Scroll to:
Polymorphisms of interferon γ and NKG2D receptor genes in predicting vertical transmission of HIV/HCV coinfection
https://doi.org/10.21886/2219-8075-2023-14-4-44-57
Abstract
Objective: to investigate single nucleotide polymorphisms in IFNγ gene variant rs2430561 and in NKG2D gene variant rs2617160 as potential risk factors for mother-to-child viral transmission among HIV/HCV-coinfected women.
Materials and methods: 65 mother-child pairs were examined, divided into 4 groups depending on the child's infection (HIV/ HCV infected, HIV or HCV infected, not infected). Methods: multiplex polymerase chain reaction (PCR), PCR for determining the viral load of HIV and HCV, flow cytometry for immunological studies, statistical analysis.
Results: the single nucleotide polymorphism in IFNγ gene variant rs2430561 had no prognostic value when determined in the mothers. When recording the TT and AT genotypes of a single nucleotide polymorphism in this gene in a child, it was combined with the probability of the child's infection with HIV/HCV, HIV or HCV, depending on the magnitude of the viral load of HIV and HCV in the mother's blood in different trimesters of pregnancy. The presence of the AA and AT genotypes of the NKG2D gene variant rs2617160 in the mothers had a prognostic value as contributing tothe child infection with HIV/HCV or HIV alone. It was noted that the mechanisms of perinatal viral transmission included a certain level of decline in the absolute number of CD4+ lymphocytes in the woman blood in the second and third trimesters of pregnancy.
Conclusions: the presence of AA and AT genotypes of the NKG2D gene variant rs2617160 in woman co-infected with HIV/HCV makes it possible to predict the risk of child infection not only during pregnancy, but also at the planning stage.
Keywords
For citations:
Khamatova A.A., Balmasova I.P., Chebotareva T.A. Polymorphisms of interferon γ and NKG2D receptor genes in predicting vertical transmission of HIV/HCV coinfection. Medical Herald of the South of Russia. 2023;14(4):44-57. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-4-44-57
Introduction
Infections caused by human immunodeficiency virus (HIV) and hepatitis C virus (HCV) remain one of the major global health problems. According to 2017 data, 2.3 million people living with HIV were simultaneously infected with HCV [1]. Particular attention of researchers in this area is focused on the problem of HIV/HCV co-infection among women of fertile age [2][3].
Data from a meta-analysis, including more than 900 sources from around the world, revealed that on average the proportion of pregnant women additionally infected with HCV was 2.4% in the general population of HIV-infected persons, [4]. In accordance with modern methodological approaches, determining the risk of vertical transmission of HCV in coinfected women requires regarding the HCV viral load and HIV status. In relation to HIV, it should be noted that the administration of highly active antiretroviral therapy and a low viral load are important prognostic factors for reducing the vertical transmission of this virus in recent years [5]. Some authors reported that the proportion of children born to mothers with HIV/HCV coinfection and infected with HCV was 5.2% in developed countries [6].
Currently, the role of the placenta as an epidemic risk factor for the vertical transmission of HIV and HCV in comorbid conditions is widely discussed [7]. The fact is that 15–35% of cases of perinatal transmission of the virus occur through transplacental pathways, including intrauterine and antenatal penetration ones; 50–75% of cases are due to infection during childbirth, and 10–20% during breastfeeding [8].
From the point of view of the significance of transplacental transmission of pathogens, the immune aspect in women deserves special attention. In particular, that is stipulated by the fact that HIV and, to a lesser extent, HCV belong to the virus category, which can exhibit an immunotropic effect [9][10]. Moreover, psychoactive substances, the abuse of which is a common concomitant of HIV and HCV infection, suppress immune responses and can affect viral pathogenesis [11].
In the light of investigation of the immune mechanism that is most important in the transplacental transmission of viruses, the population of innate immune cells, namely natural killer cells, deserves special attention. In the female reproductive organs, in particular, in the uterus (uNK) and in the decidua (dNK) at pregnancy, these lymphocytes produce a pool of tissue-resident natural killer cells with distinctive characteristics. This is related to the peculiarities of the immune system response to the development of pregnancy; since the fetus is semi-allogeneic for the mother, the pregnancy must induce mechanisms preventing its rejection [12].
The main functions of dNK are the control of trophoblast ingrowth into the decidua through the cytotoxic effects of these cells [13], as well as the production of cytokines, growth factors, angiogenic and other factors, which determine neoangiogenesis, tissue remodeling, and placental development. The importance of a high level of IFNγ produced by dNK is stipulated by its involvement in the formation of fetal resistance to viral infection [14].
The identical aspect of tissue-resident natural killer cells localized in the decidua of the uterus in the gestational process, which determines the state of the mother-fetus interface, has so far been beyond the attention of researchers. Meanwhile, the current state of scientific knowledge on this problem has been significantly expanded with findings suggesting the importance of this immune mechanism both in maintaining the physiological course of pregnancy and in the cellular immune protection of the fetus from the infection possibility [15].
The NKG2D receptor is one of the sensitive elements for the activation of natural killer cells, including those in the decidua of the uterus during pregnancy. In this case, its ligands are stress-induced molecules of the trophoblast and placenta, especially at their attack by viruses. In this context, IFNγ is the main secretory product of dNK [16].
