Scroll to:
Overcoming poor ovarian response in assisted reproductive technology programs in patients of late reproductive age
https://doi.org/10.21886/2219-8075-2024-15-1-98-107
Abstract
Objective: to increase the effectiveness of in vitro fertilization in patients of late reproductive age by optimizing prepregnancy preparation for the IVF program.
Materials and methods: The study included 67 patients of late reproductive age with a history of unsuccessful IVF attempts, divided into two groups at the second stage of the study, depending on the volume of prepregnancy preparation: group 1 (33 patients), group 2 (34 patients). The control group (50 patients) were healthy women of reproductive age. Immunohistochemical examination of the endometrium and microbiological examination of the uterine cavity, hormonal status of peripheral blood, study of the concentration of melatonin in peripheral blood and follicular fluid, antioxidant status of follicular fluid, expression of leukemia inhibitory factor (LIF) in the cervical secretion were performed.
Results: in patients with age-related dysregulation of the hypothalamic-pituitary-ovarian axis and impaired fertile function, a systemic and local (in follicles) decrease in melatonin levels was revealed. The studied effects of melatonin suggested that its use in patients of late reproductive age with reduced follicular reserve and a "poor" response to ovarian stimulation may increase the effectiveness of in vitro fertilization (IVF).
Conclusions: The inclusion in the pre-gravidar preparation of a complex of water-soluble polypeptide fractions of the epiphysis, stimulating the production of endogenous melatonin, made it possible to increase the effectiveness of IVF in patients by an average of 3.1 times according to the criterion of "clinical pregnancy" and 4.1 times according to the criterion of "live birth".
For citations:
Uryupina K.V., Kucenko I.I., Kravczova E.I., Arzumanyan K.A., Musavi M.N. Overcoming poor ovarian response in assisted reproductive technology programs in patients of late reproductive age. Medical Herald of the South of Russia. 2024;15(1):98-107. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-98-107
Introduction
The need for women of late reproductive age to realize their reproductive plans is one of the relevant problems of modern obstetrics and gynecology. In addition to complicated obstetric and gynecological history, as well as the increasing frequency of somatic pathology, the dominant factors of reproductive potential decline in women of late reproductive age include the decline in natural fertility. Taking into account the delayed realization of fertile function, assisted reproductive technologies (ART) make it possible to ensure childbearing for patients older than 35 years old. According to the reports of the Russian Association of Human Reproduction (2020), patients of late reproductive age account for about 54.4% of standard in vitro fertilization (IVF) programs. Age is undoubtedly the key pathogenetic factor of IVF failures in this cohort of patients, because the progressive reduction of the follicular reserve is associated with a poor ovarian response, determined in more than 50% of cases [1]. Reduced follicular reserve and poor ovarian response in patients of late reproductive age are accompanied by a reduced frequency of oocyte fertilization due to their poorer quality, reduced quality of embryos, high frequency of aneuploidy, and a reduced probability of embryo implantation [2]. In addition to low ovarian reserve in patients of late reproductive age, there is a decrease in the implantation potential of the endometrium. Destabilization of the hormonal background and cytokine response, including changes in the expression of leukemia inhibitory factor (LIF), and desynchronization of uterine cycle phases indicate the impaired receptivity of the endometrium, which reduces the probability of implantation even if the quality of embryos is good [3][4]. Although many methods were developed to improve the outcomes of IVF programs and live births in patients of late reproductive age, the search for means to improve oocyte and endometrial quality continues.
Studies in recent decades have shown that melatonin plays an important role in the synchronization of various reproductive processes. The regulatory, antioxidant, and immunomodulatory properties of melatonin are considered significant pathogenetic factors affecting almost all levels of the reproductive system regulatory axis [5][6]. Oocytes, granulosa cells, and luteal cells synthesize melatonin, which is concentrated in follicular fluid and, being a powerful direct free radical scavenger, protects all components of the ovary from oxidative stress, which worsens with the age of a woman [7].
Thus, the studied effects of melatonin suggest that the correction of its level in the treatment of infertility in patients of late reproductive age with reduced follicular reserve and poor response to ovarian stimulation can provide an increase in follicular reserve and oocyte quality, as well as increase the effectiveness of IVF procedures.
The aim of the study is to increase the efficiency of IVF in patients of late reproductive age by optimizing the pre-conceptional preparation for the IVF program.
