Scroll to:
Predictors of unfavorable neurological outcomes in premature children: a modern view on the problem
https://doi.org/10.21886/2219-8075-2024-15-3-77-82
Abstract
Objective: to assess the prognostic significance of antibodies to the NR2 subunit of the N-methyl-D-aspartate (NMDA) glutamate receptor and brain natriuretic propeptide (NTproBNP) as predictors of unfavorable neurological outcomes in premature children.
Materials and methods: A prospective cohort continuous study included 151 premature children, with a gestational age (GA) of 26–32 weeks and a body weight of 590–1990 gr. The children were measured the quantitation of NTproBNP level in the cord blood and level of antibodies to the NR2 subunit of the N-methyl-D-aspartate (NMDA) glutamate receptor in the blood serum on the 21st day of life. The division of children into groups was carried out in accordance with the assessment of neurological outcomes at control points of the research: 1 control point — at the time of discharge from the hospital, 2 control point — at the age of 1 year of corrected age (CA), 3 control point — 4 years of life.
Results: in the course of the study, it was found a “cascade” increase in the cohort of children with unfavorable neurological outcomes from 36.4% at the time of discharge from the hospital to 70% by the age of 4 years. High values of NT-proBNP level in cord blood and antibodies to the NR2 subunit of the N-methyl-D-aspartate (NMDA) glutamate receptor were established on 21st day of postnatal life in children with both macrostructural brain damages in the neonatal period and having an unfavorable neurological outcome at the age of 1 year of corrected age (CA) and at 4 years of life.
Conclusion: modern neurochemical markers of CNS damages N-proBNP and antibodies to the NR2 subunit of the N-methyl-D-aspartate (NMDA) glutamate receptor open up the possibilities of early diagnosis of brain damages at the cellular level and the start of neuroprotective therapy to reduce neurological disability.
Keywords
For citations:
Pavlinova E.B., Savchenko O.A. Predictors of unfavorable neurological outcomes in premature children: a modern view on the problem. Medical Herald of the South of Russia. 2024;15(3):77-82. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-3-77-82
Introduction
The percentage of premature births worldwide remains high with no downward trend. Currently, the conjugation of prematurity with impaired cognitive function and mental health in children is becoming apparent. Of particular concern is the long-term neurological deficit in children born prematurely without macrostructural brain lesions [1].
Until recently, the search for the causes of neurological deficiency in this category of children was in the area of the pathological conditions of the perinatal period [2]. Currently, the focus is shifted to the search for microstructural and biochemical disorders of the central nervous system, as well as to the study of the features of neuroontogenesis in this category of children [3]. Early diagnosis of microstructural brain disorders by modern neuroimaging methods is sometimes unavailable and remains controversial due to subjectivity and methodological flaws [4].
At present, the priority is the search for biomarkers of brain damage at the cellular level.
The aim of the study was to assess the prognostic significance of antibodies to the NR2 subunit N-methyl-D-aspartate (NMDA) of the glutamate receptor and brain natriuretic propeptide (N-terminal pro-BNP) as predictors of adverse neurological outcomes in premature infants.
Materials and methods
A prospective cohort continuous study included 151 premature newborns. The children were born and received treatment at the Omsk City Clinical Perinatal Center. The work was approved by the local ethics committee of Omsk State Medical University (protocol No. 118 of March 12, 2020).
The inclusion criteria were premature infants with a gestational age (GA) of 26–32 weeks and birth weight of 590–1990 g.
The exclusion criteria were premature infants with congenital heart defects (except for cases of impaired hemodynamic adaptation characteristic of premature infants – open ductus arteriosus and open oval window), congenital brain malformations according to neuroimaging methods, children with genetic diseases, and lack of informed voluntary consent of the child's legal representatives to participate in the study.
Enzyme-linked immunosorbent assay was used to quantify the level of brain natriuretic propeptide (N-terminal pro-brain natriuretic peptide/NT-proBNP) in umbilical cord blood at birth.