Currently, there is evidence that polymorphism of the NKG2D gene affects susceptibility to infectious diseases, cancer, and autoimmune disorders [17], while the polymorphism of the allelic variant rs2617160 (TT genotype) of the NKG2D gene is the only marker and independent factor associated with susceptibility to chronic hepatitis B. Individuals with the TT rs2617160 genotype may demonstrate a significant decrease in natural cytotoxic activity during the elimination of hepatitis B virus (HBV) compared with carriers of the TA or AA genotypes, which contributes to the persistence of the virus and leads to chronic HBV infection [18].
Furthermore, it was found that the polymorphism of the allelic variant rs2430561 can affect the expression of the IFNγ gene [19], while the existence of alleles T and A at position +874 from the translation start site is associated with high and low production of IFNγ, respectively [20]. The latter fact was discovered already at the beginning of the 21st century. Polymorphisms in the non-coding regions of the IFNγ gene are associated with autoimmune disorders [21] and chronic infectious diseases [22], including the risk of chronic viral hepatitis B and C [23][24].
Data on assessing the significance of these single nucleotide polymorphisms in the transplacental transmission of HIV/HCV coinfection from mother to child could not be found in the available scientific literature.
The purpose of the study was the examination of single nucleotide polymorphisms of the rs2430561 allele of the IFNγ gene and the rs2617160 allele of the NKG2D gene as potential risk factors for intrauterine infection of a child born to a mother coinfected with HIV/HCV.
Materials and methods
The study included 65 mother-child pairs registered in the general outpatient and pediatric outpatient departments of the Moscow City AIDS Prevention and Control Center (MGC AIDS) of the Moscow State Budgetary Healthcare Institution “Infectious Clinical Hospital No. 2 of the Moscow Health Department” in the period from 2004 to 2021.
All women under clinical supervision were warned about participation in the study and signed informed voluntary consent in accordance with the principles of the Helsinki Declaration of the World Medical Association.
The age of mothers was from 17 to 49 years, and the age of children was from 6 months to 18 years. All mothers, without exception, were coinfected with HIV/HCV, HIV infection was at stage 3-4B. The mother-child pairs included in the study were divided into 4 groups according to perinatal transmission of viruses to the child:
- group 1: mother-child pairs with perinatal infection of the child with HIV and HCV, 9 pairs (14%);
- group 2: mother-child pairs with perinatal HIV infection of the child, 12 pairs (18%);
- group 3: mother-child pairs with perinatal HCV infection of the child, 17 pairs (26%);
- group 4: mother-child pairs with an inconclusive test for HIV in the child and the absence of HCV, 27 pairs (42%).
Dependence on taking injectable psychoactive drugs, including a history of it, in each group was revealed by 21% to 31% of women.
The study was prospective-retrospective in nature. The study protocol is presented in Figure 1.
All mothers and their children were assessed for single nucleotide polymorphisms of the IFNγ and NKG2D genes using multiplex polymerase chain reaction (PCR). PCR was carried out on the basis of the PCR analyzer “Abbott m2000rt” (Abbott Molecular Inc., USA) and the automated station for sample preparation and isolation of nucleic acids and proteins “QI Asymphony SP” (QIAGEN GmbH, Germany) in accordance with the user's manual for the devices.
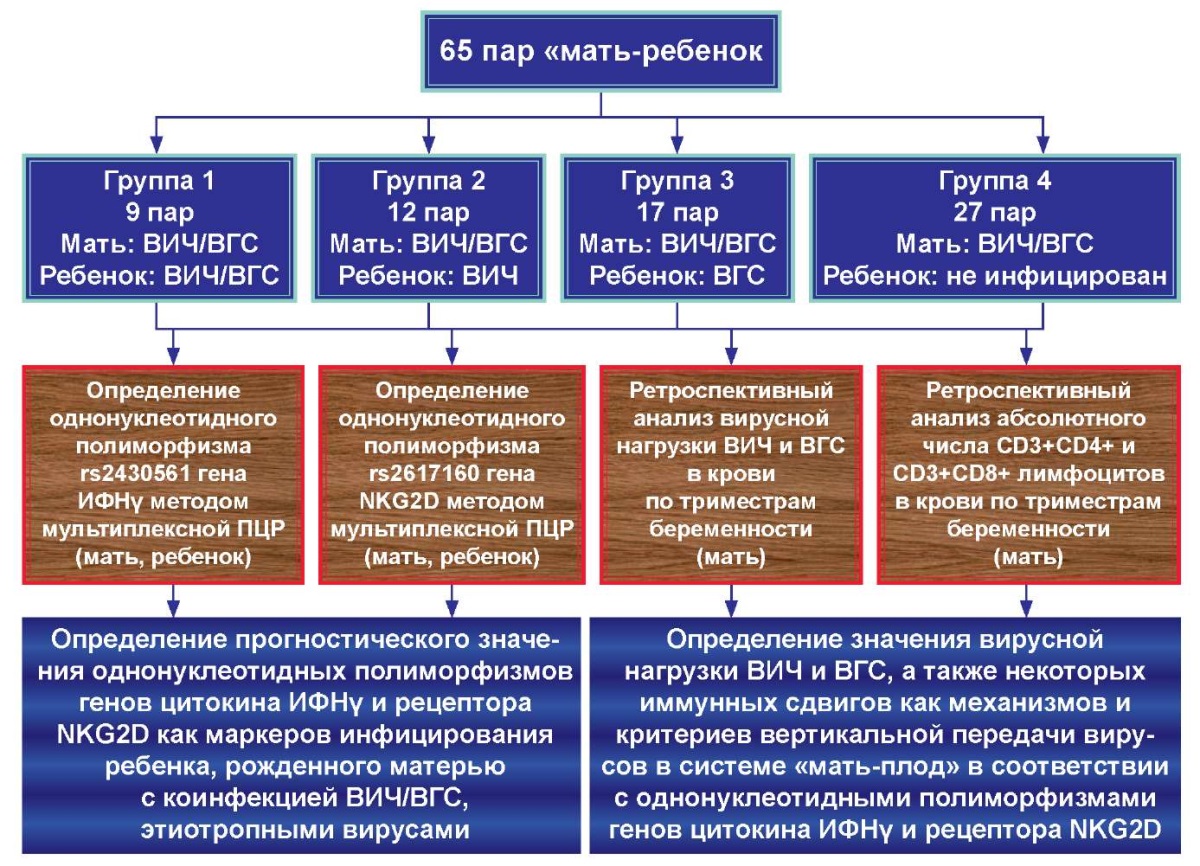
Figure 1. Study protocol.
