Scroll to:
Immunotherapy in patients with recurrent implantation failure
https://doi.org/10.21886/2219-8075-2024-15-4-79-89
Abstract
Objective: to evaluate the effect of personalized immunotherapy on the frequency of pregnancy and gestation in women with recurrent implantation failure.
Materials and methods: the clinical immunogram before and aſter courses of personalized immunotherapy in 65 women with a history of recurrent implantation failure during the period of preparation for treatment under the ART program (24 cryoprotocols in the natural cycle, 41 cryoprotocols with hormone replacement therapy without pituitary blockade): CD3+, CD3+CD4+, CD3+CD8+, CD3+CD20+, CD16+ in direct reaction of surface immunofluorescence using monoclonal antibodies; concentration of serum Ig G, A, M by ELISA; total circulating immune complexes; Nitroblue tetrazolium (NBT) dye reduction test; phagocytic activity of neutrophils.
Results: the immune status of 100% of patients with secondary infertility and 36.4% of women with primary infertility is characterized by secondary T-immunodeficiency and significant activation of both B-lymphocytes and cellular factors of nonspecific body protection — peripheral NK cells (CD16+) and neutrophils. Repeated courses of personalized immunotherapy in women with a history of recurrent implantation failure led to normalization of altered immune parameters and showed their effectiveness in 67.7% of patients who gave birth to live children.
Conclusion: one of the leading reasons for habitual ineffective implantation in the presence of euploid embryos of good quality is general and local (endometrial) immunological imbalance. A personalized approach to the selection of immunotherapy for each patient optimizes the frequency of implantation, the onset and outcome of pregnancy.
Keywords
For citations:
Trunova O.A., Gulmamedova I.D., Maylyan E.A. Immunotherapy in patients with recurrent implantation failure. Medical Herald of the South of Russia. 2024;15(4):79-89. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-4-79-89
Introduction
The main limiting factor in the efficiency of assisted reproductive technologies (ART) occurs at the implantation stage, since 70% of transferred embryos do not implant [1].
The term recurrent implantation failure (RIF) is used to identify women with an abnormally low chance of pregnancy per embryo, to provide prognostic data, and to guide treatment to optimize implantation in subsequent transfers.
Currently, there is heterogeneity in the criteria for RIF. According to an international survey of 735 physicians [2], 84% of clinicians defined RIF based on the number of embryos transferred, with the largest group (45%) defining RIF as the failure to transfer three fresh or frozen embryos. Interestingly, factors such as the location of the clinic (European or non-European) and the type of ownership (private or public) were correlated with the definition used.
The Preimplantation Genetic Testing (PGT) Consortium of the European Society of Human Reproduction and Embryology (ESHRE) defined RIF as three or more failed high-quality embryo transfers or ≥10 failed embryo transfers in multiple transfers [3]. The American Society for Reproductive Medicine (ASRM) has not published criteria for RIF. The problem with defining RIF is that there are many factors that influence implantation success: the age of oocytes and uterus, the duration of infertility, the genetic status of embryos (diagnostic method), systemic diseases, lifestyle (obesity or smoking), structural uterine abnormalities, chronic endometritis, and endometriosis.
The role of immunological factors in RIF is the subject of much debate. Early reproductive failure is the most common complication of pregnancy, with only one-third of conceptions resulting in live birth [4].
Recurrent reproductive failure is associated with the expansion of circulating NK cells, key cells for maternal tolerance, decidual angiogenesis, and embryonic growth [5]. Uterine NK cells (uNKs) are the most abundant lymphocytes found in the decidua during implantation and the first trimester of pregnancy. They are important for early placental development, particularly trophoblast invasion and transformation of spiral arteries. However, inadequate uNK function is associated with reproductive failure such as recurrent miscarriage (RM) and RIF. Significantly elevated uNK levels in the endometrium of women with RM and RIF may indicate an underlying disruption of the immune environment culminating in implantation and/or placentation failure [6].
There is a large and evolving literature on the Th1/Th2 balance during pregnancy. Although a certain local proinflammatory environment is necessary for embryo implantation and angiogenesis [7], the dominance of proinflammatory Th1 cytokines is considered to be detrimental to the onset and course of pregnancy, while anti-inflammatory Th2 cytokines regulate and improve the Th1 response [8]. It has been shown that patients with RIF have an imbalance of pro- and anti-inflammatory cytokines in the peripheral blood: increased levels of proinflammatory cytokines IFN-γ, IL-1β, and IL-6; decreased levels of the anti-inflammatory cytokine TGF-β1 and an increased ratio of proinflammatory/anti-inflammatory cytokines [9].
Over the past 20 years, various immunotherapy methods have been actively used to treat miscarriage [10] (granulocyte colony-stimulating factor [11], TNF-alpha inhibitors, leukemia inhibitor [12][13], glucocorticoids [14], intralipid [15][16][17][18], immunoglobulins [19], immunocytotherapy [20][21][22][23][24][25]).
Randomized controlled clinical trials in this area lack a personalized approach.
The study was aimed at evaluating the impact of personalized immunotherapy on the frequency of onset and maintenance of pregnancy in women with RIF.
Materials and methods
At the stage of preparation for treatment under the ART program (24 cryoprotocols in a natural cycle, 41 cryoprotocols with hormone replacement therapy without pituitary blockade), a randomized controlled prospective immunological study of 65 patients aged 25–40 years with RIF (main group) was conducted. Considering the emotional factor of inefficient ART cycles, financial costs that are difficult to bear for residents of the region, and the long duration of infertility, this study included married couples with two (not three) or more unsuccessful transfers of high-quality embryos according to the classification by Gardner (1999)1. In eight patients, the euploidy of the embryos was confirmed by preimplantation genetic diagnostics methods. Indications for ART were as follows: tubal-peritoneal factor of infertility – in 12 (18.46%) patients, endometriosis of stages 2–3– in 21 (32.3%) women, and male factor of infertility – in 32 (49.23%) cases. Women with organic pathology of the endometrium and anomalies of uterine development were excluded from the study.
The control group consisted of 20 practically healthy fertile women aged 25–40 years.