By the end of the 1st day of life, brain neurosonography with blood flow determination and Doppler echocardiography were performed (to exclude children with congenital brain anomaly and congenital heart disease from the study). On day 21 of life, serum NR2-AT levels (ng/ml) were determined by enzyme-linked immunosorbent assay according to a protocol developed by DRD Diagnostic Reagents Devices Ltd. (Skolkovo Innovation Center). During the hospital stay and at the time of discharge, a series of neurosonography studies were conducted with the determination of blood flow, as well as EEG and MRI of the brain.
The distribution of children into groups was based on the assessment of neurological outcomes in children born deeply premature at the following study control points. The moment of discharge from the hospital – the 1st control point: group I – children with structural lesions of the central nervous system, group II – without structural lesions of the central nervous system. Adjusted age – 1st year of life (2nd control point): group III – unfavorable outcome, group IV – favorable outcome. Age – 4 years of life (3rd control point): group V – unfavorable outcome, group IV – favorable outcome (Fig. 1).
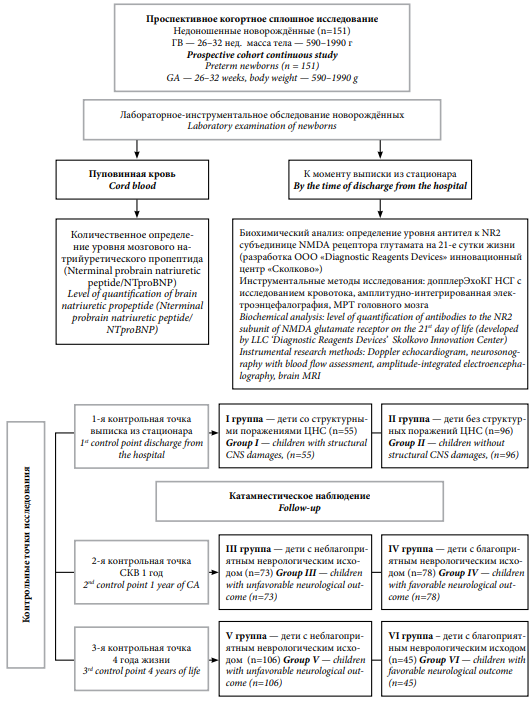
Рисунок 1. Дизайн клинического исследования
Figure 1. Clinical study design
Statistical processing of the obtained information included the formation of a database taking into account the clinical, laboratory, and morphofunctional characteristics of groups using the Microsoft Office spreadsheet program Excel 2003 (License Agreement 74017-640-0000106-57177). For statistical processing of the material, the application package Statistica v. 6.1 (license agreement BXXR006B092218FAN11) was used. Statistical hypotheses were tested by detecting differences in the compared groups using the Wald-Wolfowitz test and Mann-Whitney U test. In statistical calculations, the critical error level p was considered equal to 0.05. Comparison of binary groups, relative frequencies within one group, in two independent groups was carried out by constructing four-field tables of absolute frequencies, according to testing the zero statistical hypothesis about the equality of relative frequencies in two populations using the exact two-sided Fisher's test and χ2 criterion with Yates correction.
Results
At the 1st control point of the study, all children were divided into groups: group I – premature children with structural lesions of the central nervous system (n = 55), group II – children without structural lesions of the central nervous system (n = 96).
Children of the study groups did not have statistically significant differences in birth weight (p = 0.156) and GA (p = 0.054): in group I, body weight was 1270.5 [990; 1490] g, GA – 30 [28; 32] weeks, and in group II – 1420 g [1125; 1530] and 31 [29; 33] weeks, respectively. As a result of the study, it was found that children of group I had higher values of lactate (p = 0.048), NT-proBNP (p = 0.0005) in cord blood, and the level of antibodies to the NR2 subunit of the NMDA receptor, determined on the 21st day of life (p = 0.00001) (Table 1).
