Scroll to:
Features of the formation of hybrid humoral immunity to SARS-CoV-2
https://doi.org/10.21886/2219-8075-2025-16-2-117-122
Abstract
Objective: to assess the dynamics of changes in the levels of specific antibodies in individuals with a history of COVID-19 and those vaccinated with synthetic peptides of SARS-CoV-2. Materials and methods: 40 people who had COVID-19 and immunized with synthetic SARS-CoV-2 peptides were examined. Methods: ELISA diagnostics of specific antibodies to the S and N protein of SARS-CoV-2 and statistical. Results: with COVID-19 in the post-infectious period, the production of S-protein-specific IgG is observed throughout the year from the moment of recovery. Vaccination of recovered individuals with peptide antigens leads to increased synthesis of IgG not only to the S-protein, but also to the N protein of the coronavirus, with a stable tendency towards an increase in antibody content over 3 months of observation. Conclusions: humoral post-infectious immunity is characterized by the predominant production of IgG to the S-protein of SARS-CoV-2, which persist for a year from the moment of recovery, while hybrid immunity, along with the production of antibodies to the S-protein, promotes the predominant synthesis of Ig G to the N protein SARA -CoV-2.
For citations:
Sizyakina L.P., Zakurskaya V.Ya. Features of the formation of hybrid humoral immunity to SARS-CoV-2. Medical Herald of the South of Russia. 2025;16(2):117-122. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-2-117-122
Introduction
Over three years of the pandemic, the new coronavirus infection has demonstrated the full extent of the threat to humankind, which pathogens such as SARS-CoV-2 can carry. According to official data, the pandemic took over 6.8 million lives, which makes it one of the deadliest in history1. In total, about 676 million cases of SARS-CoV-2 infection (8.4% of the world’s population) were detected during this period; however, the true prevalence of the disease was probably greater, since not all cases have been officially registered and confirmed2. Despite the experience gained over the past period and the knowledge gained about the pathogenesis, diagnosis, and methods of therapy of this disease, COVID-19 remains relevant for the health care system of the Russian Federation and other countries2. To date, the leading mechanism for protecting the population from SARS-CoV-2 is herd immunity, determined by the presence of 50–85% of the population immunized either by natural infection with COVID-19 or by vaccination [1].
For a long time, post-infectious immunity to SARS-CoV-2 was a controversial issue, especially its duration and effectiveness. Many works were devoted to the issue of antibody retention [2–4]. The seropositivity of those who have recovered from COVID-19 is mentioned most often within 6–12 months after recovery. However, the issue of current dates of vaccination for healthy people and revaccination for recovered people still remains open. To date, 10 vaccines of various types are used in the Russian Federation3. These are viral vector vaccines, peptide vaccines, recombinant vaccines, and whole virion vaccines. Certainly, different approaches in vaccine production technology lead to differences in the development of post-vaccination immunity. Differences are not only in possible adverse events [5], their frequency and severity [6][7], but also in the effectiveness of the formed antiviral response. Thus, there is a need for a deeper study of the effectiveness and safety of various types of vaccines in order to develop further prophylactic measures among the population.
Vaccination of persons who have already recovered from COVID-19 remains another important issue. The term “hybrid immunity” is increasingly common in the literature and implies a combination of post-infectious and post-vaccination immune responses [8][9]. There are disputes about the advisability of such vaccination and its timing. The main criterion for immune intensity today is the development of specific antibodies to the pathogen and their preservation at a certain “protective” level.
The purpose of the study was to assess the dynamics of changes in the content of specific antibodies in persons with a history of COVID-19 and vaccinated with synthetic SARS-CoV-2 peptides.