Materials and Methods
A prospective cohort study included 67 patients (main group) in late reproductive age (older than 35 years) with a history of failed IVF attempts who applied for ART at the “Embrio” fertility clinic (Krasnodar and Sochi). Inclusion criteria were late reproductive age, a persisting menstrual cycle, a reduced follicular reserve, and the presence of a poor ovarian response in previous IVF cycles. Exclusion criteria were the presence of significant somatic and gynecological proliferative and inflammatory diseases, tubal-perineal infertility, endometriosis, uterine fibroids, immunohistochemical criteria of chronic endometritis without the autoimmune component, autoimmune chronic endometritis in the exacerbation stage, and the presence of pathogenic and opportunistic microflora in the uterine cavity. The control group (50 patients) included healthy women of reproductive age who were followed up in clinics within the surrogacy and oocyte donation program.
Before repeated IVF programs, an immunohistochemical examination of the endometrium and a microbiological examination of the uterine cavity were included in the scope of the examination. The LIF expression level in cervical secretion was determined by enzyme-linked immunosorbent assay (ELISA) using test systems from BenderMedsystems (USA). High-performance liquid chromatography with mass spectrometric detection was performed to identify melatonin concentration in peripheral blood (before and after pre-conceptional preparation in the secretory phase of the menstrual cycle) and melatonin concentration in peripheral blood and follicular fluid during the IVF cycle. The antioxidant status of follicular fluid was evaluated by calculating the ratio between superoxide dismutase (SOD) and malonic dialdehyde (MDA) (SOD/MDA/10 CU). SOD activity (units/mL) was measured by the inhibition of pyrogallol auto-oxidation at 420 nm using a UV/visible spectrophotometer. MDA levels (nmol/mL) were measured using the thiobarbituric acid method, by high-performance liquid chromatography.
Based on the studies and taking into account the revealed insufficiency of melatonin both in peripheral blood and follicular fluid, the patients were administered a preparation containing a complex of low-molecular-weight water-soluble polypeptide fractions isolated from bovine epiphysis, which has a stimulating effect on the synthesis of endogenous melatonin. The patients in the main group were divided into two clinical groups by simple randomization to evaluate the efficacy of the proposed therapy. In addition to standard estrogen-gestagen priming (estradiol-gel transdermally, 2 mg from day 1 to day 25 of the menstrual cycle, and micronized progesterone from day 14 to day 25 of the menstrual cycle, 400 mg daily intravaginally for 3 cycles of stimulation), women of the first group (Group I, 34 patients) were treated with intramuscular injection of low-molecular-weight water-soluble polypeptide fractions of epiphysis (10 mg daily for 10 days from day 5 to day 15 of the menstrual cycle preceding the stimulation cycle). Group II patients (33 women) were treated with standard estrogen-gestagen priming. In the IVF cycle, estrogen-gestagen priming was used to support the luteal phase. Superovulation stimulation, taking into account the reduced follicular reserve of the patients, was performed with the initial injection of recombinant human follicle-stimulating hormone (r-hFSH) 225 IU and menopausal gonadotropin at a dose of 75 IU. From day 7 to day 12 of stimulation, a gonadotropin-releasing hormone receptor antagonist (GnRH) was added to the therapy in order to suppress the secretion of endogenous gonadotropins. Recombinant chorionic gonadotropin at a single dose of 250 µg (once) was used as an ovulation trigger. The post-fertilization period was supported with micronized progesterone (600 mg per day).
Statistical analysis was performed using Microsoft Excel (2010) and STATISTICA 10 (USA). The normality of distribution was assessed using the Shapiro-Wilk and Kolmogorov-Smirnov tests. The mean was estimated by calculating the median, standard deviation and error of the mean, lower and upper quartile. The authors used parametric methods of comparison (Student's t-test) and non-parametric methods – Kruskal-Woliss and Mann-Whitney, Spearman and Pearson correlation analysis. The significance level of p<0.05 was applied after mathematical processing.
Results
The mean age of the patients was 39.5±2.1 years. The mean duration of infertility was 4.6±1.1 years. In the history, 42 (62.6%) female patients had one and 25 (37.4%) had 2 unsuccessful IVF attempts, during which a standard examination and preparation for IVF were performed.
The results of the initial ultrasound examination revealed a decrease in the antral follicle count (AFC – 3.4±1.9) and inconsistency of endometrial thickness with the menstrual cycle phase (4.5±1.3 mm in the middle secretory phase) in the patients of the main group.
According to the results of the previous unsuccessful IVF attempt, a poor ovarian response was recorded in the patients of the main group: the number of obtained follicles was 4.3±1.6, aspirated oocytes – 3.2±1.4, and the average number of oocytes in metaphase – 3.1±1.6. Finally, the mean number of normally fertilized oocytes was 2.6±0.9, the fertilization rate was 27.3±5.4, and the mean number of blastocysts per patient was 0.7±0.01. Embryo transfer occurred in 35 (52.2%) patients, biochemical pregnancy occurred in 11 (16.4%), and clinical pregnancy (according to ultrasound examination) did not occur in 100% of patients.
