestnik Perinatologii i Pediatrii (Russian Bulletin of Perinatology and Pediatrics). 2021;66(1):39-46. (In Russ.) https://doi.org/10.21508/1027-4065-2021-66-1-39-46
4. Syutkin V.E., Salienko A.A., Olisov O.D., Novruzbekov M.S. Relapse of autoimmune diseases after liver transplantation. Transplantologiya. The Russian Journal of Transplantation. 2022;14(4):421-431. (In Russ.) https://doi.org/10.23873/2074-0506-2022-14-4-421-431
5. Khatkov I.E., Avanesyan R.G., Akhaladze G.G., Beburishvili A.G., Bulanov A.Y., et al. Diagnostic and conservative treatment nuances in patients with obstructive jaundice: in the wake of Russian consensus. Terapevticheskii arkhiv. 2021;93(2):138-144. https://doi.org/10.26442/00403660.2021.02.200619
6. Khoshpouri P, Habibabadi RR, Hazhirkarzar B, Ameli S, Ghadimi M, et al. Imaging Features of Primary Sclerosing Cholangitis: From Diagnosis to Liver Transplant Follow-up. Radiographics. 2019;39(7):1938-1964. https://doi.org/10.1148/rg.2019180213
7. Podstavkina I.S., Mordasova V.I., Korotkikh N.N., Trunova P.A., Timchenko I.V., et al. The effect of liver transplantation on the course of ulcerative colitis in patients with primary sclerosing cholangitis - ulcerative colitis on clinical examples. Pharmacology & Pharmacotherapy. 2023;(2):26-36. https://doi.org/10.46393/27132129_2023_2_26
8. НNikitin A.V., Khavkin A.I., Skvortsova T.A., Volynets G.V., Atameeva A.O. Combination of ulcerative colitis with cirrhosis of the liver in the outcome of primary sclerosing cholangitis. Experimental and Clinical Gastroenterology. 2020;174(5):104-107. (In Russ.) https://doi.org/10.31146/1682-8658-ecg-177-5-104-107
9. Tsyrkunov V.M., Prokopchik N.I., Andreev V.P. Autoimmune cholestatic lesions of biliary ducts. Hepatology and Gastroenterology. 2021;5(2):99-100. (In Russ.) https://doi.org/10.25298/2616-5546-2021-5-2-99-100
10. Chen C, Ke R, Yang F, Cai Q, Liu J, et al. Risk factors for recurrent autoimmune liver diseases after liver transplantation: A meta-analysis. Medicine (Baltimore). 2020;99(20):e20205. https://doi.org/10.1097/MD.0000000000020205
11. Gumm A, Perez-Atayde A, Wehrman A. Posttransplant considerations in autoimmune liver disease: Recurrence of disease and de novo. Clin Liver Dis (Hoboken). 2022;20(4):130-135. https://doi.org/10.1002/cld.1239
12. Raikhelson K.L., Pazenko E.V., Marchenko N.V. Primary sclerosing cholangitis: review of recommendations for diagnosis and treatment of disease. Consilium Medicum. 2017;19(8):121–130. https://doi.org/10.26442/2075-1753_19.8.121-130
13. Polunina T.E. Cholestasis: algorithms for diagnosis and treatment. Academy of medicine and sports. 2021;2(4):28-36. (In Russ.) https://doi.org/10.15829/2712-7567-2021-43
14. Khatkov I.E., Maev I.V., Bordin D.S., Kucheryavyi Y.A., Abdulkhakov S.R., et al. The Russian consensus on the diagnosis and treatment of chronic pancreatitis: Enzyme replacement therapy. Terapevticheskii arkhiv. 2017;89(8):80-87. https://doi.org/10.17116/terarkh201789880-87
15. Voskanyan S.E., Syutkin V.E., Sushkov A.I., Voskanyan Yu.V., Veselkova A.Yu. Liver allograft pathology in the late posttransplant period. Transplantologiya. The Russian Journal of Transplantation. 2023;15(3):359-375. https://doi.org/10.23873/2074-0506-2023-15-3-359-375
