Scroll to:
Results of ligation the internal iliac and ovarian arteries in the experiment
https://doi.org/10.21886/2219-8075-2023-14-4-77-82
Abstract
Objective: to study in an experiment on animals pathomorphological changes in the tissues of the uterus, appendages and blood vessels after ligation of the internal iliac and ovarian arteries.
Materials and methods: An experiment was conducted with 6 mature pigs. Group I (n=3) consisted of intact pigs without surgery, group II (n=3) included animals with ligation of the internal iliac and ovarian arteries on both sides. 12 months after the operation, slaughter was performed and the organo-complex of the uterus with appendages and iliac vessels was taken for electron microscopic pathomorphological examination.
Results: comparative results of the study of the uterus, appendages found that the normal structure of the mature uterus was preserved, there were no changes in the structure of the fallopian tubes, tissues and vessels of the ovaries. All changes were found in the area of ligation of the ovarian and internal iliac arteries, although the arteries retained their lumen, but were deformed and surrounded by dense fibrous connective tissue. Conclusion: ligation of the ovarian and internal iliac arteries with absorbable suture material causes only local changes. The sufficiency of collateral blood flow is confirmed by the absence of atrophy of the uterus, fallopian tubes and ovaries, as well as organ deformities.
Keywords
For citations:
Kantsurova M.R., Rymashevsky A.N., Voloshin V.V., Sapronova N.G., Todorov S.S., Rymashevsky M.A., Kantsurov R.N. Results of ligation the internal iliac and ovarian arteries in the experiment. Medical Herald of the South of Russia. 2023;14(4):77-82. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-4-77-82
Introduction
The use of uterine-sparing techniques in massive bleeding is not new to obstetric and gynecological practice [1–5]. A variety of methods have been developed to achieve hemostasis and reduce intraoperative blood loss [1][6–7]. Sufficient domestic and foreign experience in surgical hemostasis has been accumulated. Uterine devascularization procedures include uterine artery (UA), ovarian artery (OA), and internal iliac artery (IIA) ligation, uterine artery embolization (UAE), tourniquet application, clipping, etc. [1][8].1
Since the 1960s, many studies have been conducted to examine the internal genitalia after pelvic artery ligation, such as those by Tsitsishvili (1961) [9], Rembez and Klinskaya (1964), who examined the pelvic organs after OA and IIA ligation in rabbits and dogs [10].
Over the past half century, experimental work has become uncommon. In general, researchers focused on studying the reproductive system of farm animals. The scarcity of experimental work was attributed to the complications associated with selecting experimental animals that were expected to represent a human functional model, characterizing and investigating the simulated process, as well as conducting an experiment itself. Pigs are an ideal biological model for human health and diseases. Anatomical and physiological similarities between humans and pigs, ease of pig livestock management, and availability for various manipulations make pigs an attractive option for modeling. The human and porcine female reproductive tracts share a number of similarities.
The endometrium, myometrium, and perimetrium are the three layers of the porcine uterine wall. The uterine horns terminate at the oviducts (fallopian tubes), prominent tortuous tubes. They are the site of fertilization and embryo transfer to the uterus. The porcine genitals are supplied by branches derived from the abdominal aorta and IIA (paired OAs, middle UA, and posterior UA). Similar to human anatomy, porcine OAs originate from the abdominal aorta, whereas the middle UA is well-developed and considered an extension of the umbilical artery, and the posterior UA originates from the paired urogenital artery [11].
There are only a few experimental studies in the literature that address the pathomorphological assessment of uterine and appendage tissues after intraoperative hemostasis, specifically IIA ligation. This has become the focus of this research.
This study aims to evaluate the pathomorphological changes in the tissues of the uterus, uterine appendages, and vessels in an experiment with pigs after IIA and OA ligation and to assess the development of collateral circulation.
Materials and Methods
This study was performed in line with the principles of World Medical Association's (WMA) Declaration of Helsinki (2000), International Guiding Principles for Biomedical Research Involving Animals (1985), moral and ethical principles for biomedical research on animals developed by the Council for International Organizations of Medical Sciences (CIOMS), European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes, including the main provisions of the CIOMS Guidelines (Strasbourg, France, 1986), Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes2. The pig-keeping routine was compliant with Order No. 114 of the Ministry of Agriculture of the Russian Federation of March 29, 2016, which approves veterinary regulations for the production, breeding, and sale of pigs3.