At the preanalytical stage, venous blood was collected into tubes with the potassium salt of ethylenediaminetetraacetic acid (EDTA) to prevent coagulation; after mixing, 250 μl of whole blood was collected into labeled tubes and the fraction of leukocyte cells was washed using the reagent “HEMOLYTIC” (RU No. FSR 2010/09505 dated 09/04/19, AmpliSens LLC). The resulting cell suspension was taken to isolate total DNA using the AmpliPrime DNA-sorb-V reagent kit (RU No. FSR 2009/05220 dated 05/03/2019, NextBio LLC).
After DNA extraction, a study was carried out to identify single nucleotide polymorphisms using multiplex PCR. To determine polymorphisms in the NKG2D gene, the “TaqMan” reagent kit manufactured by Applied Biosystems was used; for genotyping polymorphic markers in the IFNg gene, the “rs2430561 IFNG gene” reagent kit manufactured by TestGen (series: 202111-411) was used.
This study analyzed retrospective data from outpatient monitoring cards of mothers in each trimester of pregnancy to identify their HIV and HCV viral loads assessed by PCR. In addition, using the results of an immunological study carried out by flow cytometry, the absolute number of lymphocytes with phenotypes CD3+CD4+ (T-helper) and CD3+CD8+ (cytotoxic T-lymphocytes, CTL) was determined to calculate the immunoregulatory index (IRI) as the ratio of these cells (CD3+CD4+/CD3+CD8+).
Statistical data processing was performed using the SPSS statistical software package (version 23). For statistical processing of frequency data, one-way analysis of variance (ONE WAY ANOVA) was used. The homogeneity or heterogeneity of the distribution of the occurrence frequency of a trait in the compared groups was assessed with the Fisher criterion. Virological and immunological data were analyzed based on determining their 95% confidence intervals of numerical values of indicators and their statistical comparison using the χ2 test at p<0.05.
Results
The results of the investigation of the representation of various variants of single nucleotide polymorphism in the allelic variant rs2430561 of the IFNγ gene in both mother and child are presented in Table 1.
As it follows from the data in Table 1, determining the prognostic value of single nucleotide polymorphism of one of the alleles of the IFNγ gene showed ambiguous results. In particular, intergroup comparisons of the frequency of occurrence of this polymorphism in pregnant women coinfected with HIV/HCV revealed the absence of influence of this factor on the perinatal infection of the child. Meanwhile, the same single nucleotide polymorphism in a child turned out to be significant in the development of perinatal infection with HIV or HCV, including the two viruses simultaneously.
Thus, infection of a child with HIV as well as HIV/HCV is associated with the frequency of occurrence of the polymorphism of the rs2430561 allele in the IFNγ gene. In infected children, the AA variant was registered significantly less often than in children born healthy. The occurrence of the AT variant in infected children in both groups was higher. Approximately a quarter of children infected with HIV had a TT variant that was not found in healthy children, and therefore, the existence of this variant can presumably be considered a risk factor for perinatal HIV infection.
The identical analysis using comparative statistics showed that in the group of children infected with HCV, the AA variant was also registered less frequently than in healthy children; the TT variant was not registered at all in any of the comparison groups; the AT variant was determined 2.2 times more often in the group children infected with HCV, and presumably, it can serve as a nonspecific marker of infection with this virus.