Таблица / Table 1
Значения биомаркеров повреждения ЦНС у недоношенных детей с благоприятными и неблагоприятными неврологическими исходами в различные возрастные периоды
Biomarker values of CNS damages in premature children with favorable and unfavorable neurological outcomes at various age periods
|
Контрольные точки
Control points
|
Исход
Outcome |
At к NR2 субъединице NMDA, 21-й день жизни, нг/мл
At to NR2 NMDA subunit 21st day of life, ng/mL
|
р |
NT –proBNP пуповинная кровь, пг/мл
NT –proBNP cord blood, pg/mL |
р |
Лактат пуповинной крови, ммоль/л.
Cord blood lactate, mmol/L |
р |
|
Выписка из стационара
Discharge from hospital
|
1 |
0,210 [ 0,120;0,343] 0,458 [ 0,247;2,563] |
0,0000.. |
631,6 [ 293,9;2136,6] |
0,0005* |
2,9 [ 1,9;3,9] |
0,048* |
|
2 |
0,180 [ 0,130;0,299] 0,209 [ 0,139;0,331] |
251,0 [ 83,5;391,9] |
2,0 [ 1,5;3,0] |
||||
|
скоррегированный возраст (СКВ) 1 год corrected age 1 year |
1 |
0,247 [ 0,128;0,435] 0,325 [ 0,213;1,301] |
0,001* |
804,0 [ 323,2;2397,7] |
0,009* |
2,8 [ 1,7;3,8] |
0,041* |
|
2 |
0,146 [ 0,09;0,247] 0,200 [ 0,120;0,266] |
207,1 [ 67,7;345,6] |
2,2 [ 1,4;2,7] |
||||
|
4 года жизни
4 years of life |
1 |
0,247 [ 0,119;0,367] |
0,03* |
801,3 [ 273,1;2136,6] |
0,0004* |
2,2 [ 1,5;3,1] |
0,579 |
|
2 |
0,1467 [ 0,070; 0,174] |
128,9 [ 58,6;333,1] |
2,0 [ 1,4;3,1] |
Примечание: *различия между группами статистически значимы (p<0,05); сравнение двух независимых групп критерием Манна-Уитни. Исход 1 — неблагоприятный. Исход 2 — благоприятный.
Note: * differences between groups are statistically significant (p < 0.05); comparison of two independent groups by the Mann-Whitney criterion. Outcome 1 — unfavorable. Outcome 2 — favorable.
By the time of discharge from the hospital, 55 children (36.4%) had a structural brain lesion: intraventricular effusion (IVE) of grades 1–2 (25.5%), periventricular leukomalacia (PVL) (10.9%), ventriculodilation (30.9%), brain lesion characteristic for the intrauterine infection (9%), grade 2–3 IVE with PVL (11%) and multicystic damage to the brain substance (3.6%), parenchymal hemorrhage (5.4%), ischemic stroke (3.6%).
At the 2nd control point of the study (1 year of corrected age (COA)), children were divided into groups according to neurological outcomes: group III – unfavorable (n = 73) and group IV – favorable (n = 78). Children of these groups did not have statistically significant differences in birth weight (p = 0.578) and GA (p = 0.864): in group III, body weight was 1100 [ 858; 1490] g, GA – 30 [ 28; 32] weeks, and in group IV – 1200 g [ 990; 1350] and 31 [ 29; 30] weekы, respectively. It was found that children of group III had higher values of lactate (p = 0.041), NT-proBNP (p = 0.009) in umbilical cord blood, and the level of antibodies to the NR2 subunit of the NMDA receptor on the 21st day of life (p = 0.001) (Table 1).
By the 1st year of COA, the cohort of children with neurological disorders increased by 18 children and amounted to 73 children (48%). In this cohort of children, sensorineural hearing loss was diagnosed in 1 child (1.4%), epilepsy – in 2 children (2.7%), delay in speech development (DSD) – in 39 children (53.5%), motor development delay – in 10 children (13.6%), delay of psychomotor development – in 20 children (27.4%), and cerebral palsy – in 1 child (1.4%).