The experimental groups included six Large White female pigs each. The animals were 4 months old and weighed between 17 and 23 kg. The experimental animals were divided into two groups: Group I (n=3) consisted of intact pigs without surgery, and Group II (n=3) included animals that underwent a procedure of bilteral OA and IIA ligation.
Twelve hours before the operation, the animals were fasted and provided with enough water to keep the animals from becoming dehydrated. Perioperative stress and physical exertion were minimized. The surgical procedure was performed in a veterinary clinic. After weighing, the animals were pre-medicated with atropine i.m. at 0.1 mg/kg of body weight, xylazine hydrochloride (Xylanit) at 0.1–0.5 mL/kg of body weight, tiletamine and zolazepam (Zoletil 100) at 10 mg/kg of body weight. Sedation occurred 12–14 minutes after administration. The animal was positioned supine on the operating table. The external jugular vein was catheterized, and the skin was treated with an alcohol-based antiseptic. Sterile linen was used to protect the operating field.
The peritoneal access was created aseptically in layers through a lower midline incision. The intestinal loops were elevated upwards and the bladder was brought downwards using a wet gauze. Next, a vaginal speculum was inserted. The surgeon opened the parietal peritoneum within an aortic bifurcation to expose the common iliac artery and IIA. The IIA was then separated with a dissector and ligated bilaterally using an absorbable suture material (polyglycolide). The uterus was exposed, and the OA was also ligated bilaterally using the above-mentioned suture material. Hemostasis was monitored. The anterior abdominal wall was tightly sutured in layers, and the surgical wound was covered with an aseptic dressing. The amount of intraoperative blood loss was within 35-40 mL, and the surgery length averaged around 25–30 minutes. No postoperative deaths have been reported in the experimental animals. The early and late postoperative periods were unremarkable.
After 12 months, the animals were euthanized in a specialized room. The iliac arteries and uterus with uterine appendages were removed for electron microscopic pathology. The specimens were fixed in formalin and delivered to the Morphology Department of the Rostov State Medical University Clinic of the Russian Ministry of Health. For histology, transverse sections were taken of the uterine body at 1.0 cm from the horns, the oviducts at 3.0 cm from the ampulla, the IIA and OA in the ligation zone, and at 1.0 cm in the distal direction. A longitudinal section was taken from the ovaries through the entire thickness in the largest dimension.
The sections were cut into pieces with a maximum size of 2.0×1.5×0.5 cm and fixed in buffered 10% formalin solution, followed by histological processing using an STP120-2 Spin Tissue Processor (Thermo Scientific, USA). The material was then poured into HistoSafe embedding cassettes containing Histomix paraffin medium using an EG1150H Modular Tissue Embedding Center (Leica, Germany). The paraffin blocks were sectioned on a Rotary 3003 Microtome (PFM Medical, Germany) to obtain 3.0-µm serial sections. The specimens were stained with hematoxylin and eosin, Van Gieson's picrofuchsin, and Masson's trichrome to identify connective tissue components. Microscopy and photography of the obtained histologic specimens were performed using a Leica 1000DM microscope (Germany).
Results
The endometrial microscopy in Group I demonstrated the glandular epithelium composed of several layers of epithelial cells, endometrial glands of various sizes from small to cystically dilated, large spiral arteries, and the inner annular and outer longitudinal layers of smooth muscle cells, between which the vascular layer characterizing the normal structure of the mature uterus could be distinguished. The uterine horns had a similar structure. In Group II, the postoperative histological pattern of the uterine walls and horns did not differ from Group I (Figure 1 [a, b]).