This clinical study was performed in accordance with the "Rules of Good Clinical Practice in the Russian Federation" and the Helsinki Declaration of the World Medical Association and approved by the Ethics Committee of the State Educational Institution of Higher Professional Education "Donetsk National Medical University named after M. Gorky" (protocol No. 27/5-1 dated April 14, 2021).
The immunological study included an analysis of individual components of the immune system (T and B lymphocytes (LF)), as well as the functional state of neutrophils. The absolute numbers of leukocytes and lymphocytes were determined in the peripheral blood; immunophenotyping of lymphocytes was performed indicating the absolute and relative number of CD3+ (T lymphocytes), CD3+CD4+ (T helpers), CD3+CD8+ (cytotoxic T cells/suppressors), CD3+CD20+ (B lymphocytes), CD16+ (NK cells), as well as the immunoregulatory index (T helpers/T suppressors) (CD3+4+/CD3+8+) in a direct surface immunofluorescence reaction using monoclonal antibodies (mAb) (Beckman Coulter, France) followed by microscopy with a microscope (Granum R50) with a fluorescent attachment (Granum F2). The secretory activity of B lymphocytes was judged by the concentration of serum immunoglobulins (Ig) of classes G, A, and M using the ELISA method (Granum, Ukraine). In order to determine the severity and activity of the immunopathological process, the level of total circulating immune complexes (CIC) was measured in the blood serum using the nephelometric method. The method is based on nephelometry of different solubility of Ig monomers in the immune complexes in the presence of polyethyleneglycol (PEG-6000) in the medium. The intensity of oxygen-dependent metabolism of neutrophils was studied in the nitroblue tetrazolium (NBT) reduction reaction in the spontaneous and induced NBT test with a suspension of Serratia marcescens (2·10⁹/ml). When assessing the phagocytic capacity of neutrophils, the percentage of cells that showed phagocytic activity (PhA) and the average number of latex particles absorbed by one neutrophil (PhP) were determined.
The study was performed in phase II of the menstrual cycle (days 19–22).
At the stage of planning the cryoprotocol program, the patients' systemic immunity parameters were examined and, depending on the detected deviations in the immune status, one, two, or three individual courses of immunotherapy were prescribed, taking into account the achievement of positive dynamics or in the case of inefficient subsequent implantation. The interval between courses of immunotherapy was 6–12 months. One course of individual immunotherapy was received by 37 women, two courses were required by 19 patients, and three courses were provided to another nine patients.
Depending on the identified deviations in the immunogram, the following immunomodulators were prescribed: aminodihydrophthalazinedione sodium, arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine diacetate, glucosaminylmuramyl dipeptide (GMDP, a synthetic analog of the structural fragment of the membrane (peptidoglycan) of bacterial cells), azoximer bromide, arginyl-alpha-ribonucleic acid, dioxomethyltetrahydropyrimidine, as well as vitamin complexes containing zinc, selenium, and copper.
One of the causes of dysfunction of the immune system of the examined women was chronic stress caused by living in conditions of military action. Fear of a repeated ineffective attempt was also a stress irritant. All patients in the main group were prescribed a sedative herbal collection.
Statistical data processing was performed using variation statistics methods in the Excel 2016 program. The reliability of average group indicators was assessed using Student's t-test for unrelated (main group – control) and related (dynamics during treatment) samples.
Results
The duration of the infertility period in the main group ranged from 1 to 20 years and averaged 7 years. Primary infertility was observed in 26 (40%) women of the main group, secondary infertility — in 39 (60%). Only 10 (15.38%) of women with secondary infertility had a history of childbirth, in two cases (3.08%) the childbirth was pathological. In the group of women with RIF, attention was drawn to the high frequency of both artificial (18–27.69%) and spontaneous (14–21.54%) abortions, as well as ectopic pregnancies (16–24.6%).
Indications for ART were the tubal-peritoneal factor of infertility in 12 (18.46%) patients and endometriosis of stages 2–3 in 21 (32.3%) women.
In 32 (49.23%) couples, such male factor of infertility as pathozoospermia (oligo-, astheno-, teratozoospermia and their combinations) was observed, which could not be treated. The concentration of spermatozoa was less than ≤2 million, motility was less than ≤5%, morphologically normal spermatozoa were less than ≤5%. With this pathology, an embryo genetically related to the man can only be obtained by the method of intracytoplasmic sperm injection (ICSI). These couples had a history of two IVF/ICSI cycles with the receipt of morphologically and genetically healthy embryos, but there was no implantation, and pregnancy did not occur. This was explained by the fact that infertility in some couples was caused not by one, but by several reasons.
The uterine factor was observed in 54 (83.08%) women (endometrial hyperplasia, hypoplasia, chronic endometritis confirmed by immunohistochemistry, implantation window displacement), and endocrine disorders were detected in 41 (63.08%) women. Thyroid dysfunction was identified in 21 (32.31%) women with RIF. Somatic history was aggravated in 42 (64%) patients. Urogenital infections were noted in the history of 73% of women.
From one to four surgeries were performed on the pelvic organs in 41 (63.08%) women, endoscopic surgeries – in 37 (56.9%) patients. Surgeries on the ovaries were performed in 25 (38.46%) patients, and surgeries on the fallopian tubes – in 38 (58.46%) patients.
All patients had a history of two or more ineffective cycles of artificial insemination. Of these, 57 (87.7%) had a history of ineffective ART cycles, and 8 (12.3%) had biochemical pregnancies or spontaneous abortions.
The above anamnestic data may be the cause of chronic stress and imbalance of the immune system, and the high frequency of reproductive losses in the anamnesis also indicates possible initial deviations of the immune system. Before inclusion in the ART program, the identified gynecological and somatic pathology was treated.
In the main group, compared to the control group, there was a relative (p=0.026) and absolute (p=0.018) lymphocytosis, a decrease in the percentage of the T lymphocyte population (CD3+) (p=0.000001), the T helper subpopulation (CD3+ CD4+) (p=0.00034), the absolute number of cytotoxic T cells/suppressors (CD3+ CD8+) (p=0.05), as well as the value of the immunoregulatory index (p=0.010). Moreover, the relative (p=0.00004) and absolute (p=0.0001) number of B lymphocytes (CD20+), as well as NK cells (CD16+) (relative – p=0.000001, absolute – p=0.000001) significantly exceeded the control values (Table 1).