Materials and methods
The study included 40 people who had a history of COVID-19 infection and then were vaccinated with synthetic peptides SARS-CoV-2. The average age of the examined persons was 42 ± 10.4 years. According to gender, patients were distributed as follows: men – 13; women – 27. The clinical study was open-label, prospective and conducted in accordance with the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research with Human Participation” with amendments of 2000, WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects (2013), “Rules of Good Clinical Practice in the Russian Federation” approved by Order of the Ministry of Health of Russia dated April 1, 2016 No 200n. Each patient signed a voluntary informed consent to participate in the study. After recovering from a COVID-19 infection, the content of specific antibodies to SARS-CoV-2 was assessed at the 1st, 3rd, 6th, and 12th months from the moment of recovery. Subsequently, in the case of a decrease in the level of specific antibodies below the threshold value (PR < 4), all patients underwent two-stage immunization with synthetic peptides SARS-CoV-2, developed by the State Scientific Center of Virology and Biotechnology “Vector” of Rospotrebnadzor. The interval between two administrations (V1 and V2) was 21 days. The dynamics of the formation of hybrid immunity were monitored at the following control points: one day before vaccination, 21 days after the second dose, and 3 months after vaccination. Specific IgGs to S-protein (Spike-protein) SARS-CoV-2 in blood serum were determined semi-quantitatively by the method of a solid-phase enzyme immunoassay using Vector-Best test systems. The results were calculated in the positivity rate (PR) using the formula: PR = Asamp./Acrit. The result was considered positive at PR of the sample ≥ 1.2; negative – at PR < 0.8; borderline – at PR < 1.2. Additionally, in patients, antibodies of the IgG class to the N-protein SARS-CoV-2 were determined quantitatively by an enzyme immunoassay in serum using the test system developed and produced by the Pasteur Research Institute of Epidemiology and Microbiology. Antibody concentration was expressed in standard units per mL of buffer solution (cu/mL) and evaluated as follows: samples with a calculated concentration below 100 cu/mL (limit of quantification) were evaluated as negative, and a concentration above 100 cu/mL was evaluated as positive. Herewith, concentration above 3000 CU/mL was evaluated as a very high level of specific IgG, concentration in the range of 3000–1501 CU/mL was evaluated as a high level, concentration in the range of 1500–751 CU/mL – as an average level, concentration in the range of 750–187 CU/mL – a low level, and concentration in the range of 186–100 CU/mL – as a very low level.
Statistical data processing was performed using STATISTICA 10 software (StatSoft Inc., USA). Descriptive statistics of quantitative features were presented as a central trend of median and interquartile range (25th and 75th percentiles), presented in the text as Me [LQ; UQ]. A pairwise comparison of medians in groups was carried out using the Mann-Whitney test. A comparison of the overall dynamics of changes within the group was calculated using the Friedman U-test. Differences were considered statistically significant at the p<0.05 level.
Results
When assessing the intensity of post-infectious immunity, a dynamic decrease in the IgG content to the SARS-CoV-2 S protein was noted during the year of observations (Table 1). However, seropositivity was reliably maintained in most subjects by the 12th month. When assessing the dynamics of antibodies to the nuclear protein, no statistically significant changes were detected. Levels of IgG to N-protein were below the threshold value at most study checkpoints and showed no clear trend in those who had recovered. Even a month after recovery, the IgG to N-protein did not exceed the limit of quantitation.
Immunization of the people who had previously recovered from COVID was the second stage of the study. The initial content of specific IgG to nuclear and spike antigens was determined before immunization (Table 2). Twenty-one days after vaccination, their content increased significantly; the trend continued throughout 3 months of observations after vaccination. The increase in the titer of antibodies to S-protein was 2.6 times by the third month, while IgG to N-protein increased by 9.7 times over the same period.
It was interesting to compare the results obtained in the two groups at the same control points (1 and 3 months). Pairwise comparison demonstrated that during immunization with peptide antigens, the content of IgG to the SARS-CoV-2 S protein was significantly lower than in the group of recovered persons; however, their dynamic trend was multidirectional (Fig. 1). In the post-vaccination period during the formation of hybrid immunity, an increase was noted, while in those who have recovered, there was a decrease in the concentration.
A similar analysis of IgG to N-protein content showed the opposite result; specifically, a significant increase in the concentration of antibodies was noted in a group of persons with hybrid immunity in contrast to the negative results in the group of people who had recovered (Fig. 2). At the same time, a tendency toward an increase in the content of IgG to the nuclear protein was also observed in the first group.