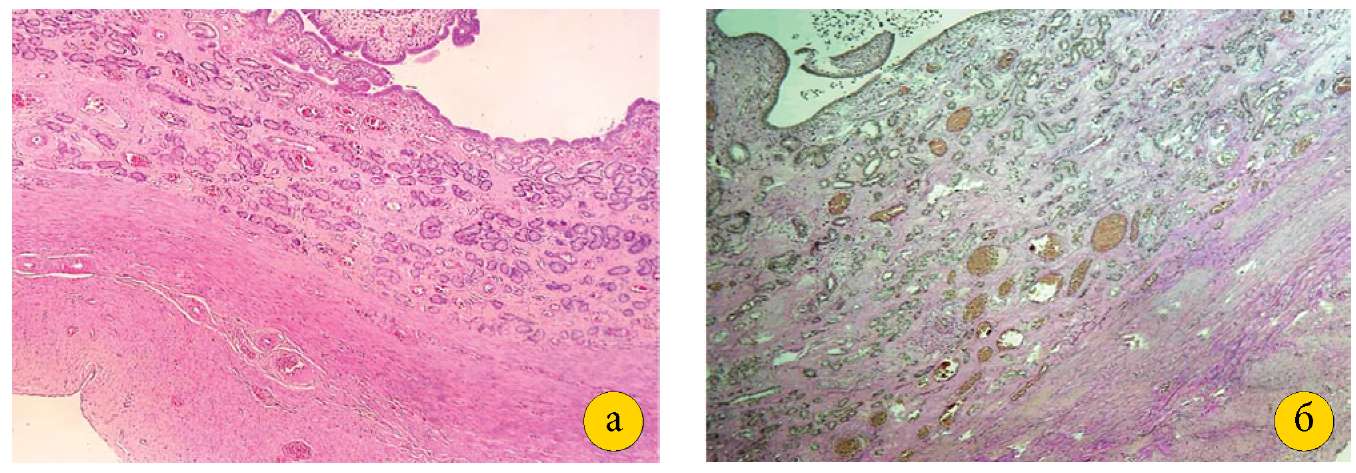
Figure 1. A — tissue of the pig uterine horn of group I
(magnification 4×10, stained with hematoxylin and eosin).
B — wall of the pig uterine horn of group II
(magnification 4×10, stained with hematoxylin and eosin).
The ovarian cortex in Group I was specific for numerous primordial, primary, secondary, and tertiary follicles, and a yellow body, which provided morphological evidence that the animals were ready for fertilization. Numerous branches of spiral arteries and venous plexuses were found in the ovarian medulla. The structure of the ovarian arteries and veins was normal, including the adventitia, both middle and inner layers. Histologically, the surgical outcomes in Group II were specific for changes noted at the vascular ligation sites. The ligated OA (0.1–0.15 cm in diameter) retained its lumen (0.05–0.07 cm in diameter). However, the dense fibrous connective scar tissue was noted to develop around the OA and adjacent vessels (Figure 2 [a, b]).
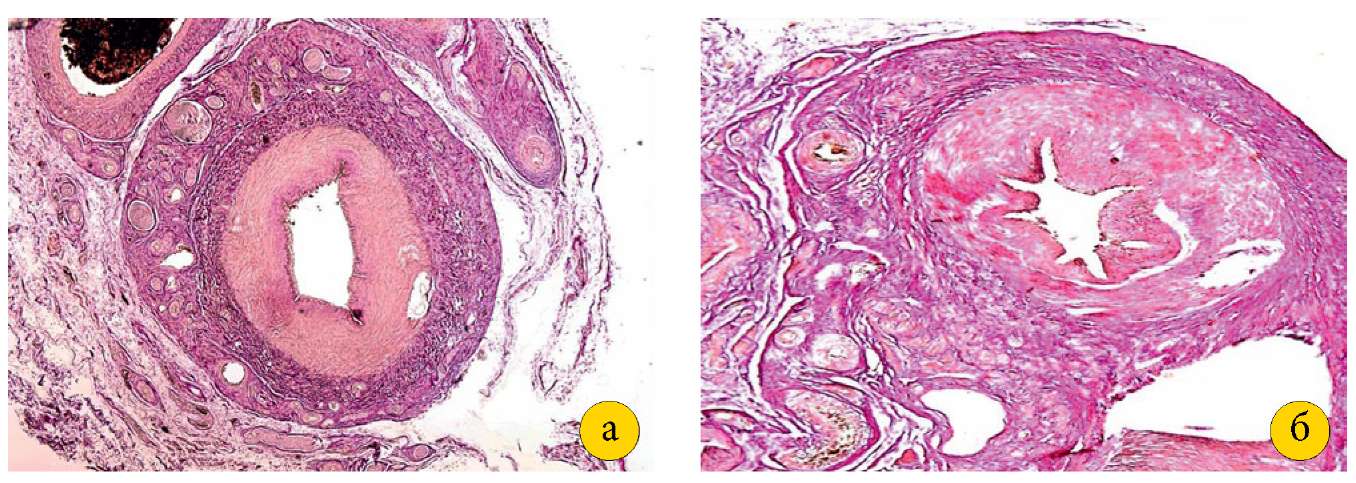
Figure 2. A — ovarian artery and part of the vein of an intact pig
(magnification 2.5×10, stained with hematoxylin and eosin).