Table 1
Correlation of the frequency
of various single nucleotide polymorphisms in the IFN-γ gene variant rs2430561
in different mother-child groups
|
“Mother – child” group |
Polimorphism variant (pers./%) |
ONE WAY ANOVA |
|||
|
AA |
AT |
TT |
F |
p |
|
|
Single nucleotide polymorphism in the mother, HIV/HCV perinatal infection of the child |
|||||
|
Group 1 – Mother:HIV/HCV; child:HIV/HCV |
- |
6 persons 67% |
3 persons 33% |
3.795 |
0.060 |
|
Group 4 – Mother: HIV/HCV; child: healthy |
12 persons 44% |
9 persons 33% |
6 persons 23% |
||
|
Single nucleotide polymorphism in the mother, HIV perinatal infection of the child |
|||||
|
Group 2 – Mother: HIV/HCV; child: HIV |
6 persons 50% |
6 persons 50% |
- |
1.206 |
0.279 |
|
Group 4 – Mother: HIV/HCV; child: healthy |
12 persons 44% |
9 persons 33% |
6 persons 23% |
||
|
Single nucleotide polymorphism in the mother, HCV perinatal infection of the child |
|||||
|
Group 3 – Mother: HIV/HCV; child: HCV |
11 persons 65% |
3 persons 17,5% |
3 persons 17,5% |
1.005 |
0.322 |
|
Group 4 – Mother: HIV/HCV; child: healthy |
12 persons 44% |
9 persons 33% |
6 persons 23% |
||
|
Single nucleotide polymorphism in the child, HIV/HCV perinatal infection of the child |
|||||
|
Group 1 – Mother:HIV/HCV; child:HIV/HCV |
3 persons 33% |
6 persons 67% |
- |
6.188 |
0.023* |
|
Group 4 – Mother: HIV/HCV; child: healthy |
18 persons 67% |
9 persons 33% |
- |
||
|
Single nucleotide polymorphism in the child, HIV perinatal infection of the childGroup 2 – Mother: HIV/HCV; child: HIV |
|||||
|
3 persons 25% |
6 persons 50% |
3 persons 25% |
11.385 |
0.002* |
|
|
Group 4 – Mother: HIV/HCV; child: healthy |
18 persons 67% |
9 persons 33% |
- |
||
|
Single nucleotide polymorphism in the child, HCV perinatal infection of the child |
|||||
|
Group 3 – Mother: HIV/HCV; child: HCV |
5 persons 29% |
12 persons 71% |
- |
7.281 |
0.015* |
|
Group 4 – Mother: HIV/HCV; child: healthy |
18 persons 67% |
9 persons 33% |
- |
||
Note: F – Fisher criterion,
p – the probability of differences in data distribution according to Fisher criterion,
* – statistical significance of intergroup differences at p<0.05
Further, single nucleotide polymorphisms of the rs2617160 allele of the NKG2D gene were investigated in the mother and child by comparing data from examined groups consisting of the infected children (groups 1, 2, 3) with group 4, which did not contain perinatally infected children. The results of this study are presented in Table 2.
Table 2
Correlation of the frequency
of various single nucleotide polymorphisms in the NKG2D gene variant rs2617160
in different mother-child groups
|
“Mother – child” group |
Polimorphism variant (pers./%) |
ONE WAY ANOVA |
|||
|
AA |
AT |
TT |
F |
p |
|
|
Single nucleotide polymorphism in the mother, HIV/HCV perinatal infection of the child |
|||||
|
Group 1 – Mother:HIV/HCV; child:HIV/HCV |
3 persons 33% |
6 persons 67% |
- |
7.286 |
0.011* |
|
Group 4 – Mother: HIV/HCV; child: healthy |
- |
12 persons 50% |
12 persons 50% |
||
|
Single nucleotide polymorphism in the mother, HIV perinatal infection of the child |
|||||
|
Group 2 – Mother: HIV/HCV; child: HIV |
6 persons 50% |
3 persons 25% |
3 persons 25% |
5.165 |
0.029* |
|
Group 4 – Mother: HIV/HCV; child: healthy |
- |
12 persons 50% |
12 persons 50% |
||
|
Single nucleotide polymorphism in the mother, HCV perinatal infection of the child |
|||||
|
Group 3 – Mother: HIV/HCV; child: HCV |
- |
8 persons 47% |
9 persons 53% |
1.038 |
0.314 |
|
Group 4 – Mother: HIV/HCV; child: healthy |
- |
12 persons 50% |
12 persons 50% |
||
|
Single nucleotide polymorphism in the child, HIV/HCV perinatal infection of the child |
|||||
|
Group 1 – Mother:HIV/HCV; child:HIV/HCV |
3 persons 33% |
6 persons 67% |
- |
9.466 |
0.004* |
|
Group 4 – Mother: HIV/HCV; child: healthy |
3 persons 11% |
9 persons 33% |
15 persons 56% |
||
|
Single nucleotide polymorphism in the child, HIV perinatal infection of the child |
|||||
|
Group 2 – Mother: HIV/HCV; child: HIV |
6 persons 50% |
- |
6 persons 50% |
2.462 |
0.125 |
|
Group 4 – Mother: HIV/HCV; child: healthy |
3 persons 11% |
9 persons 33% |
15 persons 56% |
||
|
Single nucleotide polymorphism in the child, HCV perinatal infection of the child |
|||||
|
Group 3 – Mother: HIV/HCV; child: HCV |
3 persons 18% |
6 persons 35% |
8 persons 47% |
0.446 |
0.508 |
|
Group 4 – Mother: HIV/HCV; child: healthy |
3 persons 11% |
9 persons 33% |
15 persons 56% |
||
Note: F – Fisher criterion,
p – the probability of differences in data distribution according to Fisher criterion,
* – statistical significance of intergroup differences at p<0.05
As it follows from Table 2, there is reason to suggest a relationship between the single nucleotide polymorphism of the rs2617160 allelic variant of the NKG2D gene and perinatal infection of the child with HIV and/or HCV if the mother has HIV/HCV co-infection. This polymorphism is important for both the mother and the child.
Indicatively, one-way analysis of variance revealed a relationship between single nucleotide polymorphism of the rs2617160 allele of the NKG2D gene in the mother and perinatal infection of the child with HIV and HCV simultaneously, as well as HIV alone. In this respect, the AA variant was recorded only in mothers with HIV/HCV co-infection, whose children were perinatally infected, and its frequency of occurrence was approximately a third of women in group 1 and a half of women in group 2 who were under observation. The remaining women were carriers of the AT variant, the frequency of which was not significantly different from that in group 4 consisted of healthy born children. The TT variant was recorded only in group 4.