The analysis of the hormonal background in the patients of the main group compared to the results of the control group showed a higher level of gonadotropic hormones (FSH – 11.9±5.2 IU/L, LH – 11.5±2.6 IU/L, control – 5.5±1.6 IU/L, p<0.001 for both indicators). The levels of peripheral steroid hormones (estradiol, progesterone) were within the reference intervals for women of the indicated age, but compared to the control group, they were significantly reduced (main group: estradiol – 154.6±42.8 pg/mL, progesterone – 28.7±6.4 ng/mL, control group: estradiol – 250.6±126.9 pg/mL, progesterone – 34.9±8.4 ng/mL, p<0.001 for both parameters). The species composition of endometrial microbiota obtained by cultural tests and PCR did not show the presence of pathogenic and opportunistic microflora in the endometrium. The predominantly detected microflora included Lactobacillus spp. (53 (79.1%) patients). The biopsy microbial content level was 4.01/104±1.02/105 CFU/mL.
Immunohistochemical criteria of chronic endometritis without detectable autoimmune component and autoimmune chronic endometritis in the stage of exacerbation in the patients of the main group were not revealed, but in 32 (47.7%) patients, criteria confirming the presence of weakly expressed autoimmune chronic endometritis were found. At the same time, 56 (83.6%) patients showed decreased expression of estrogen receptors and progesterone receptors in the stroma and endometrial glands. All patients in the main group showed a decrease in the mean expression level of LIF (a marker of endometrial receptivity) in the endometrium (4.6±0.3 points and 6.0±0.2 points, p<0.001) and in cervical secretion (23.6±3.3 pg/mL vs. 35.8±7.2 pg/mL, p<0.001) compared to the control group. There was a strong positive correlation between these parameters (r(endometrial LIF/LIF of cervical secretion)=0.91).
The study revealed a statistically significant decrease in melatonin levels in both peripheral blood (10.9±2.4 pg/mL vs. 28.9±2.4 pg/mL in the control group, p<0.001) and follicular fluid (22.9±2.1 pg/mL vs. 35.6±1.5 pg/mL in the control, p<0.001).
In this study, the concentration of SOD (the main enzyme included in the antioxidant defense system of the body) in follicular fluid in the group of patients of late reproductive age with ovarian insufficiency was significantly reduced compared to the control group (32.4 [ 30.6; 34.1] units/mL vs. 56.6 [ 56.5;58.7] units/mL in controls, p<0.001). The concentration of MDA, which is a marker of oxidative stress, on the contrary, was elevated and amounted to a statistically significant difference with the result of the control group (0.89 [ 0.85;0.93] nmol/mL), p<0.001. The level of antioxidant defense in follicular fluid is most accurately reflected by the calculated SOD/MDA ratio, which amounted to 9.3 [ 9.3;9.8] CU in the group of healthy women. In the group of age-matched patients with a history of unsuccessful IVF attempts, this index was significantly lower due to both inhibition of antioxidant properties of follicular fluid and an increase in pro-oxidants (3.6 [ 3.1;4.1] CU, p<0.001). The index of the oxidative stress level in the follicle (SOD/MDA) had a strong inverse correlation with melatonin concentration in follicular fluid (r=0.92).
As a result of complex pre-conceptional preparation with the use of epiphysis-containing medication, the level of melatonin in peripheral blood in patients of Group I amounted to a statistically significant difference with the initial results 34.8±1.6 pg/mL (p<0.001) and practically equaled the results of the control group (p=0.066), whereas the level of melatonin in Group II without stimulation of endogenous melatonin practically did not change and amounted to 11.2±2.1 pg/mL (p=0.0436) compared to the initial results. Similar dynamics of melatonin content were observed in follicular fluid. In Group I, a statistically significant increase (p<0.001) almost to the results of the control group (25.3±1.2 pg/mL, p=0.009) was observed. In Group II, a statistically significant increase in the melatonin level was not revealed (23.5±1.2, p=0.065) (Table 1).