B — ovarian artery after ligation,
surrounded by dense fibrous connective tissue
(magnification 4×10, stained with hematoxylin and eosin).
Next to the ligated artery, there were several smaller collateral arteries that formed a kind of vascular bundle, with a wide lumen and thickened muscle membrane (Figure 3).
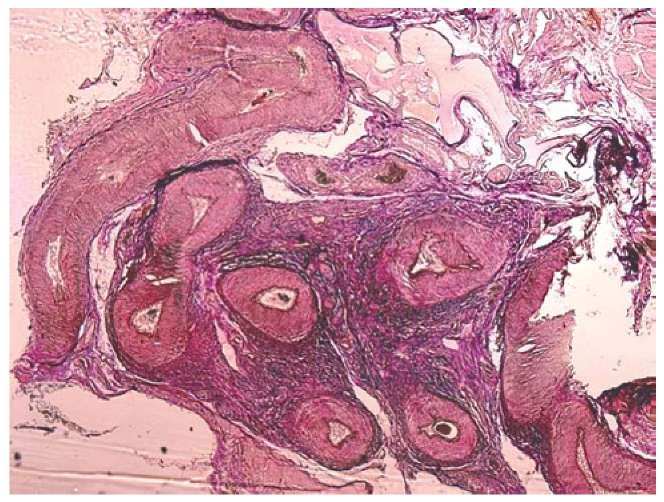
Figure 3. Arteries and veins in the form of a bundle,
surrounded by dense fibrous connective tissue
next to the ligated ovarian artery in group II
(magnification 2.5×10, Van Gieson picrofuchsin staining).
The OA structure was indistinguishable from the control at 1.0 cm distal to the intervention area.
The ovaries of Group II were also normal. Numerous primordial, primary, secondary and tertiary follicles, and yellow and white bodies localized in the ovarian cortex confirmed that the animals were ready for fertilization.
The IIA in Group I was 0.3–0.4 cm in diameter, had a wide lumen (0.2–0.3 cm in diameter), and was surrounded by loose fibrous connective tissue. As shown by the examination of the group II specimens, the IIA had a diameter of 0.25–0.3 cm, retained its lumen size (0.1–0.15 cm in diameter), but was deformed and surrounded by dense fibrous connective tissue within a radius of 0.1 cm (Figure 4 [a, b]).