Таблица / Table 1
Показатели иммунограммы в основной группе женщин с ПНИ и в контрольной группе
Immunogram indicators in the main group of women with recurrent implantation failure and in the control group (X̄±Sx̄)
|
Показатели / Indicators |
Основная группа / Main group |
Контрольная группа / Control group |
р = |
|
Лейкоциты, Г/л / Leukocytes (gigalitre) |
6,01±0,24 |
5,88±0,21 |
0,684 |
|
Лимфоциты / Lymphocytes, % |
37,96±1,25 |
33,9±1,3 |
0,026 |
|
Лимфоциты, Г/л / Lymphocytes (gigalitre) |
2,24±0,1 |
1,96±0,06 |
0,018 |
|
Т-лимфоциты / T-lymphocytes (СD3+), % |
41,90±1,79 |
55,6±1,9 |
0,000001 |
|
Т-лимфоциты, Г/л / T-lymphocytes (СD3+) (gigalitre) |
0,93±0,059 |
1,09±0,08 |
0,113 |
|
Т-хелперы / T-helpers (СD3+СD4+), % |
24,16±1,27 |
35,3±2,7 |
0,00034 |
|
Т-хелперы, Г/л / T-helpers (СD3+СD4+) (gigalitre) |
0,53±0,04 |
0,65±0,05 |
0,050 |
|
Т- цитотоксические клетки/супрессоры / T-cytotoxic cells/suppressors (СD3+СD8+), % |
21,93±1,0 |
21,3±0,9 |
0,640 |
|
Т- цитотоксические клетки/супрессоры, Г/л / T-cytotoxic cells /suppressors (СD3+СD8+) (gigalitre) |
0,49±0,034 |
0,41±0,03 |
0,050 |
|
Т-хелперы/Т-супрессоры / T-helpers/T-suppressors |
1,26±0,082 |
1,64±0,12 |
0,010 |
|
В-лимфоциты (СD20+) / B-lymphocytes, % |
21,60±1,34 |
13,8±1,2 |
0,00004 |
|
В-лимфоциты, Г/л / B-lymphocytes (СD20+) (gigalitre) |
0,50±0,053 |
0,29±0,02 |
0,0001 |
|
NK-клетки / NK-cells (СD16+), % |
26,27±1,54 |
15,7±1,3 |
0,000001 |
|
NK-клетки, Г/л / NK-cells (СD16+) (gigalitre) |
0,59±0,047 |
0,27±0,02 |
0,000001 |
|
Фагоцитарная активность нейтрофилов / Phagocytic activity of neutrophils, % |
49,76±1,85 |
54,3±3,6 |
0,265 |
|
Фагоцитарное число / Phagocytic number |
3,07±0,17 |
2,53±0,19 |
0,037 |
|
Спонтанный НСТ-тест / Spontaneous Nitroblue tetrazolium (NBT) dye reduction test, % |
27,25±1,15 |
15,0±0,8 |
0,000001 |
|
Спонтанный ИАН / Spontaneous neutrophil activation index |
0,33±0,019 |
0,25±0,02 |
0,005 |
|
Индуцированный НСТ-тест / Induced Nitroblue tetrazolium (NBT) dye reduction test, % |
34,46±1,35 |
38,6±1,57 |
0,049 |
|
Индуцированный ИАН / Induced neutrophil activation index |
0,42±0,018 |
0,48±0,09 |
0,516 |
|
Циркулирующие иммунные комплексы, усл. ед. / Circulating immune complexes (standard unit) |
20,21±1,93 |
11,5±2,11 |
0,003 |
|
Иммуноглобулин А, г/л / Immunoglobulin A (g/l) |
2,13±0,104 |
1,9±0,08 |
0,076 |
|
Иммуноглобулин М, г/л / Immunoglobulin M (g/l) |
2,06±0,12 |
1,15±0,06 |
0,000001 |
|
Иммуноглобулин G, г/л / Immunoglobulin G (g/l) |
12,05±0,49 |
11,5±0,5 |
0,434 |
The hyperimmunoglobulinemia M (p=0.000001) was also manifested. At the same time, the concentrations of immunoglobulins A and G were within the physiological norm. The total number of circulating immune complexes was increased compared to the control (p=0.003).
Neutrophil phagocytic activity as an indicator of the cellular link of nonspecific reactivity of the body in women of the main group did not have a statistically significant difference with the control (p = 0.265). However, according to the data of the spontaneous NST test, a violation of homeostasis of the body of women with RIF was revealed (NST test spont. – 27.25 ± 1.15%; control – 15.0 ± 0.8%; p = 0.000001; IAN spont. – 0.33 ± 0.019; control – 0.25 ± 0.02; p = 0.005). The remaining indicators were within the physiological norm.
In 30% of patients, a combination of a decrease in the absolute number of CD3+ cells and an increase in the number of NK cells was observed.
Depending on the leading link in the disruption of immune homeostasis, the clinical immunologist proposed the following algorithm for immunotherapy.
In the case of severe immunodeficiency of hypo-T-helper, hypo-T-suppressor type with a decrease in the relative and absolute quantity of T cells, a low value of the immunoregulatory index, arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine diacetate was prescribed at 1 ml of 0.005% solution intramuscularly once every 2 days (the course included 15 injections) or GMDP (10 mg once per day, the course lasted 10 days) and arginyl-alpha-ribonucleic acid at 250 mg 4 times per day (the course lasted 20 days).
In the case of depletion of reserves for oxygen-dependent metabolism of neutrophils, patients took aminodihydrophthalazinedione sodium in suppositories once every 3 days (the course lasted 15 days) and dioxomethyltetrahydropyrimidine 500 mg 4 times a day (the course lasted 20 days).
In the case of immune system imbalance, manifested by a decrease in T lymphocytes (CD3+) and their subpopulations with a simultaneous increase in B lymphocytes (CD20+), NK cells (CD16+), hyperimmunoglobulinemia A and M, as well as activation of phagocytic activity with depletion of the reserve capacity of neutrophils for a respiratory burst, azoximer bromide was prescribed at 12 mg once a day intramuscularly (a course of 15 injections) or aminodihydrophthalazinedione sodium in suppositories once a day every 2 days on the third day (a course of 15 days).