The existence of AT and, to a lesser extent, AA genotypes of allele rs2617160 of the NKG2D gene was also typical for children perinatally coinfected with HIV/HCV, although this fact had no prognostic significance.
Thus, perinatal infection of a child with HIV (isolated or simultaneously with HCV) was associated with the existence of a single nucleotide polymorphism in the form of AT or AA genotypes of the rs2617160 allele of the NKG2D gene in a mother coinfected with HIV/HCV, which can be interpreted as a possibility of predicting the risk of a child becoming infected with HIV or HIV/HCV even before pregnancy.
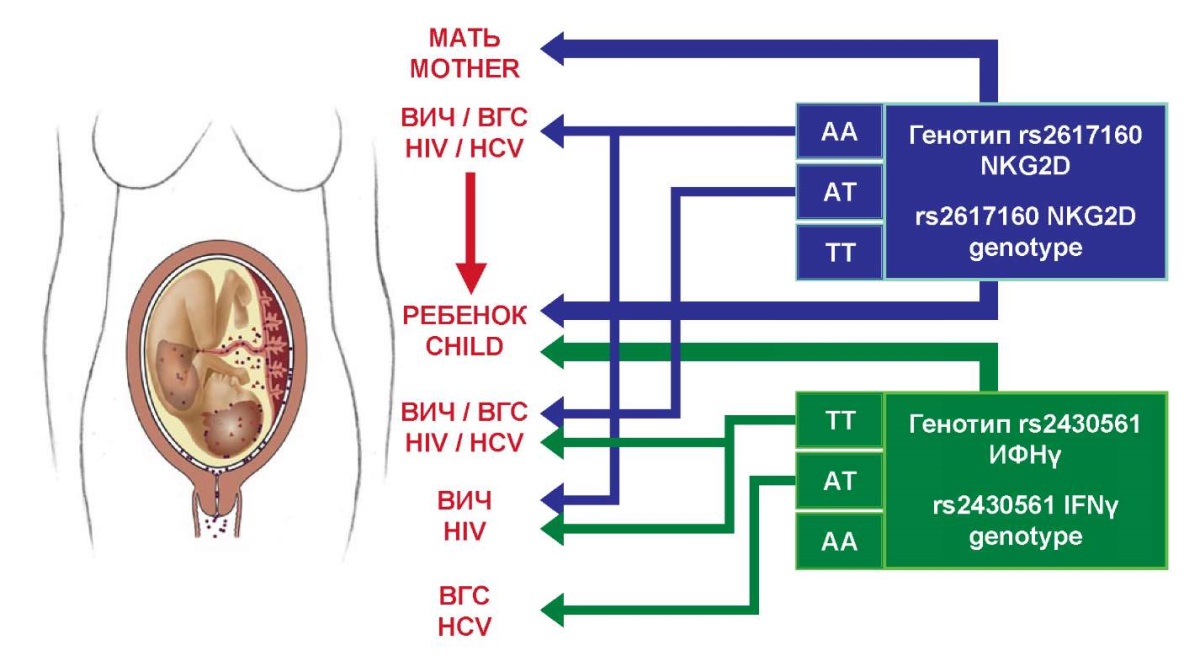
Figure 2. The relationship between IFNγ and NKG2D genes polymorphisms
and perinatal child infection with HIV and HCV.
A summary diagram of the relationship between the examined polymorphisms and the possibility of a child becoming infected with HIV and HCV is presented in Figure 2. The figure illustrates that the examined polymorphism of the IFNγ gene is significant only when detected in a child, that is, essentially it does not have prognostic significance without an invasive method of sampling for research. From this point of view, the NKG2D gene polymorphism is more promising, since it is informative when detected in both mother and child that requires further confirmation at the population level.
In order to elucidate the main mechanisms that determine the prognostic significance of these polymorphisms for intrauterine infection of the fetus, the viral load of HIV and HCV, as well as the absolute content of subpopulations of T-helper cells (CD3+CD4+) and cytotoxic T-lymphocytes (CTL, CD3+CD8+) were estimated in the blood of mothers of the examined groups in each trimester with the subsequent calculation of the immunoregulatory index as the ratio CD3+CD4+/CD3+CD8+.