Таблица 1 / Table 1
Динамическая характеристика специфических антител к различным антигенам SARS-CoV-2 у лиц, переболевших СOVID-19
Dynamic characteristics of specific antibodies to various SARS-CoV-2 antigens in individuals who have recovered from COVID-19
|
Показатель Index |
1 месяц 1 month |
3 месяца 3 months |
6 месяцев 6 months |
12 месяцев 12 months |
Значение Р P value |
|
IgG к S-белку, КП IgG to S-protein, PR |
16,69 [ 10,71; 17,46] |
15,8 [ 5,67; 17] |
8,53 [ 2,36; 15,05] |
3,7 [ 1,04; 8,4] |
*p˂0,01 |
|
IgG к N-белку, у.е./мл IgG to N-protein, u.u./ml |
84,9 [ 32,8; 184] |
65,2 [ 27,5; 178,6] |
137,6 [ 64,4;351,45] |
47,5 [ 35,87;82,27] |
p>0,05 |
Примечание: * статистическая значимость различий (p˂0,05) рассчитана с помощью U-критерия Фридмана.
Note: * statistical significance of differences (p˂0.05) was calculated using the Friedman U test.
Таблица / Table 2
Динамическая характеристика специфических антител к различным антигенам SARS-CoV-2 у лиц, переболевших СOVID-19 и вакцинированных пептидной вакциной
Dynamic characteristics of specific antibodies to various SARS-CoV-2 antigens in individuals who have recovered from COVID-19 and were vaccinated with a peptide vaccine
|
Показатель Index |
До вакцинации Before vaccination |
Через 21 день после вакцинации 21 days after vaccination |
Через 3 месяца после вакцинации 3 months after vaccination |
Значение Р P value |
|
IgG к S-белку, КП IgG to S-protein, PR |
1,98 [ 0,99; 2,69] |
3,76 [ 1,09; 5,2] |
5,24 [ 2,91; 8,32] |
*p˂0,01 |
|
IgG к N-белку, у.е./мл IgG to N-protein, u.u./ml |
51,25 [ 25,89; 108,6] |
396,15 [ 191,6; 662,7] |
496,85 [ 181,2; 1088] |
*p˂0,01 |
Примечание: * статистическая значимость различий (p˂0,05) рассчитана с помощью U-критерия Фридмана.
Note: * statistical significance of differences (p˂0.05) was calculated using the Friedman U test.
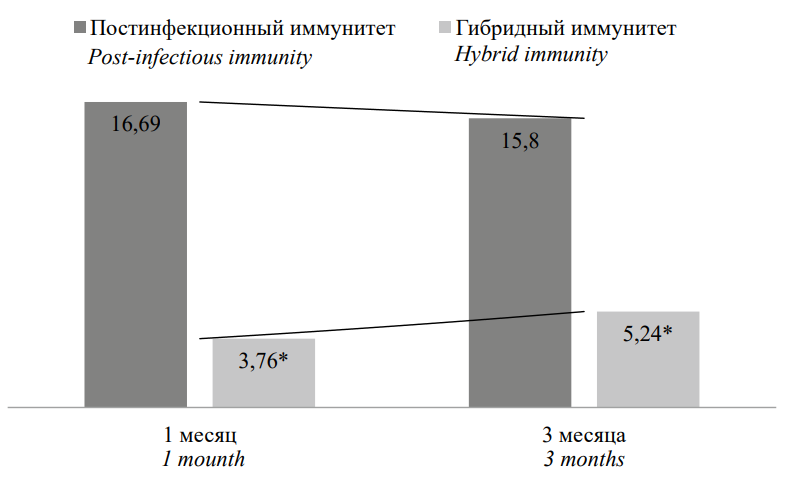
Рисунок 1. Сравнительная характеристика содержания IgG к S-белку, КП
Примечание: * статистическая значимость различий (p˂0,05) рассчитана с помощью критерия Mann H.B., Whitney D.R.
Figure 1. Comparative characteristics of IgG content to S-protein, PR
Note: * statistical significance of differences (p˂0.05) was calculated using the Mann H.B., Whitney D.R test.