Таблица / Table 1
Динамика уровня мелатонина периферической крови
и фолликулярной жидкости после предгравидарной подготовки
Dynamics of melatonin levels in peripheral blood
and follicular fluid after pregravidar preparation
|
Параметры Parameters |
I группа, n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
Мелатонин периферической крови,пг/мл Peripheral blood melatonin, pg/ml |
Исходно Initially |
11,2±2,8 |
10,7±2,1 |
28,9±2,4 |
<0,001 |
0,436 |
|
После ПГ After PG |
25,3±1,2 |
11,2±2,1 |
||||
|
Мелатонин фолликулярной жидкости, пг/мл Melatonin of follicular fluid, pg/ml |
Исходно Initially |
23,5±1,2 |
22,7±0,5 |
35,6±1,5 |
<0,001 |
0,065 |
|
После ПГ After PG |
34,8±1,6 |
23,5±1,2 |
||||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
The antioxidant activity coefficient (SOD/MDA/10) in Group I patients increased 2-fold due to a significant increase in SOD (52.7 [ 51.2;56.4] units/mL, p<0.001) compared to baseline data) and a decrease in MDA (0.67 [ 0.67;0.83] nmol/mL, p<0.001) compared to baseline data). At the same time, despite a significant increase, the antioxidant activity coefficient did not reach the results of the control group (7.4 [ 7.3;7.8] CU, p<0.001) (Table 2).
Таблица / Table 2
Динамика уровня анти- и прооксидантных ферментов
в фолликулярной жидкости после предгравидарной подготовки
Dynamics of the level of anti- and pro-oxidant enzymes
in the follicular fluid after pregravidar preparation
|
Параметры Parameters |
I группа,n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
SOD, (ед/мл) SOD, (units/ml) |
Исходно Initially |
32,4 [ 30,6;34,1] |
32,4 [ 30,6;34,1] |
56,6 [ 56,8;58,7] |
<0,001 |
0,043 |
|
После ПГ After PG |
52,7 [ 51,2;56,4] |
33,7 [ 29,3;37,7] |
||||
|
MDA,(нмоль/мл) MDA, (nmol/ml) |
Исходно Initially |
0,89 [ 0,85;0,93] |
0,89 [ 0,83;0,92] |
0,59 [ 0,54;0,69] |
<0,001 |
0,045 |
|
После ПГ After PG |
0,67 [ 0,67;0,83] |
0,87 [ 0,84;0,96] |
||||
|
SOD/MDA/10, (у.е.) SOD/MDA/10, (units) |
Исходно Initially |
3,6 [ 3,3;3,9] |
3,6 [ 3,3;3,9] |
9,3 [ 9,3;9,8] |
<0,001 |
0,032 |
|
После ПГ After PG |
7,4 [ 7,3;7,8] |
3,8 [ 3,5;3,9] |
||||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
The FSH and LH levels decreased in Group I to 7.1±1.4 IU/L and 7.9±1.7 IU/L, respectively, versus 11.9±3.9 IU/L and 11.4±3.4 IU/L in baseline measurements (p<0.001), whereas in Group II, due to the use of estrogen-gestagen therapy alone, there was also a statistically significant decrease, but with a smaller difference from baseline. The dynamics of peripheral hormones were also more favorable in Group I in patients with additional melatonin priming (Table 3).
Таблица / Table 3
Динамика уровня гонадотропных и половых гормонов
в периферической крови после предгравидарной подготовки
Dynamics of gonadotropin and sex hormones levels
in peripheral blood after pregravidar preparation
|
Параметры Parameters |
I группа, n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
ФСГ, МЕ/л FSH, IU/l |
Исходно Initially |
11,9±3,9 |
11,8±4,1 |
5,5±1,6 |
<0,001 |
<0,001 |
|
После ПГ After PG |
7,1±1,4 |
9,1±1,8 |
||||
|
ЛГ, МЕ/л LH, ME/l |
Исходно Initially |
11,4±3,1 |
11,4±3,4 |
7,6±1,5 |
<0,001 |
<0,001 |
|
После ПГ After PG |
7,9±1,7 |
9,9±1,5 |
||||
|
Е2 (эстрадиол), пг/мл E2 (estradiol), pg/ml |
Исходно Initially |
153,2±141,1 |
153,2±41,1 |
250,6±66,9 |
<0,001 |
<0,001 |
|
После ПГ After PG |
265,1±23,4 |
208,1±27,2 |
||||
|
Прогестерон, нг/мл Progesterone, ng/ml |
Исходно Initially |
28,5±6,4 |
24,5±3,2 |
34,9±8,4 |
<0,001 |
<0,001 |
|
После ПГ After PG |
31,2±5,7 |
30,3±4,8 |
<0,001 |
<0,001 |
||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
The number of antral follicles determined by ultrasound increased 2-fold in Group I patients (to 7.6±1.1) compared to AFC in Group II patients (4.6±1.1, p<0.001), but did not reach the results of the control group (12.5±1.8, p<0.001).
Endometrial thickness in both groups increased relative to baseline parameters, but there was no statistically significant difference between Groups I and II (Group I – 8.5±1.1 mm, Group II – 8.2±1.3 mm, p=0.326) (Table 4).