All patients took a complex of vitamins and microelements (including those containing zinc, selenium, and copper) for 2 months.
In most patients, after the first course, there was an improvement in their condition, accompanied by a significant increase in both the proportion (30.30±1.59%, before treatment – 24.16±1.27%, p=0.003) and the absolute number (0.70±0.052 g/l, before treatment – 0.53±0.04 g/l, p=0.008) of the subpopulation of T helpers (CD3+) (CD4+) reduced before treatment (Table 2, Fig. 1).
Таблица / Table 2
Показатели иммунограммы женщин основной группы до и после курсов иммунотропной терапии, (X̄±Sx̄)
Immunogram indicators of women in the main group before and after courses of immunotropic therapy, (X̄±Sx̄)
|
Показатели / Indicators |
До лечения / Before treatment |
После І курса лечения / After the first course of treatment |
р 0–I |
После ІІ курса лечения / After the second course of treatment |
р 0–II |
После ІІІ курса лечения / After the third course of treatment |
р 0–III |
|
Лейкоциты, Г/л / Leukocytes (gigalitre) |
6,01±0,24 |
5,95±0,22 |
0,850 |
5,88±0,29 |
0,730 |
5,68±0,606 |
0,620 |
|
Лимфоциты / Lymphocytes, % |
37,96±1,25 |
38,69±1,33 |
0,690 |
37,28±1,94 |
0,760 |
41,33±3,78 |
0,390 |
|
Лимфоциты, Г/л /Lymphocytes ((gigalitre) |
2,24±0,1 |
2,28±0,11 |
0,790 |
2,19±0,16 |
0,790 |
2,32±0,303 |
0,800 |
|
Т-лимфоциты / T-lymphocytes (СD3+), % |
41,90±1,79 |
45,93±2,13 |
0,150 |
52,86±3,51 |
0,007 |
56,67±5,81 |
0,017 |
|
Т-лимфоциты, Г/л / T-lymphocytes (gigalitre) (СD3+) |
0,93±0,059 |
1,08±0,079 |
0,136 |
1,16±0,12 |
0,089 |
1,27±0,181 |
0,077 |
|
Т-хелперы / T-helpers (СD4+), % |
24,16±1,27 |
30,30±1,59 |
0,003 |
33,21±2,63 |
0,002 |
36,33±4,12 |
0,006 |
|
Т-хелперы, Г/л / T-helpers (gigalitre) (СD4+) |
0,53±0,04 |
0,70±0,052 |
0,008 |
0,71±0,08 |
0,047 |
0,83±0,136 |
0,042 |
|
Т- цитотоксические клетки/супрессоры / T-cytotoxic cells/suppressors (СD8+), % |
21,93±1,0 |
23,03±0,98 |
0,433 |
24,90±1,76 |
0,145 |
24,00±2,69 |
0,473 |
|
Т-цитотоксические клетки/супрессоры, Г/л / T-cytotoxic cells /suppressors (СD8+) (gigalitre) |
0,49±0,034 |
0,53±0,036 |
0,425 |
0,54±0,058 |
0,457 |
0,58±0,117 |
0,469 |
|
Т-хелперы/Т-супрессоры / T-helpers/T-suppressors |
1,26±0,082 |
1,35±0,065 |
0,398 |
1,50±0,148 |
0,161 |
1,53±0,133 |
0,081 |
|
В-лимфоциты (СD20+) / B-lymphocytes, % |
21,60±1,34 |
22,22±1,19 |
0,729 |
21,62±1,6 |
0,992 |
24,89±3,03 |
0,323 |
|
В-лимфоциты, Г/л / B-lymphocytes (gigalitre) (СD20+) |
0,50±0,053 |
0,52±0,043 |
0,755 |
0,47±0,051 |
0,672 |
0,57±0,089 |
0,498 |
|
NK-клетки / NK-cells (СD16+), % |
26,27±1,54 |
25,13±1,33 |
0,576 |
28,34±2,61 |
0,496 |
27,44±3,63 |
0,767 |
|
NK-клетки, Г/л / NK-cells (gigalitre) (СD16+) |
0,59±0,047 |
0,59±0,05 |
1,000 |
0,62±0,082 |
0,984 |
0,65±0,11 |
0,969 |
|
Фагоцитарная активность нейтрофилов / Phagocytic activity of neutrophils, % |
49,76±1,85 |
50,85±2,07 |
0,696 |
50,28±2,87 |
0,879 |
48,50±3,25 |
0,737 |
|
Фагоцитарное число / Phagocytic number |
3,07±0,17 |
3,36±0,23 |