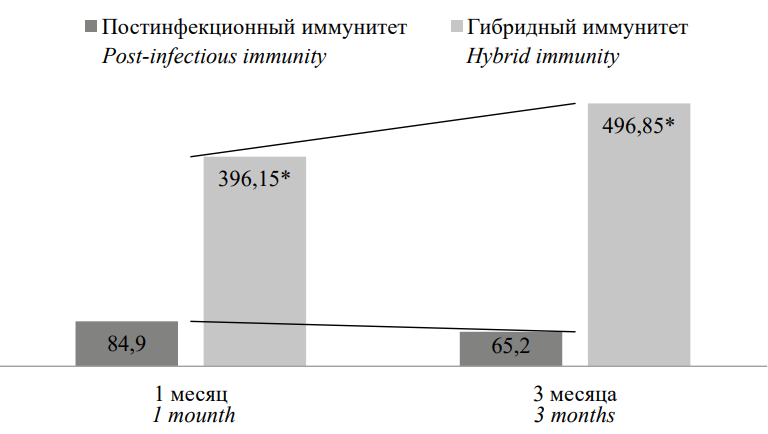
Рисунок 2. Сравнительная характеристика содержания IgG к N-белку, у.е./мл
Примечание: * статистическая значимость различий (p˂0,05) рассчитана с помощью критерия Mann H.B., Whitney D.R.
Figure 2. Comparative characteristics of IgG content to N-protein, u.u./ml
Note: * statistical significance of differences (p˂0.05) was calculated using the Mann H.B., Whitney D.R test.
Discussion
Despite the continuing contradictions regarding the role of the humoral immune response in COVID-19, especially in conditions of the high variability of the virus [10], the content of specific antibodies is still actively used in real clinical practice to solve a number of problems. First of all, this is used for retrospective detection of past infection. There are other, no less important goals: to evaluate the intensity of anti-infectious immunity and to make a decision on the need for vaccination [11]. The results demonstrated that by the 12th month from the date of recovery from COVID-19, specific antibody response was maintained; however, the concentration of IgG was low, and vaccination was required. This is consistent with a number of previous studies and refutes the need for specific prevention of the new coronavirus infection earlier than a year after recovery [12][13]. An important observation was the absence or low concentration of specific IgG to the nuclear protein of coronavirus. It is known that N-protein preferentially induces T-cell specific immune response, and stimulation of humoral defense mechanisms is not a leading one for this protein [14]. Therefore, in this case, the lack of antibodies to this protein does not allow making an unambiguous conclusion about the intensity of the cellular immune response and requires further research. Observation for the formation of hybrid immunity during vaccination with a peptide vaccine was an important stage of the study. The results demonstrated a less pronounced humoral response to the spike protein and, at the same time, a pronounced antibody response to the nuclear protein. Such differences can be explained by the presence of peptide SARS-CoV-2 N-antigens in a form accessible to immune cells. At the same time, the vaccine contains peptides with the most conservative regions of the S-protein, but not with its full structure, which influences the magnitude of the demonstrated humoral response. Similar features of the peptide vaccine probably contribute to a better T-cell-specific immune response to SARS-CoV-2. It is known that long-term immunological memory for other known coronavirus infections (MERS, SARS-CoV) was determined precisely by specific T-lymphocytes, which were detected in the blood of those who had recovered even 10 years after recovery [18][19]. In addition, the trend of antibody synthesis was opposite to the post-infection period. By the 3rd month of observations, a steady increase in the content of specific IgG was detected in persons with “hybrid immunity”, while a slight downward trend was already detected in those who had recovered.
Conclusion
Post-infectious humoral immunity to SARS-CoV-2 persists for 12 months from the date of recovery and is characterized by the predominant synthesis of specific antibodies to the S-protein of the virus. When vaccinated with SARS-CoV-2 peptide antigens of persons who have previously had COVID-19, hybrid immunity is formed mainly due to the increased synthesis of specific IgG to the coronavirus N-protein, which could indirectly indicate the accumulation of a higher number of specific to SARS-CoV-2 T-lymphocytes, providing long-term protection against the virus.