Таблица / Table 4
Динамика параметров ультразвукового исследования
после предгравидарной подготовки
Dynamics of ultrasound parameters after pregravidar preparation
|
Параметры Parameters |
I группа, n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
Количество антральных фолликулов Number of antral follicles |
Исходно Initially |
3,4±1,8 |
3,4±1,8 |
12,5±1,8 |
p<0,001 |
p<0,001 |
|
После ПГ After PG |
7,6±1,1 |
4,6±1,1 |
||||
|
Толщинаэндометрия, мм Endometrial thickness, mm |
Исходно Initially |
4,4±1,2 |
4,4±1,2 |
13,4±1,2 |
p<0,001 |
p<0,001 |
|
После ПГ After PG |
8,5±1,1 |
8,2±1,3 |
||||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
In both groups, LIF concentration increased significantly compared to the baseline parameters (p<0.001), but there was no statistically significant difference in LIF levels between the groups (29.1±9.4 pg/mL – Group I, 28.9±6.5 pg/mL – Group II, p=0.721), and the index did not reach the results of the control group (35.8±7.2 pg/mL, p<0.001) (Table 5).
Таблица / Table 5
Динамика LIF в цервикальном секрете после предгравидарной подготовки
Dynamics of LIF in the cervical secretion after pregravidar preparation
|
Параметры Parameters |
I группа, n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
LIF, пг/мл рg/ml |
Исходно Initially |
23,6±3,3 |
22,9±2,9 |
35,8±7,2 |
<0,001 |
<0,001 |
|
После ПГ After PG |
29,1±9,4 |
28,9±6,5 |
<0,001 |
<0,001 |
||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
After pre-conceptional preparation, all patients were admitted to the ovulation stimulation cycle according to the unified protocol.
As a result, no statistically significant changes in the induced cycle parameters and the parameters of oogenesis and embryogenesis were observed in group II patients. In group I patients, after additional therapy with a preparation containing low-molecular-weight polypeptide fractions of epiphysis, the number of total follicles increased more than 2-fold and, accordingly. The number of aspirated follicles and the average number of oocytes in metaphase II increased 2-fold (Table 6).
Таблица / Table 6
Параметры индуцированного цикла после проведения предгравидарной подготовки
Parameters of the induced cycle after pregravidar preparation
|
Параметры Parameters |
I группа, n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
Общее количество фолликулов Total number of follicles |
Исходно Initially |
4,2±1,3 |
4,2±1,5 |
28,3±2,2 |
p<0,001 |
p=0,34 |
|
После ПГ After PG |
10,3±1,3 |
4,6±1,3 |
||||
|
Общее количество аспирированных ооцитов Total number of aspirated oocytes |
Исходно Initially |
3,3±1,3 |
3,3±1,3 |
17,3±1,5 |
p<0,001 |
p=0,53 |
|
После ПГ After PG |
10,2±2,3 |
3,5±1,5 |
||||
|
Среднее число ооцитовМII The average number of oocytes MII |
Исходно Initially |
3,1±1,1 |
3,1±1,1 |
14,2±1,5 |
p<0,001 |
p=0,76 |
|
После ПГ After PG |
8,2±1,4 |
3,2±1,4 |
||||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
As a result, the average number of normally fertilized oocytes in Group I patients increased to 7.5±1.2 and amounted to a 2-fold difference compared to the identical index in Group II patients (3.0±1.2, p<0.001). The fertilization rate increased in Group 1 to 52.3±6.8%, and the average number of blastocysts and the average number of excellent/good quality blastocysts increased more than 2-fold compared to the initial data, which was also a statistically significant difference with the indicators of Group II patients (p<0.001) (Table 7).
Таблица / Table 7
Особенности оогенеза и эмбриогенеза после проведения предгравидарной подготовки
Features of oogenesis and embryogenesis after pregravidar preparation
|
Параметры Parameters |
I группа, n=34 Group 1, n=34 |
II группа, n=33 Group 2, n=33 |
III группа (контроль), n=50 Group 3 (control), n=50 |
р (I группа) исходно/после ПГ p (group 1) initially/after PG |
р (II группа) исходно/после ПГ p (group 1) initially/after PG |
|
|
Среднее число нормально оплодотворившихся ооцитов (2PN) The average number of normally fertilized oocytes (2PN) |
Исходно Initially |
2,6±0,9 |
2,6±0,9 |
11,3±1,4 |
p<0,001 |
p=0,5 |
|
После ПГ After PG |
7,5±1,2 |
3,0±1,2 |
||||
|
Частота оплодотворения (%) Fertilization rate (%) |
Исходно Initially |
27,3±5,4 |
27,3±5,4 |
82,2±8,3 |
p<0,001 |
p=0,07 |
|
После ПГ After PG |
52,3±6,8 |
32,3±6,8 |
||||
|
Морфологическая оценка эмбрионов на 3-и сутки (баллы) Morphological assessment of embryos on day 3 (points) |
Исходно Initially |
2,2±0,7 |
2,2±0,7 |
3,1±0,9 |
p=0,035 |
p=0,35 |
|
После ПГ After PG |
2,6±0,5 |
2,6±0,5 |
||||
|
Среднее число бластоцист на пациентку The average number of blastocysts per patient |
Исходно Initially |
1,5±0,01 |
0,7±0,01 |
11,3±1,4 |
p<0,001 |
p=0,15 |
|
После ПГ After PG |
4,2±0,02 |
0,7±0,02 |
||||
|
Среднее число бластоцист отличного/хорошего качества The average number of blastocysts of excellent/ good quality |
Исходно Initially |
0,5±0,2 |
0,5±0,2 |
10,2±0,7 |
p<0,001 |
p=0,29 |
|
После ПГ After PG |
3,5±0,05 |
0,4±0,2 |
||||
Примечание: *ПГ — предгравидарная подготовка.