0,312 |
3,71±0,37 |
0,119 |
2,46±0,265 |
0,050 |
|
Спонтанный НСТ-тест / Spontaneous Nitroblue tetrazolium (NBT) dye reduction test, % |
27,25±1,15 |
27,15±1,17 |
1,000 |
31,55±2,29 |
0,050 |
34,50±4,34 |
0,110 |
|
Спонтанный ИАН / Spontaneous neutrophil activation index |
0,33±0,019 |
0,36±0,037 |
0,503 |
0,41±0,037 |
0,076 |
0,38±0,05 |
0,356 |
|
Индуцированный НСТ-тест / Induced Nitroblue tetrazolium (NBT) dye reduction test, % |
34,46±1,35 |
34,58±1,26 |
0,948 |
38,52±2,12 |
0,109 |
40,00±4,07 |
0,199 |
|
Индуцированный ИАН / Induced neutrophil activation index |
0,42±0,018 |
1,02±0,59 |
0,0001 |
0,48±0,029 |
0,099 |
0,45±0,056 |
0,636 |
|
Циркулирующие иммунные комплексы, усл. ед. / Circulating immune complexes (standard unit) |
20,21±1,93 |
23,24±2,34 |
0,319 |
24,17±3,95 |
0,370 |
15,00±3,29 |
0,176 |
|
Иммуноглобулин А, г/л / Immunoglobulin A (g/l) |
2,13±0,104 |
2,26±0,12 |
0,406 |
2,23±0,191 |
0,642 |
2,59±0,41 |
0,279 |
|
Иммуноглобулин М, г/л / Immunoglobulin M (g/l) |
2,06±0,12 |
2,16±0,12 |
0,556 |
2,28±0,196 |
0,348 |
2,09±0,27 |
0,919 |
|
Иммуноглобулин G, г/л / Immunoglobulin G (g/l) |
12,05±0,49 |
12,73±0,55 |
0,358 |
13,61±0,79 |
0,097 |
11,89±1,18 |
0,900 |
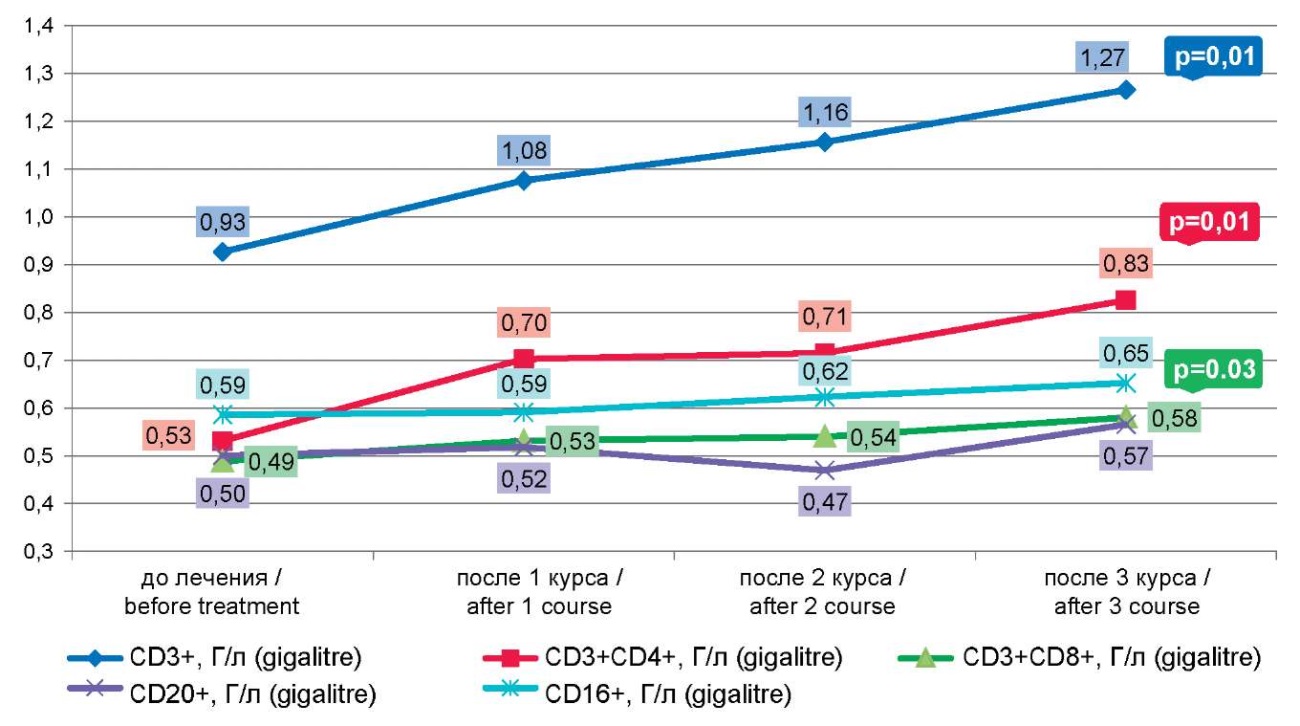
Рисунок 1. Динамика количества CD 3+, 4+, 8+, 16+, 20+ лимфоцитов после курсов иммунокоррекции
Figure 1. Dynamics of the number of CD 3+, 4+, 8+, 16+, 20+ lymphocytes after immunocorrection courses
Repeated courses led to even more pronounced stimulation of the T-link and a significant increase in the proportion of T lymphocytes (CD3+): after the second course – 52.86±3.51% (p=0.007), after the third course – 56.67±5.81% (p=0.017) (Table 2, Fig. 1).
The immunoregulatory index (CD3+4+/CD3+8+) tended to increase with each course of immunocorrection, apparently due to a statistically significant increase in the proportion and absolute number of T helpers (CD3+) (CD4+): I course p=0.003; II course p=0.002; III course p=0.007.
The number and functional activity of B lymphocytes (CD20+), as well as NK cells (CD16+), did not change statistically significantly.
There were no significant changes in the phagocytic activity of neutrophils after the first course of treatment; however, their ability to “respiratory burst”, according to the induced NBT test, statistically significantly increased after the first course of immunocorrection (1.02±0.59, before treatment – 0.42±0.018, p=0.0001).
Stimulation of the indices of phagocytic and metabolic activity of neutrophils, calculated per one cell, was also observed after the first course of immunotherapy (phagocytic number and induced neutrophil activation index) (Table 2, Fig. 3).
The second course of immunocorrection led to an increase in the spontaneous NBT test (31.55±2.29%, before treatment – 27.25±1.15%, p=0.050 (Table 2, Fig. 2). The third course, obviously, depleted the reserve capacity of neutrophils, since the phagocytic number statistically significantly decreased (Table 2, Fig. 3).