1. Johns Hopkins University Information Center. https://coronavirus.jhu.edu/map.html
2. WHO epidemiological data on COVID-19. https://www.who.int/ru/15-09-2023
3. https://вакцина.стопкоронавирус.рф
References
1. Mohamed K, Rzymski P, Islam MS, Makuku R, Mushtaq A,et al. COVID-19 vaccinations: The unknowns, challenges, and hopes. J Med Virol. 2022;94(4):1336-1349. https://doi.org/10.1002/jmv.27487
2. Yunusova M.A., Lutsenko E.S., Tsapkova N.N., Brazhnikov A.Yu., Saltykova T.S., Yudina V.S. The Level of IgG to Coronavirus Infection among the Medical Institution Employees. Epidemiology and Vaccinal Prevention. 2022;21(5):14-20. (In Russ.) https://doi.org/10.31631/2073-3046-2022-21-5-14-20
3. Novikova E.A., Petrova A.G., Moskaleva E.V., Vanyarkinа A.S., Rychkova L.V. Retrospective of International Serological Studies on the Formation and Dynamics of the Humoral Immune Response to SARS-CoV-2: from 2020 to 2021. Acta Biomedica Scientifica. 2021;6(2):47-57. (In Russ.) https://doi.org/10.29413/ABS.2021-6.2.5
4. Zhao J, Yuan Q, Wang H, Liu W, Liao X, et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027-2034. https://doi.org/10.1093/cid/ciaa344
5. Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147(11):867-876. https://doi.org/10.1161/CIRCULATIONAHA.122.061025
6. Zaçe D, La Gatta E, Petrella L, Di Pietro ML. The impact of COVID-19 vaccines on fertility-A systematic review and meta-analysis. Vaccine. 2022;40(42):6023-6034. https://doi.org/10.1016/j.vaccine.2022.09.019
7. Lai CC, Chen IT, Chao CM, Lee PI, Ko WC, Hsueh PR. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20(8):1013-1025. https://doi.org/10.1080/14760584.2021.1949293 .
8. Sizyakina L.P., Andrreeva I.I., Kharitonova M.V., Zaitseva N.S., Lyubimov D.S., et al. Mechanisms of formation of hybrid immunity in people who recovered from COVID-19 and were vaccinated with SARS-CoV-2 peptide antigens. Medical Immunology (Russia). 2022;24(3):629-640. (In Russ.) https://doi.org/10.15789/1563-0625-MOF-2490
9. Sizyakina L.P., Andrreeva I.I., Kharitonova M.V., Zaitseva N.S., Lyubimov D.S., et al. Post-vaccination immunity phenotypes upon usage of EpiVacCorona vaccine in the persons who suffered COVID-19. Medical Immunology (Russia). 2022;24(2):367-378. (In Russ.) https://doi.org/10.15789/1563-0625-PVI-2457
10. Yaugel-Novoa M, Bourlet T, Paul S. Role of the humoral immune response during COVID-19: guilty or not guilty? Mucosal Immunol. 2022;15(6):1170-1180. https://doi.org/10.1038/s41385-022-00569-w
11. Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185(14):2434-2451.e17. https://doi.org/10.1016/j.cell.2022.05.022
12. Semenova E.V., Pavliuk V.V., Uvarova M.A., Ivanov A.V. Features of humoral immunity after COVID-19. Medical Immunology (Russia). 2022;24(2):337-350. (In Russ.) https://doi.org/10.15789/1563-0625-FOH-2452
13. Toptygina A.P., Semikina E.L., Zakirov R.S., Afridonova Z.E. Comparison of the humoral and cellular immunity in COVID-19 convalescents. Russian Journal of Infection and Immunity. 2022;12(3):495-504. https://doi.org/10.15789/2220-7619-COT-1809
14. Bai Z, Cao Y, Liu W, Li J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses. 2021;13(6):1115. https://doi.org/10.3390/v13061115
About the Authors
L. P. SizyakinaRussian Federation
Lyudmila P. Sizyakina, Dr. Sci. (Med.), Professor, head of the Department of Clinical Immunology and Allergology
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
V. Ya. Zakurskaya
Russian Federation
Vita Ya. Zakurskaya, Assistant of the Department of Clinical Immunology and Allergology
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Sizyakina L.P., Zakurskaya V.Ya. Features of the formation of hybrid humoral immunity to SARS-CoV-2. Medical Herald of the South of Russia. 2025;16(2):117-122. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-2-117-122







