At the 3rd checkpoint (4 years of life), children were divided into groups according to neurological outcomes: group V – unfavorable (n = 106), group VI – favorable (n = 45). Children of the study groups did not have statistically significant differences in birth weight (p = 0.617) and GA (p = 0.564): in group V, body weight was 1285 [ 950; 1500] g, GA – 30 [ 28; 32] weeks, and in group VI – 1200 g [ 980; 1350] and 30 [ 29; 32] weeks, respectively. As a result of the study, children of group V had higher NT-proBNP values (p = 0.0004) and the level of antibodies to the NR2 subunit of the NMDA receptor on the 21st day of life (p = 0.03) (Table 1).
By 4 years of age, the cohort of children with neurological disorders increased by 33 children (22%) and amounted to 70% (106 children). By 4 years of life, the structure of neurological pathology was as follows: autism spectrum disorders (ASD) – in 8 children (8%), DSD (sensory, motor, mixed alalia) – 65 children (61.3%), general intellectual failure – 20 children (22%), epilepsy – 6 children (5.7%), and cerebral palsy – 3 people (2.8%).
Discussion
Prematurity remains the leading cause of childhood neurological morbidity and disability. This category of children has a decrease in the intelligence coefficient, social adaptation, and a high frequency of mental disorders [5].
The long-term adverse neurological outcomes in premature infants without perinatal macrostructural brain changes are of particular concern.
According to the results of our study, a "cascading" increase in the cohort of children with unfavorable neurological outcomes from the moment of discharge from the hospital to 4 years of age was revealed: by the time of discharge from the hospital, 55 children (36.4%) had structural brain lesions; by the 1st year of life, the cohort with unfavorable outcomes in the form of motor, cognitive, and behavioral disorders increased by 18 children and amounted to 73 children (48%), and by 4 years of age, 106 children (70%) had unfavorable neurological outcomes.
Thus, out of 96 premature children discharged from the hospital without structural brain lesions, by the age of 4 years, only 45 children (30%) did not have neurological disorders. The leading positions in the structure of neurological pathology were occupied by ASD (8%), DSD (61.3%), and general intellectual insufficiency (22%).
Diagnosis of neuronal damage by detailed neuroimaging methods (MRI morphometry, infrared spectroscopy of the brain, tractography, functional MRI) is not always possible; therefore, at the present stage, the search for biochemical markers of central nervous system damage at the cellular level is important [6].
Currently, the atypical course of neuroontogenesis in premature infants, which results in a decrease in the volume of brain regions, calls into question the phenomenon of "catch-up" structural brain growth in children, their neurocognitive and behavioral development in the future [7].
An early predictor of this process is the natriuretic peptide system, which from the second trimester of intrauterine development regulates not only fetal-placental blood flow, but also participates in neuroontogenesis. Without directly regulating the activity of neurons, it modulates their work through receptors expressed in the glia and acts as a neurotransmitter [8].
Receptors of the N-terminal precursor of the brain natriuretic peptide (NT-proBNP) are widely represented in the limbic system and the cerebral cortex, the high level of which directly correlates with a small volume of gray matter in the brain and a low cognitive level of people in all age groups [9]. NT-proBNP is known to be involved in the regulation of neurovascular and blood-brain barrier integrity, synaptic transmission, and neuroinflammation at the microglial level [10]. A direct correlation of the NT-proBNP level with small brain volume, gray matter, and the abnormal microstructural organization of white matter was established. NT-proBNP is considered a predictor of subclinical brain damage, epilepsy, autism, and affective disorders [11].
Excitotoxicity is one of the mechanisms of pre- and oligodendrocytes damage in the developing brain, mediated through the NMDA population of glutamatergic ionotropic receptors. Excessive stimulation of these receptors during hypoxia leads to post-traumatic oxidative stress and cell death over a long period, which can determine a distant neurological deficit in a child [12].
According to the results of our study, high values of the NT-proBNP level in cord blood and NR2 antibodies to NMDA receptors on the 21st day of postnatal life were found in children with both macrostructural brain lesions in the neonatal period and an unfavorable neurological outcome in 1st year of COA, and in 4 year of life. Umbilical cord blood lactate as a marker of the severity of intrauterine hypoxia loses prognostic significance in assessing the risk of adverse neurological outcome in children by 4 years of age.