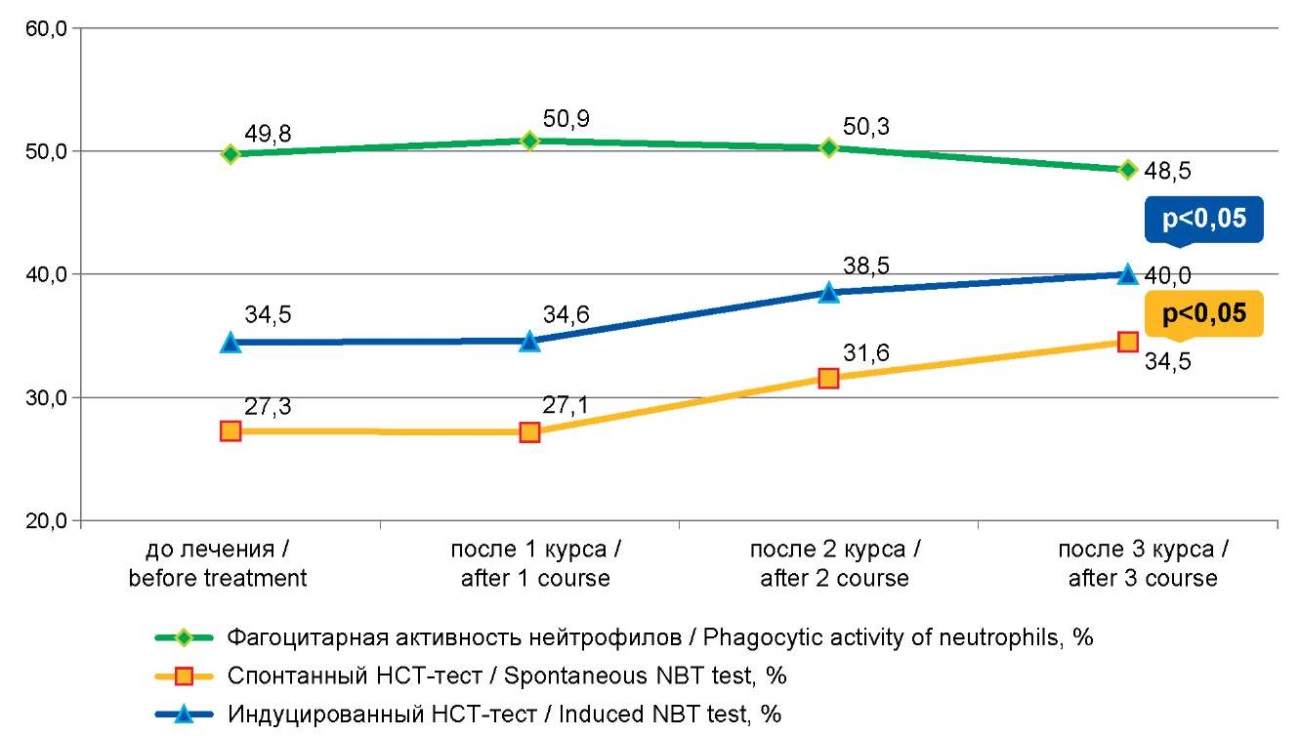
Рисунок 2. Динамика фагоцитарной и метаболической активности нейтрофилов после курсов иммунокоррекции
Figure2. Dynamics of phagocytic and metabolic activity of neutrophils after immunocorrection courses
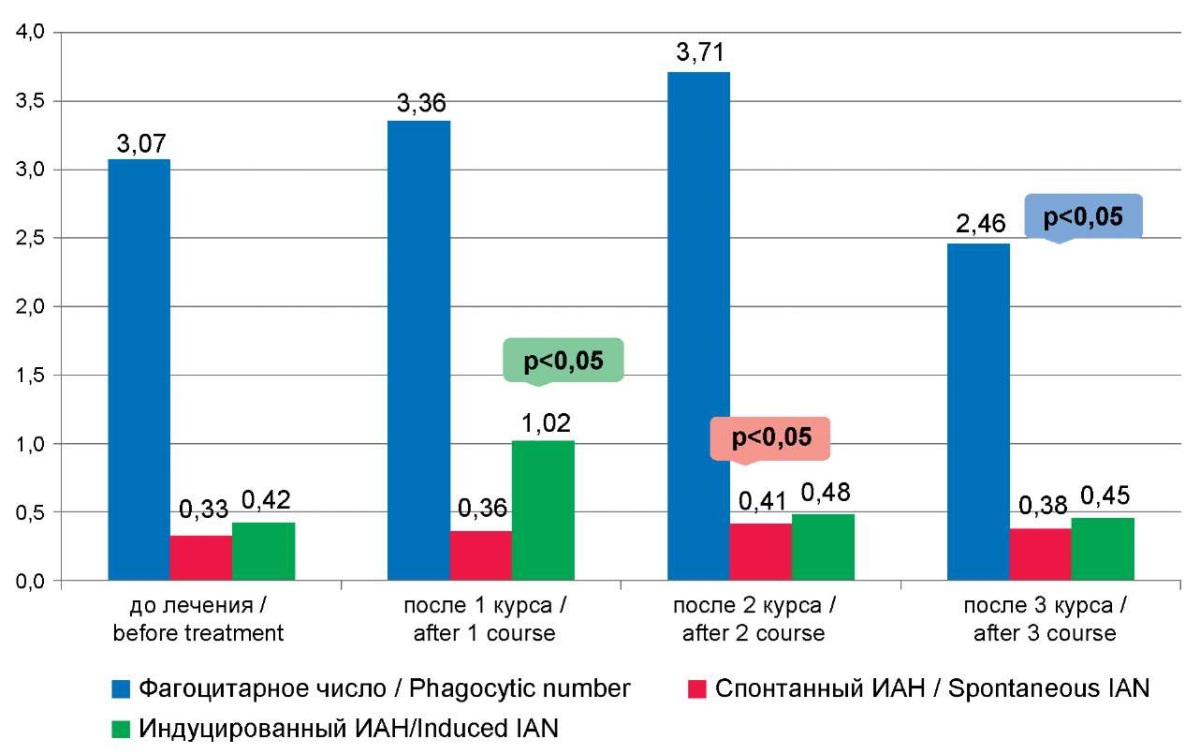
Рисунок 3. Показатели фагоцитарного числа, спонтанного и индуцированного индексов активации нейтрофилов после курсов иммунокоррекции
Figure 3. The indicators of phagocytic number, spontaneous and induced neutrophil activation indices after immunocorrection courses
Courses of individual immunocorrection using arginyl-alpha-ribonucleic acid resulted in reliable normalization of the percentage of B lymphocytes (p=0.02), concentration of serum IgM (p=0.01), and phagocytic activity of neutrophils (p=0.04). Azoximer bromide increased decreased T helpers (CD3+4+) (p=0.05), and arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine diacetate increased the number of decreased T lymphocytes (CD3+) (p=0.02) and reduced increased CIC to normal (p=0.05).
After the first course of individual immunocorrection followed by the IVF/ICSI procedure, labor occurred in 51.35% of cases (19 out of 37 women). Two courses resulted in successful delivery in 84.2% of patients (16 out of 19 women). Three courses allowed all 9 women (100%) to become mothers.