Note: *PG — pregravidar preparation.
As a result, embryo transfer took place in all patients of Group I – 33 (100%). In Group II, embryo transfer took place in 14 (41.2%) patients (p<0.001, OR=47.1 (5.7–38.8)). In Group I, biochemical pregnancy occurred in 22 (66.7%) patients per initiated cycle and embryo transfer, whereas in Group II, the rate of biochemical pregnancy per initiated cycle was 26.5% and per embryo transfer 64.3% (p<0.001, OR=5.5 (1.9–15.6)).
The rate of clinical pregnancy in Group I was 12 (36.4%) per initiated cycle and 12 (36.4%) per embryo transfer, which was significantly higher than in Group II (11.8% and 28.6%), respectively (p<0.001, OR=4.2 (1.2–15.2)). Both groups had 1 spontaneous miscarriage each at 6–7 weeks of gestation. As a result, live births in Group I were more than 4 times higher than in Group II and amounted to 33.3% of cases per cycle and per transfer, while in Group II, they remained at the minimum age-standardized mean (RAHR) levels (8.8%) (p<0.001, OR=5.2 (1.3–20.7)) (Figure 1).
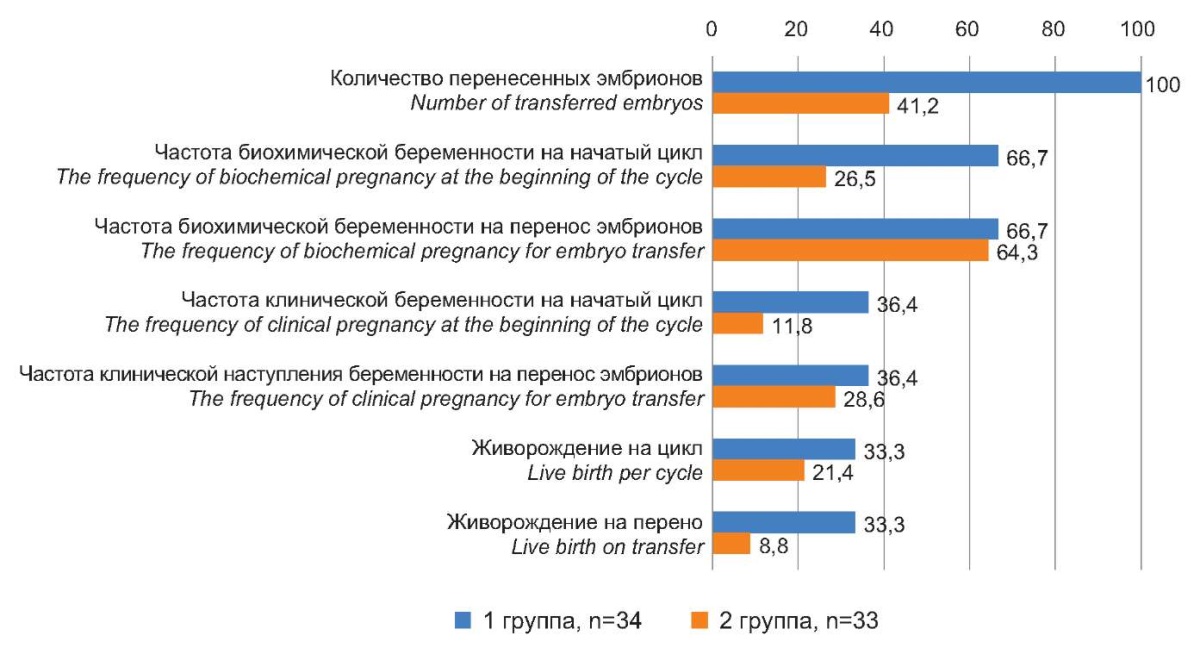
Figure 1. The outcomes of IVF programs in patients
after various pre-pregnancy preparations, %.