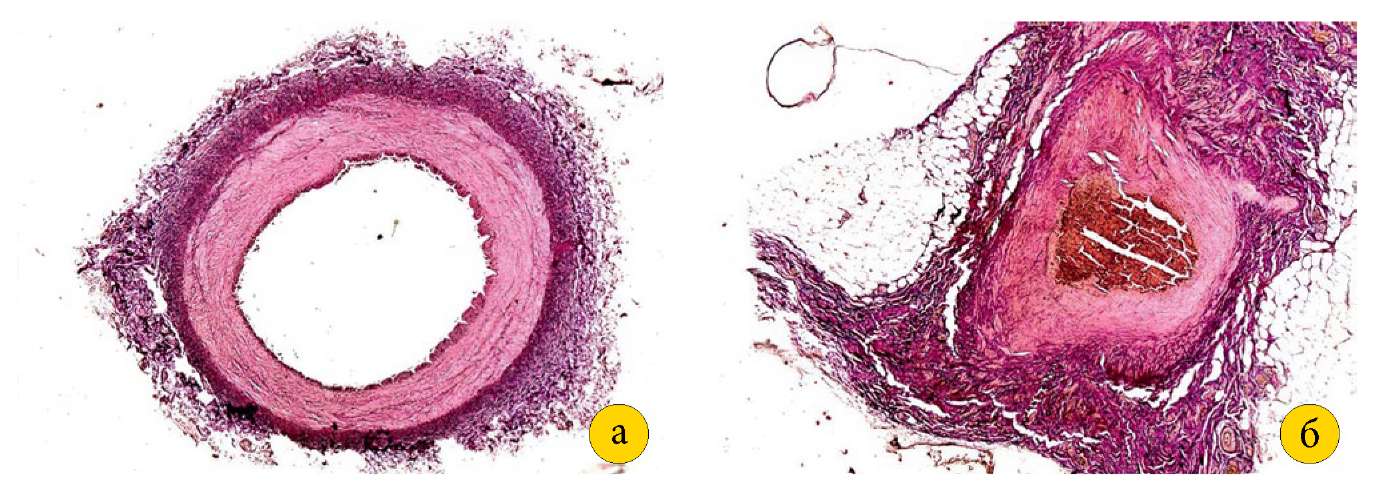
Fig. 4. A — internal iliac artery of a group I pig
(magnification 2.5×10, picrofuchsin staining according to Van Gieson).
B — internal iliac artery of a group II pig with wall deformation,
surrounded by dense fibrous connective tissue
(magnification 2.5×10, picrofuchsin staining according to Van Gieson).
Signs of collateralization within the vascular bed were observed in the oviducts in Group II animals. In the serous membrane, outside the attachment zone, there were "bundles" of vessels containing several arteries with a wide lumen and a thickened muscle membrane. The mucous membrane and middle layer were structurally similar to the control.
Discussion
Thus, a comparative analysis of the uterine body and horns microscopy in the intact pigs and in the animals with the bilateral ligation of the IIA and OA preserved the normal structure characteristic of the mature uterus. The oviducts, ovarian tissues and vessels remained structurally unchanged. All three observations in Group II showed numerous primordial, primary, secondary, and tertiary follicles, and yellow and white bodies localized in the ovarian cortex confirmed the animals to be fertile. All changes were observed within the IIA ligation, although the arteries retained their lumens, but were deformed and surrounded by dense fibrous connective tissue. The OAs also retained their lumens, but there were pronounced scar deformities around them and in the adjacent vessels. Blood supply was remarkable for the formation of smaller caliber arteries adjacent to the ligated OA, which provided collateral circulation. In addition, collateralization of the oviductal vascular bed was also reported in Group II animals.
The IIA and OA ligation produces only localized effects, such as perivascular fibrosis, wall deformation, and lumen narrowing. No vascular lumen obliterations were seen. In addition, collaterals were reported, i.e. a higher number of small caliber arteries around the ligated OA and in the serous membrane of the oviduct. Collateral blood flow was adequate, as no atrophic sites or deformities of the uterus, oviducts, or ovaries were specified.
Conclusion
The IIA and OA ligation produces only localized effects and does not pose a risk to the reproductive function of animals. This was evidenced by the presence of primordial, primary, secondary, and tertiary follicles, and yellow and white bodies in the ovaries of both groups. Collateral vessels supply the organs after the vascular ligation, which is confirmed by the absence of atrophic sites and deformities in the uterus with appendages.