Discussion
The data of scientific medical research indicate that generally accepted methods of examination of married couples do not allow clarifying the cause of infertility in 20–30% of cases. This study was aimed at offering a practicing physician an accessible, efficient, and minimally invasive method of immunodiagnostics and immunocorrection in women with RIF, in whose anamnesis there were a large number of treatment and diagnostic interventions.
Despite the fact that the main events associated with implantation failure occur in the endometrium, indicators of systemic immunity of the peripheral blood to a certain extent correlate with indicators of local immunity, and also determine the ability of the woman's immune system to provide the necessary tolerance to the "allograft" (embryo).
Increased rates of spontaneous abortions and IVF failures are to some extent due to NK cell cytotoxicity. Detection of increased levels and activity of circulating NK cells is useful for identifying individuals at risk of failure to implant embryos and loss of a karyotypically normal pregnancy [26]. Zagaynova et al. (2022) found an inverse correlation between the number of pregnancies in the anamnesis and the absolute content of activated NK cells (rs=- 0.55; p<0.01) [27].
This study did not provide evidence of effective correction of NK cell (CD16+) counts. A similar conclusion was reached in a systematic review by Von Woon et al. (2020) assessing the efficiency of immunotherapy to improve pregnancy outcomes in women with recurrent miscarriage or implantation failure, specifically selected based on abnormal NK cell levels and/or activity. The authors concluded that immunotherapy might have some benefit, but further studies were needed using scientifically validated immunological biomarkers in well-designed, large-scale randomized controlled trials [28].
The results of this study are consistent with a meta-analysis from Medline, Embase, and the Cochrane Library by Mekinian et al. (2016) on the benefits of immunomodulatory drugs in recurrent miscarriages and implantation failures, which found a moderate benefit of progesterone for live birth with an odds ratio of 1.38. TNF-α antagonists in combination with low doses of aspirin, heparin, and intravenous immunoglobulins provided a live birth rate of 71%, and the administration of G-CSF resulted in a live birth in 82.8% [18]. Kolanska et al. (2021) report that the use of immunomodulatory therapy and steroids in patients with a history of pregnancy and unexplained recurrent miscarriages helped improve obstetric outcomes, as it doubled the chances of delivering a live baby [29].
Conclusion
The immune status of infertile women is characterized by secondary T-immunodeficiency and significant activation of both B lymphocytes and cellular factors of non-specific body defense (peripheral NK cells (CD16+) of the blood and phagocytes-neutrophils). Such changes in the immune profile occurred in 100% of patients with secondary infertility and in 36.4% of women with primary infertility. In cases where these deviations reach critical values, they negatively affect the prognosis for achieving pregnancy. Failures of ART in the presence of good-quality embryos are due to immunological imbalance. The male factor can be combined with deviations in the woman's immune system, which is one of the causes of RIF. This combination was observed in this study and was successfully corrected.
A personalized approach to the selection of immunomodulatory drugs for each patient optimizes the frequency of implantation, the onset and outcome of pregnancy.
Therefore, repeated courses of personalized immunomodulatory therapy in women with a history of RIF led to the normalization of altered immune parameters and demonstrated their efficiency in 67.7% of patients in the presence of good-quality embryos, increasing the chances of implantation and the possibility of carrying a pregnancy and childbirth.
1. 2021. Evaluation of oocytes and embryos in the ART laboratory: Guidelines. Russian Association of Human Reproduction Section "Clinical Embryology". 2021.
References
1. Cheloufi M, Kazhalawi A, Pinton A, Rahmati M, Chevrier L, et al. The Endometrial Immune Profiling May Positively Affect the Management of Recurrent Pregnancy Loss. Front Immunol. 2021;12:656701. https://doi.org/10.3389/fimmu.2021.656701
2. Cimadomo D, Craciunas L, Vermeulen N, Vomstein K, Toth B. Definition, diagnostic and therapeutic options in recurrent implantation failure: an international survey of clinicians and embryologists. Hum Reprod. 2021;36(2):305-317. https://doi.org/10.1093/humrep/deaa317
3. Ata B, Kalafat E, Somigliana E. A new definition of recurrent implantation failure on the basis of anticipated blastocyst aneuploidy rates across female age. Fertil Steril. 2021;116(5):1320-1327. https://doi.org/10.1016/j.fertnstert.2021.06.045
4. Craciunas L, Gallos I, Chu j, Bourne T, quenby S, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(2):202-223. https://doi.org/10.1093/humupd/dmy044
5. Ramos-Medina R, García-Segovia A, Gil j, Carbone j, Aguarón de la Cruz A, et al. Experience in IVIg therapy for selected women with recurrent reproductive failure and NK cell expansion. Am J Reprod Immunol. 2014;71(5):458-466. https://doi.org/10.1111/aji.12217
6. Von woon E, Greer O, Shah N, Nikolaou D, johnson M, Male V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: a systematic review and meta-analysis. Hum Reprod Update. 2022;28(4):548-582. https://doi.org/10.1093/humupd/dmac006
7. Mor G. Clinical aspect of reproductive immunology. Am J Reprod Immunol. 2011;66(6):451. https://doi.org/10.1111/j.1600-0897.2011.01091.x
8. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601-610. https://doi.org/10.1111/j.1600-0897.2010.00852.x
9. Liang PY, Diao LH, Huang CY, Lian RC, Chen x, et al. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod Biomed Online. 2015;31(6):823-826. https://doi.org/10.1016/j.rbmo.2015.08.009
10. Achilli C, Duran-Retamal M, Saab w, Serhal P, Seshadri S. The role of immunotherapy in in vitro fertilization and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2018;110(6):1089-1100. https://doi.org/10.1016/jfertnstert.2018.07.004
11. Laufer N, Simon A. Recurrent implantation failure: current update and clinical approach to an ongoing challenge. Fertil Steril. 2012;97:1019–1020. https://doi.org/10.1016/j.fertnstert.2012.03.033
12. Das M, Holzer HE. Recurrent implantation failure: gamete and embryo factors. Fertil Steril. 2012;97:1021–1027. https://doi.org/10.1016/j.fertnstert.2012.02.029
13. Penzias AS. Recurrent IVF failure: other factors. Fertil Steril. 2012;97:1033–1038. https://doi.org/10.1016/j.fertnstert.2012.03.017
14. Kwak-Kim j, Han AR, Gilman-Sachs A, Fishel S, Leong S, Shoham Z. Current trends of reproductive immunology practices in in vitro fertilization (IVF)—a first world survey using IVF-worldwide.com. Am J Reprod Immunol. 2013; 69:12–20. https://doi.org/10.1111/j.1600-0897.2012.01183.x
15. Polanski LT, Baumgarten MN, quenby S, Brosens j, Camp-bell BK, RaineFenning Nj. what exactly do we mean by ‘‘recurrent implantation failure’’? A systematic review and opinion. Reprod Biomed Online. 2014;28:409–423. https://doi.org/10.1016/j.rbmo.2013.12.006
16. Plaçais L, Kolanska K, Kraiem YB, Cohen j, Suner L, Bornes M, et al. Intralipid therapy for unexplained recurrent miscarriage and implantation failure: Case-series and literature review. Eur J Obstet Gynecol Reprod Biol. 2020;252:100-104. https://doi.org/10.1016/j.ejogrb.2020.06.017
17. Coulam CB. Intralipid treatment for women with reproductive failures. Am J Reprod Immunol. 2021;85(4):e13290. https://doi.org/10.1111/aji.13290
18. Mekinian A, Cohen j, Alijotas-Reig j, Carbillon L, Nicaise-Roland P, et al. Unexplained Recurrent Miscarriage and Recurrent Implantation Failure: Is There a Place for Immunomodulation? Am J Reprod Immunol. 2016;76(1):8-28. https://doi.org/10.1111/aji.12493