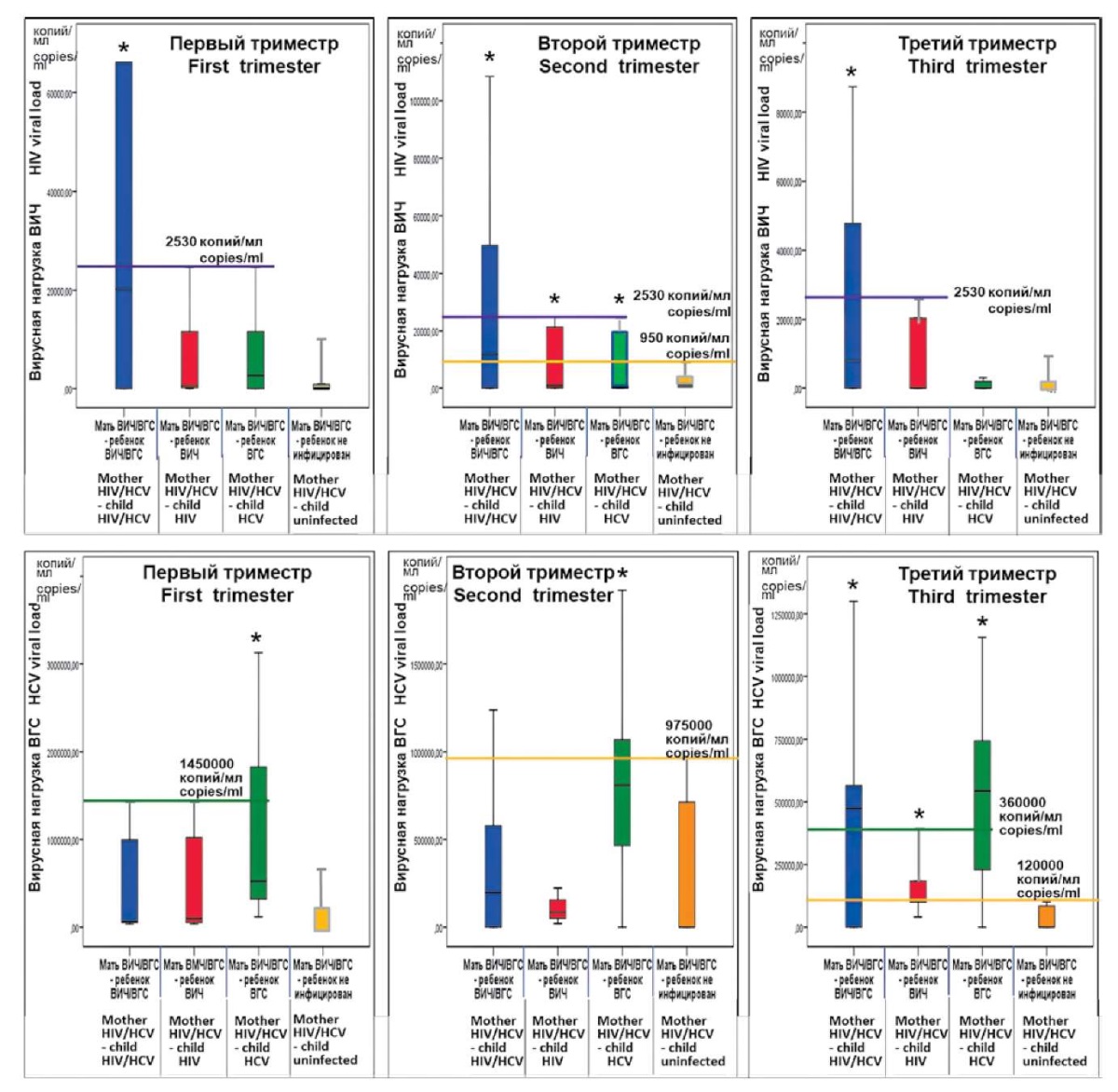
Figure 3. 95% confidence intervals of HIV (top row) and HCV (bottom row) viral loads
in different pregnancy trimesters in women of the study groups
(* — statistical significance of the difference from control group 4).
The results of the estimation of the viral load of HIV and HCV in the blood of pregnant women of different examined groups in the form of 95% confidence intervals of the indicators are presented in Figure 3.
The data show that the viral load of HIV or HCV in pregnant women with HIV/HCV coinfection seems to have a prognostic value although it manifests itself differently for each virus and depends on the pregnancy trimester.
In the second trimester of pregnancy, the viral load of HIV in groups where the child was perinatally infected with HIV, HCV, or both viruses (group 1–3) was statistically different from this indicator in group 4, where vertical transmission of viruses from the mother did not occur. Thus, HIV viral load indicators in a woman, coinfected with HIV/HCV, in the second trimester of pregnancy make it possible to determine the risk of infection of the unborn child if the HIV viral load is above 950 copies/ml. In the first and third trimesters, at the same criterion level of viral load (>950 copies/ml), the prognostic value of this indicator applies only to group 1, in which children coinfected with HIV/HCV were born.
Indicators of the HCV viral load in a coinfected woman were informative for the prognosis of perinatal HCV infection of the child when they exceeded 620,000 copies/ml in the first trimester of pregnancy or exceeded 975,000 copies/ml in the second trimester; in the third trimester of pregnancy if these values were above 120,000 copies/ml, any variant of infection was possible for the child.
Characteristics of immunological data, namely subpopulations of T-lymphocytes in the blood of pregnant women with HIV/HCV coinfection in accordance to the examined groups as 95% confidence intervals of indicators are given in Figure 4.
The main feature of immunological changes in pregnant women coinfected with HIV/HCV related to the ability to provoke intrauterine infection of the fetus is the absence of intergroup differences for each indicator in the first trimester of pregnancy.
In the second trimester, all three indicators revealed statistically significant changes. In particular, there was a decrease in the absolute number of CD3+CD4+ lymphocytes (T-helper cells) below 290 cells × 109/l in group 2, which made this group with perinatal HIV infection of children significantly different from group 4 with no infection. This decline led to a statistically significant fall in the immunoregulatory index below 0.27 in group 2. In group 3, with perinatal infection of a child with HCV, in the second trimester of pregnancy, there was a decrease in the absolute number of cytotoxic T-lymphocytes (CD3+CD8+) below 680 cells 109/l.