1. Clinical Guidelines for Management of Postpartum Hemorrhage approved by the Ministry of Health of the Russian Federation (No.01-02/366 of May 24, 2021) Last Assessed: January 1, 2022 http://www.consultant.ru/document/cons_doc_LAW_410221/
2. Borisenko EA, Kisyora YuK. Bioethics: a collection of regulations governing biomedical research involving animals. 2015:39-47
3. http://publication.pravo.gov.ru/Document/View/0001201607070043
References
1. Baev O.R., Prikhodko A.M., Pestrikova T.Y., Fedorova T.A., Shmakov R.G. Action plan for early (primary) postpartum hemorrhages (following the clinical recommendations of the Ministry of Health of Russia «Preventive measures, management tactics, anesthesia and intensive care in postpartum hemorrhages, 2019»). Obstetrics and gynecology. 2019;(12S):3-8. (In Russ.) https://doi.org/10.18565/aig.2019.12suppl.3-8
2. Loginov A.V., Zainulina M.S. The golden pages in the russian history of obstetrics: from the times of ekaterine to the present. Obstetrics, Gynecology and Reproduction. 2017;11(2):69-74. (In Russ.) https://doi.org/10.17749/2313-7347.2017.11.2.069-074
3. Mitsiuk N.A., Pushkareva N.L. Formation of the system of clinical obstetrics in Russia (late 18th - early 20th centuries). History of medicine. 2021;7(2):153-160. (In Russ.) eLIBRARY ID: 48243235
4. Khayrullina G.R., Druzhkova E.B., Fatkullina L.S., Fatkullin F.I., Budyak Y.V. Efficiency of organ-saving operations conducted about early postpartum uterine bleeding and their influence on the quality of women’s lives. Medical Herald of the South of Russia. 2020;11(2):111-116. (In Russ.) https://doi.org/10.21886/2219-8075-2020-11-2-111-116
5. Tskhai V.B., Bakunina A.A. Spontaneous rupture of uterine blood vessels during pregnancy and childbirth. Literature. Women's health and reproduction. 2021;3(50)35-46. (In Russian) eLIBRARY ID: 47270787
6. Savelyeva G.M., Sukhikh G.T., Serova V.N., Radzinsky V.E. Obstetrics: national guidelines. 2019:867-882 (In Russian)
7. Mochalova M.N., Sidorkina A.G., Mudrov V.A. Modern methods for prediction and diagnosis of postpartum bleeding. Siberian Medical Review. 2022;(4):13-21. https://doi.org/10.20333/25000136-2022-4-13-21
8. Kamenskikh G.V., Novikova V.A., Olenev A.S. Modern trends of obstetric hemorrhages prevention. Practical Medicine. 2018;16(6):26-33. (In Russ.) https://doi.org/10.32000/2072-1757-2018-16-6-26-33
9. Tsitsishvili J.R. Ligation of the vessels of the uterus as a method of stopping atonic bleeding. Clinical and experimental study. 1961:45-51. (In Russ.)
10. Rembez N.N., Klinskaya N.P. Ligation of the main arteries of the small pelvis to stop bleeding. Lecture notes. 1964:67-94. (In Russ.)
11. Popesko P. Atlas of topographic anatomy of farm animals. Volume 2. Torso. 1974. (In Russ.)
About the Authors
M. R. KantsurovaRussian Federation
Maria R. Kantsurova – assistant of the Department of obstetrics and gynecology № 1
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
A. N. Rymashevsky
Russian Federation
Alexander N. Rymashevsky – Dr. Sci. (Med.), Professor, head of the Department of obstetrics and gynecology № 1
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
V. V. Voloshin
Russian Federation
Viktorovich V. Vladimir – Cand. Sci. (Med.), Associate Professor, Associate Professor of the Department of Pathological Anatomy
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
N. G. Sapronova
Russian Federation
Natalia G. Sapronova – Dr. Sci. (Med.), Professor, head of the Department of surgical diseases № 1
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
S. S. Todorov
Russian Federation
Sergey S. Todorov – Dr. Sci. (Med.), Associate Professor, Head of the Department of Pathological Anatomy
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
M. A. Rymashevsky
Russian Federation
Mikhail A. Rymashevsky – Cand. Sci. (Med.), assistant of the department of obstetrics and gynecology № 1
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
R. N. Kantsurov
Russian Federation
Roman N. Kantsurov – Can. Sci. (Med.), cardiovascular surgeon, Department of Vascular Surgery
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
Review
For citations:
Kantsurova M.R., Rymashevsky A.N., Voloshin V.V., Sapronova N.G., Todorov S.S., Rymashevsky M.A., Kantsurov R.N. Results of ligation the internal iliac and ovarian arteries in the experiment. Medical Herald of the South of Russia. 2023;14(4):77-82. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-4-77-82







