19. Human Fertilisation and Embryology Authority. Fertility treatment 2014—Trends and figures. 2016.
20. Assisted Reproductive Technology. National Summary Report. CDC; 2015.
21. Кречетова Л.В., Вторушина В.В., Инвияева Е.В., Ванько Л.В., Николаева М.А., Тетруашвили Н.К. Влияние иммуноцитотерапии на состояние иммунной системы женщин с идиопатическим привычным выкидышем. Медицинская иммунология. 2020;22(4):751-764. https://doi.org/10.15789/1563-0625-EOI-1860
22. Кречетова Л.В., Вторушина В.В., Ванько Л.В., Николаева М.А., Инвияева Е.В., Тетруашвили Н.К. Значимость оценки экспрессии CD69 лимфоцитами периферической крови для прогноза исходов беременности у женщин с привычным выкидышем. Биомедицинская химия. 2020;66(6):477-484. https://doi.org/10.18097/PBMC20206606477.
23. Менжинская И.В., Ионанидзе Т.Б., Ванько Л.В., Тетруашвили Н.К., Кречетова Л.В. Аутоантитела как факторы риска угрожающего выкидыша у женщин с ранними потерями беременности. Акушерство и гинекология. 2021;8:94-101. https://doi.org/10.18565/aig.2021.8.94-101
24. Вторушина В.В., Кречетова Л.В., Инвияева Е.В., Тетруашвили Н.К. Фактор, in vitro подавляющий миграцию макрофагов, в крови женщин с привычным выкидышем при беременности, развивающейся после иммуноцитотерапии. Российский иммунологический журнал. 2021;24(3):399-408. https://doi.org/10.46235/1028-7221-1040-MIF
25. Николаева М.А., Арефьева А.С., Степанова Е.О., Голубева Е.Л., Вторушина В.В., и соавт. Сроки наступления беременности после предгестационной аллоиммунизации и цитокиновый профиль клеток периферической крови у женщин с привычным выкидышем в анамнезе. Акушерство и гинекология. 2021;(1):79-87. https://doi.org/10.18565/aig.2021.1.79-87.
26. Загайнова В.А., Коган И.Ю., Сельков С.А., Беспалова О.Н., Крихели И.О., и соавт. NK-клетки периферической крови у пациенток с неэффективными протоколами вспомогательных репродуктивных технологий: количество, субпопуляционный состав и маркеры активации. Акушерство и гинекология. 2022;(9):102-113. https://doi.org/10.18565/aig.2022.9.102-113.
27. Kolanska K, Alijotas-Reig j, Cohen j, Cheloufi M, Selleret L, et al. Endometriosis with infertility: A comprehensive review on the role of immune deregulation and immunomodulation therapy. Am J Reprod Immunol. 2021;85(3):e13384. https://doi.org/10.1111/aji.13384
28. woon EV, Day A, Bracewell-Milnes T, Male V, johnson M. Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: A systematic review and meta-analysis. J Reprod Immunol. 2020;142:103189. https://doi.org/10.1016/j.jri.2020.103189
29. Kolanska K, Dabi Y, Dechartres A, Cohen j, Ben Kraiem Y, et al. Unexplained recurrent miscarriages: predictive value of immune biomarkers and immunomodulatory therapies for live birth. Am J Reprod Immunol. 2021;86(2):e13425. https://doi.org/10.1111/aji.13425
30.
About the Authors
O. A. TrunovaRussian Federation
Olga A. Trunova, Dr. Sci. (Med.), Professor of the Department of Higher Education Organization, Health Management and Epidemiology
Donetsk, DNR
Competing Interests:
Authors declares no conflict of interest.
I. D. Gulmamedova
Russian Federation
Irina D. Gulmamedova, Dr. Sci. (Med.), Associate Professor, Professor of the Department of Obstetrics, Gynecology, Perinatology, Pediatric and Adolescent Gynecology
Donetsk, DNR
Competing Interests:
Authors declares no conflict of interest.
E. A. Maylyan
Russian Federation
Eduard A. Maylyan, Dr. Sci. (Med.), Associate Professor, Head of the Department of Microbiology, Virology, Immunology and Allergology
Donetsk, DPR
Competing Interests:
Authors declares no conflict of interest.
Review
For citations:
Trunova O.A., Gulmamedova I.D., Maylyan E.A. Immunotherapy in patients with recurrent implantation failure. Medical Herald of the South of Russia. 2024;15(4):79-89. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-4-79-89







