16. Korobka V.L., Pasetchnikov V.D., Korobka R.V., Pak E.S., Shapovalov A.M. Use of endoscopic band ligation alone and in combination with nonselective beta blockers for prevention of variceal bleeding in ascites patients on the liver transplant waiting list. Russian Journal of Transplantology and Artificial Organs. 2022;24(3):42-50. https://doi.org/10.15825/1995-1191-2022-3-42-50
17. Korobka V.L., Pak E.S., Shapovalov A.M., Kostrykin M.U., Tkachev A.V. Analysis of four-year management of the waiting list for liver transplantation in Rostov region: prospects for reducing mortality of candidates listed for liver transplantation. Medical Herald of the South of Russia. 2019;10(3):32-39. (In Russ.) https://doi.org/10.21886/2219-8075-2019-10-3-32-39
18. Visseren T, Erler N.S., Heimbach J.K., Eaton J.E., Selzner N, et al. Inflammatory conditions play a role in recurrence of PSC after liver transplantation: An international multicentre study. JHEP Rep. 2022;4(12):100599. https://doi.org/10.1016/j.jhepr.2022.100599
19. Steenstraten IC, Sebib Korkmaz K, Trivedi PJ, Inderson A, van Hoek B, et al. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment Pharmacol Ther. 2019;49(6):636-643. https://doi.org/10.1111/apt.15148
20. Bajer L, Slavcev A, Macinga P, Sticova E, Brezina J, et al. Risk of recurrence of primary sclerosing cholangitis after liver transplantation is associated with de novo inflammatory bowel disease. World J Gastroenterol. 2018;24(43):4939-4949. https://doi.org/10.3748/wjg.v24.i43.4939
21. Visseren T, Erler NS, Polak WG, Adam R, Karam V, et al. Recurrence of primary sclerosing cholangitis after liver transplantation – analysing the European Liver Transplant Registry and beyond. Transpl Int. 2021;34(8):1455-1467. https://doi.org/10.1111/tri.13925
About the Authors
E. S. PakRussian Federation
Ekaterina S. Pak, Cand. Sci. (Med.), Assistant of the Department of Reconstructive, Cardiovascular, Thoracic, Maxillofacial Surgery and Organ Transplantation;
Head of the Gastroenterology Department, of the Regional Center of Surgery and Donor Coordination
Rostov-on-Don
T. M. Petrova
Russian Federation
Tatyana M. Petrova, gastroenterologist of the Gastroenterology Department, of the Regional Center of Surgery and Donor Coordination
Rostov-on-Don
R. V. Korobka
Russian Federation
Roman V. Korobka, Cand. Sci. (Med.), Associate Professor of the Department of Reconstructive, Cardiovascular, Thoracic, Maxillofacial Surgery and Organ Transplantation;
Director of the Regional Center of Surgery and Donor Coordination
Rostov-on-Don
A. A. Ushakov
Artyom A. Ushakov, gastroenterologist of the Gastroenterology Department, of the Regional Center of Surgery and Donor Coordination
Rostov-on-Don
O. B. Kucherenko
Russian Federation
Olga В. Kucherenko, assistant of the Department of Radiation Diagnostics;
head of the X-ray diagnostic department
Rostov-on-Don
V. Yu. Katsiyaev
Russian Federation
Vladimir Yu. Katsiyaev, pathologist, Head of laboratory of Immunohistochemistry
Rostov-on-Don
O. V. Bukhtin
Russian Federation
Oleg V. Bukhtin, Resident of the Department of Internal Diseases No. 1
Rostov-on-Don
Review
For citations:
Pak E.S., Petrova T.M., Korobka R.V., Ushakov A.A., Kucherenko O.B., Katsiyaev V.Yu., Bukhtin O.V. Recurrence of primary sclerosing cholangitis in the graft. Medical Herald of the South of Russia. 2024;15(3):97-105. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-3-97-105







