Conclusion
The use of modern neurochemical markers of central nervous system damage opens up the possibility of early diagnosis of brain damage at the cellular level and the start of neuroprotective therapy in order to reduce neurological disability.
References
1. Finch-Edmondson M, Morgan C, Hunt RW, Novak I. Emergent Prophylactic, Reparative and Restorative Brain Interventions for Infants Born Preterm With Cerebral Palsy. Front Physiol. 2019;10:15. https://doi.org/10.3389/fphys.2019.00015
2. Penn AA, Gressens P, Fleiss B, Back SA, Gallo V. Controversies in preterm brain injury. Neurobiol Dis. 2016;92(Pt A):90-101. https://doi.org/10.1016/j.nbd.2015.10.01
3. Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, et al. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33(2):411-423. https://doi.org/10.1523/JNEUROSCI.4445-12.2013
4. Oltman SP, Rogers EE, Baer RJ, Jasper EA, Anderson JG, et al. Newborn metabolic vulnerability profile identifies preterm infants at risk for mortality and morbidity. Pediatr Res. 2021;89(6):1405-1413. https://doi.org/10.1038/s41390-020-01148-0
5. Weider S, Lærum AMW, Evensen KAI, Reitan SK, Lydersen S, et al. Neurocognitive function and associations with mental health in adults born preterm with very low birthweight or small for gestational age at term. Front Psychol. 2023;13:1078232. https://doi.org/10.3389/fpsyg.2022.1078232
6. Vo Van P, Alison M, Morel B, Beck J, Bednarek N, et al. Advanced Brain Imaging in Preterm Infants: A Narrative Review of Microstructural and Connectomic Disruption. Children (Basel). 2022;9(3):356. https://doi.org/10.3390/children9030356
7. Ma Q, Wang H, Rolls ET, Xiang S, Li J, et al. Lower gestational age is associated with lower cortical volume and cognitive and educational performance in adolescence. BMC Med. 2022;20(1):424. https://doi.org/10.1186/s12916-022-02627-3
8. Miyoshi T, Hosoda H, Minamino N. Significance of Atrial and Brain Natriuretic Peptide Measurements in Fetuses With Heart Failure. Front Physiol. 2021;12:654356. https://doi.org/10.3389/fphys.2021.654356
9. Abdelalim EM, Takada T, Torii R, Tooyama I. Molecular cloning of BNP from heart and its immunohistochemical localization in the hypothalamus of monkey. Peptides. 2006;27(7):1886-1893. https://doi.org/10.1016/j.peptides.2006.01.001
10. Gallo G, Bianchi F, Cotugno M, Volpe M, Rubattu S. Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension. J Clin Med. 2020;9(7):2265. https://doi.org/10.3390/jcm9072265
11. Zonneveld HI, Ikram MA, Hofman A, Niessen WJ, van der Lugt A, et al. N-Terminal Pro-B-Type Natriuretic Peptide and Subclinical Brain Damage in the General Population. Radiology. 2017;283(1):205-214. https://doi.org/10.1148/radiol.2016160548
12. Savchenko O.A., Pavlinova E.B. Long-term neurological outcomes in premature children with excitotoxic brain damage. S.S. Korsakov Journal of Neurology and Psychiatry. 2022;122(9-2):37-41. (In Russ.) https://doi.org/10.17116/jnevro202212209237
About the Authors
E. B. PavlinovaElena B. Pavlinova, Dr. Sci. (Med.), Associate Professor, Head of the Department of Hospital Pediatrics with Postgraduate Course, Vice-Rector for Academic Affairs
Omsk
O. A. Savchenko
Russian Federation
Olga A. Savchenko, Cand. Sci. (Med.), Associate Professor, Hospital Pediatrics with Postgraduate Course
Omsk
Review
For citations:
Pavlinova E.B., Savchenko O.A. Predictors of unfavorable neurological outcomes in premature children: a modern view on the problem. Medical Herald of the South of Russia. 2024;15(3):77-82. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-3-77-82







