Рисунок 1. Исходы программ ЭКО у пациенток
после проведения различной предгравидарной подготовки, %.
As a result, the efficiency of IVF in patients of late reproductive age with pre-conceptional preparation with correction of pineal dysfunction (Group I) maximally approached the average statistical efficiency of IVF, which is 30–35% (RAHR 2021), which is quite high for patients of late reproductive age.
Discussion
Melatonin plays an important role in the synchronization and regulation of fertility, affecting all levels of the regulatory axis of the menstrual-ovarian cycle. According to recent studies, topical melatonin contained in follicular fluid neutralizes free radicals and protects reproductive material from oxidative stress and mitochondrial dysfunction [8].
In the present study, the main group of women initially had a significant decrease in melatonin levels in peripheral blood and follicular fluid compared to the control group. A decrease in melatonin concentration has an indirect effect on a woman's fertile function, since ovarian aging, as well as many other degenerative processes, is largely determined by the level of oxidative stress, and melatonin and its metabolites inhibit prooxidant enzymes, presumably affecting oocyte maturation and quality [9].
In patients of late reproductive age who received melatonin priming, the oxidant profile in follicular fluid improved. Based on these data, it can be assumed that one of the additional pathogenetic moments of oogenesis disturbance in patients of late reproductive age, when oocyte dysfunctions and implantation insufficiency of the endometrium are combined, is the deficiency of pineal and extrapineal melatonin secretion. For this reason, correction of pineal deficiency, introduced into the complex of pre-conceptional preparation, can improve the efficiency of IVF programs. As a stimulator of endogenous melatonin, low-molecular-weight water-soluble polypeptide fractions of epiphysis were administered intramuscularly to the patients of Group I in addition to standard estrogen-gestagen priming. Supplementation of standard pre-conceptional preparation with epiphysis preparations increased the level of melatonin in peripheral blood and follicular fluid. Probably, after increasing the concentration of melatonin, which is a modulator of indirect oxidative damage by increasing the activity of antioxidant enzymes and decreasing the activity of prooxidant enzymes, the antioxidant status of follicular fluid changed significantly in Group I patients.
Stimulation of endogenous melatonin, which led to its increase systemically and locally, was accompanied by more pronounced favorable changes in the level of gonadotropic hormones, which was manifested in a decrease in the concentrations of FSH and LH in peripheral blood. The maximum improvement of the hormonal background during stimulation of epiphysis penialocytes both at central and peripheral levels positively correlated with the activation of ovarian function and to a lesser extent – with the growth of the functional layer of the uterus. In particular, the ultrasound examination showed an increased number of antral follicles. However, the effect of melatonin on the implantation potential of the endometrium was not as positive. No significant increase in endometrial thickness was observed, and the level of LIF expression in cervical secretion also changed insignificantly.
Nevertheless, as a result, the overall efficiency of the IVF procedure in women of late reproductive age after pre-conceptional preparation with the administration of pineal polypeptides approached the average statistical efficiency (30–35%), according to the data of the Russian Association of Human Reproduction from 2021.
Conclusion
Negative IVF attempts in women of late reproductive age are explained by a statistically significant decrease in the follicular reserve, a decrease in the number of mature oocytes and quality embryos, and receptor deficiency of endometrial implantation factor, even in a cohort of conventionally healthy women. Defects in the functioning of the entire axis of reproductive system regulation in patients of late reproductive age in the presence of a combination of oocyte factor (“poor response”) and implantation failure of the endometrium (“thin endometrium”) are accompanied by decreased melatonin levels in peripheral blood and follicular fluid. In the follicular fluid of this cohort, there was an increase in oxidative stress correlating with decreased melatonin levels.
The inclusion of a complex of water-soluble polypeptide fractions of epiphysis stimulating the production of endogenous melatonin in the complex of the pre-conceptional preparation showed a positive effect on the normalization of the functioning of the hypothalamic-pituitary-ovarian axis of reproductive system regulation. Elevated melatonin levels in peripheral blood and follicular fluid decreased the level of oxidative stress and increased antral and post-stimulated and aspirated follicles count. This effect is probably realized due to the proven influence of melatonin on gonadotropin-inhibitory hormone (GnIH) neurons, with melatonin stimulating not only the synthesis of GnIH but also its release, thereby reducing the concentration of LH in plasma, and indirectly, affecting the level of FSH and peripheral ovarian hormones [10]. Melatonin is also known to indirectly reduce oxidative stress by increasing antioxidant enzymes, decreasing pro-oxidant enzymes, and stimulating the synthesis of other endogenous antioxidants such as glutathione [8]. Apparently, this mechanism explains the reduction of oxidative stress in follicles, which contributes to an increase in the average number of MII oocytes and the average number of blastocysts of excellent/good quality. As a result, correction of pineal insufficiency made it possible to improve the results of ART in patients of late reproductive age by an average of 3.1 times by the criterion “clinical pregnancy”, and by 4.1 times by the criterion “live birth”.