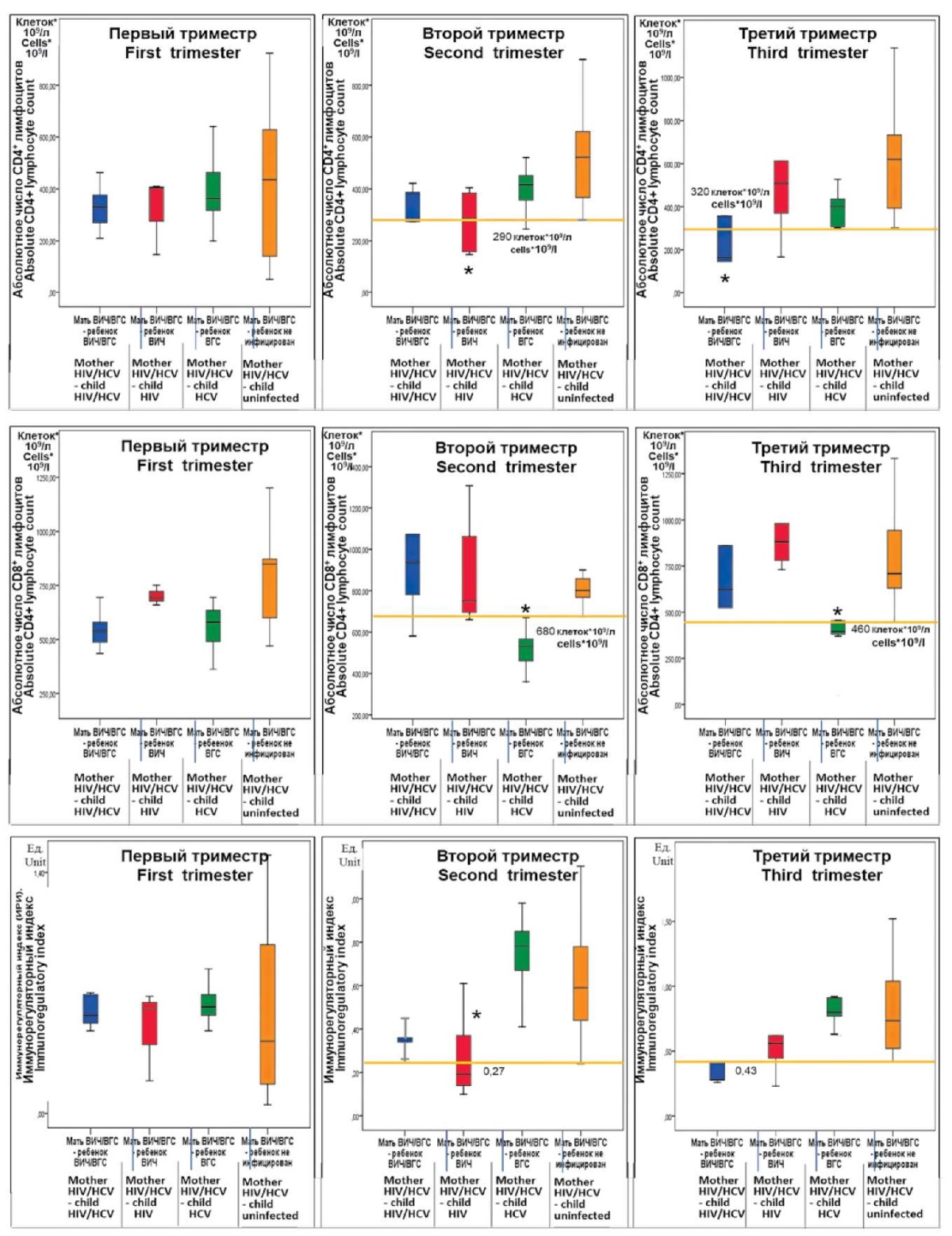
Figure 4. 95% confidence intervals of immunological parameters
in different pregnancy trimesters in women of the study groups
(* — statistical significance of difference from control group 4).
In the third trimester of pregnancy, immunological discrepancies from control were somewhat different and manifested in various manners in the examined groups. In group 1 where the children were perinatally infected with HIV/HCV, the values of C3+CD4+ T-lymphocytes were below 320 cells × 109/l, and the immunoregulatory index decreased to 0.43. A drop in the absolute number of CD3+CD8+ cytotoxic T lymphocytes below 480 cells × 109/l was recorded in group 3, in which children were infected with HCV.
Thus, in mothers co-infected with HIV/HCV, various changes were found in both the viral load of HIV and HCV, and immunological parameters, which differed significantly among each other in the examined groups and manifested in various manners in the trimesters of pregnancy.
Summarizing the findings, it becomes obvious that several factors are important for the perinatal infection of a child. They include, in particular, the state of the mother’s body, as evidenced by shifts in immunological indicators that affect the pattern of infection in different groups. In addition, this process depends on the state of the interface “mother-fetus” (placenta), and, perhaps, on the condition of the fetus’s body, capable of counteracting the transition of viral agents from the mother to fetus in accordance with the level of viral load of pathogens.
In accordance with the assumptions made, parallels were drawn between single nucleotide polymorphisms of the IFNγ and NKG2D genes and the identified shifts in the studied parameters. First, this was done in relation to the viral load of HIV and HCV, and second, in relation to immunological parameters (Figure 5).