However, in this study, the authors did not find a significant effect of pineal correction on the endometrial implantation factor, despite a significant increase in estradiol and progesterone levels, the absence of pathogenic and opportunistic infection in the uterine cavity, and the presence of mild autoimmune chronic endometritis in only a part of the patients. This problem requires further investigation to clarify and correct predominantly endometrial dysfunctions.
References
1. Sugurova A.T., Yashchuk A.G., Khusainov R.I. Clinical and genetic aspects of the problem of ovarian response when using assisted reproductive technologies. Russian Bulletin of Obstetrician-Gynecologist. 2020;20(6):48-55. (In Russ.) https://doi.org/10.17116/rosakush20202006148.
2. Dolgushina N.V., Adamyan L.V., Sheshko E.L. Late reproductive age of a woman: risks of reproductive dysfunction (literature review). Russian Journal of Human Reproduction. 2023;29(4):99-106. (In Russ.) https://doi.org/10.17116/repro20232904199
3. Posiseeva L.V., Perepechay M.I., Petrova O.A., Petrova U.L. Opportunities of Pregravid Preparation for Women with Low Ovarian Reserve. Effektivnaya farmakoterapiya. 2020;16(7):6-9. (In Russ.) https://doi.org/10.33978/2307-3586-2020-16-7-6-9
4. Marron K, Harrity C. Endometrial lymphocyte concentrations in adverse reproductive outcome populations. J Assist Reprod Genet. 2019;36(5):837-846. https://doi.org/10.1007/s10815-019-01427-8
5. Gomes PRL, Motta-Teixeira LC, Gallo CC, Carmo Buonfiglio DD, Camargo LS, et al. Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen Comp Endocrinol. 2021;300:113633. https://doi.org/10.1016/j.ygcen.2020.113633.
6. Yong W, Ma H, Na M, Gao T, Zhang Y, et al. Roles of melatonin in the field of reproductive medicine. Biomed Pharmacother. 2021;144:112001. https://doi.org/10.1016/j.biopha.2021.112001
7. Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int J Mol Sci. 2020;21(3):1135. https://doi.org/10.3390/ijms21031135
8. Reiter RJ, Sharma R, Romero A, Manucha W, Tan DX, et al. Aging-Related Ovarian Failure and Infertility: Melatonin to the Rescue. Antioxidants (Basel). 2023;12(3):695. https://doi.org/10.3390/antiox12030695
9. Yang D, Mu Y, Wang J, Zou W, Zou H, et al. Melatonin enhances the developmental potential of immature oocytes from older reproductive-aged women by improving mitochondrial function. Heliyon. 2023;9(9):e19366. https://doi.org/10.1016/j.heliyon.2023.e19366
10. Adamyan LV, Sibirskaya EV, Shcherina AV. Pathogenetic aspects of premature ovarian failure. Russian Journal of Human Reproduction. 2021;27(1):6-12. (In Russ.) https://doi.org/10.17116/repro2021270116
About the Authors
K. V. UryupinaРоссия
Kristina V. Uryupina, Junior Researcher, Department of Obstetrics, Gynecology and Perinatology
Krasnodar
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
I. I. Kucenko
Россия
Irina I. Kutsenko, Dr. Si. (Med.), Professor, head of the Department of obstetrics, gynecology and Perinatology
Krasnodar
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
E. I. Kravczova
Россия
Elena I. Kravtsova, Cand. Si. (Med.), associate Professor of obstetrics, gynecology and Perinatology
Krasnodar
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
K. A. Arzumanyan
Россия
Kamilla A. Arzumanyan, 6th year student of the Faculty of Medicine
Krasnodar
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
M. N. Musavi
Россия
Margarita N. Musavi, 6th year student of the Faculty of Medicine
Krasnodar
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
Review
For citations:
Uryupina K.V., Kucenko I.I., Kravczova E.I., Arzumanyan K.A., Musavi M.N. Overcoming poor ovarian response in assisted reproductive technology programs in patients of late reproductive age. Medical Herald of the South of Russia. 2024;15(1):98-107. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-98-107
JATS XML







