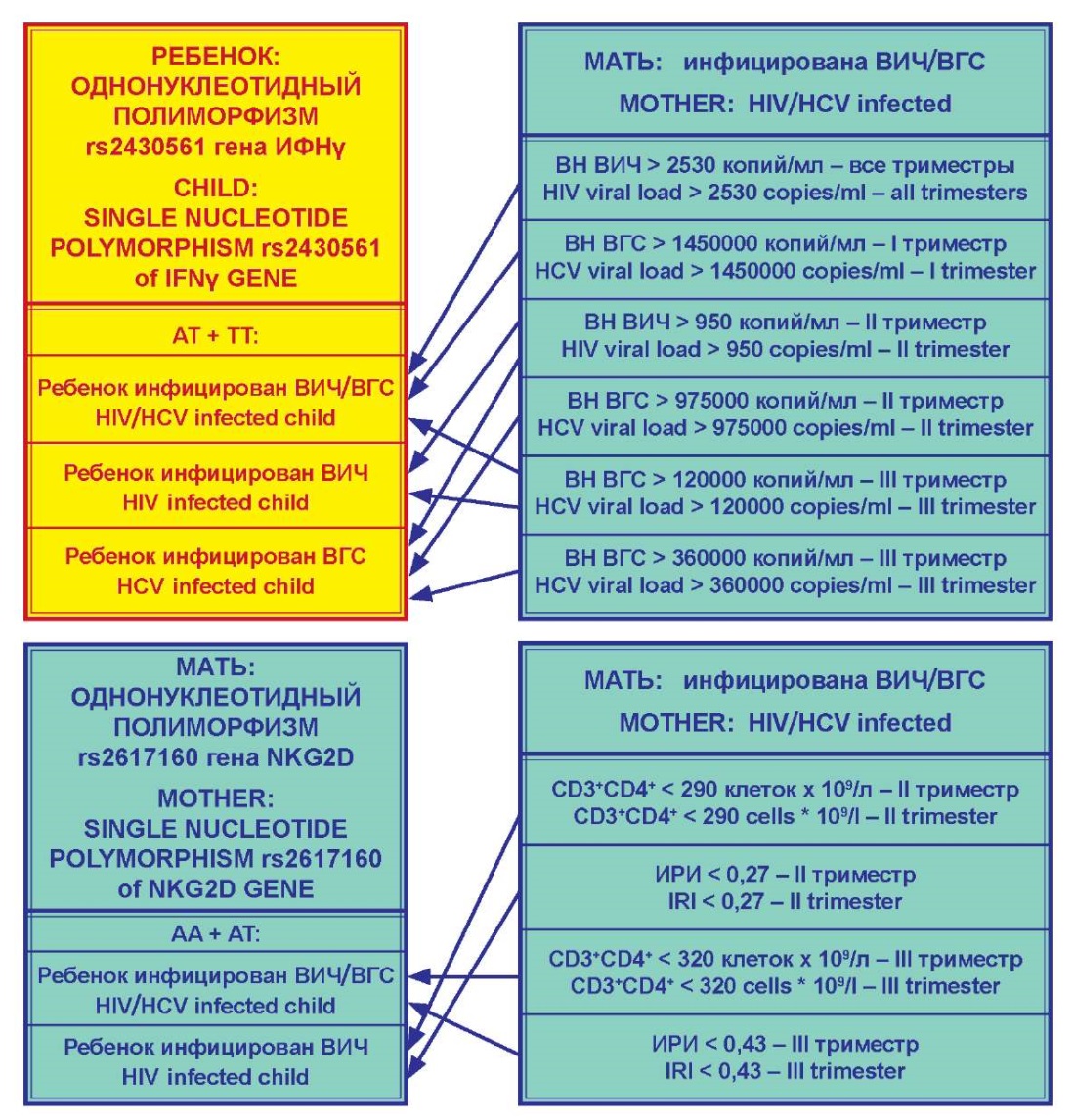
Figure 5. Correspondence of polymorphisms
in the IFN-γ gene in the child and the NKG2D gene in the mother
to the virological and immunological parameters of pregnant women.
As shown above, single nucleotide polymorphism of the IFNγ gene in the allelic variant rs2430561, which was considered in the context of predicting the risk of having an infected child in a mother co-infected with HIV/HCV, did not have a prognostic value when determined in a pregnant woman. Meanwhile, the existence of this single-nucleotide polymorphism in a child was connected with the possibility of infection. Nevertheless, this fact cannot have a prognostic value, as determining this trait in the fetus during pregnancy would require an invasive intervention that would contribute to its infection. However, in the aspect of elucidating the mechanism of a child infection from an infected mother, this is of some interest. Immunological indicators of a mother as a risk factor for child infection were not considered, since the cellular elements of the immune systems of the mother and fetus function separately due to the placental barrier, although indirectly the immune factors have an impact on the viral load with HIV and HCV. In the authors’ opinion, investigation of the viral load of HIV and HCV by itself in the mother’s blood was appropriate in this case, taking into account the capacity of the viruses for vertical transmission.
As follows from Figure 4, coinfection of a child having the TT and AT genotypes of the rs2430561 allele in the IFNγ gene with HIV/HCV was highly probable if the mother, who was coinfected with HIV/HCV, had an HIV viral load of at least 2530 copies/ml during the entire pregnancy. These conditions must be added by the HCV viral load in the mother exceeding 1,450,000 copies/ml in the first trimester and 120,000 copies/ml in the third trimester.
The risk of a child becoming infected with HIV at the same single nucleotide polymorphism variant was associated with other conditions. In particular, when in the second trimester of pregnancy, the maternal HIV viral load was more than 950 copies/ml and in the third trimester, the HCV viral load exceeded 120,000 copies/ml. In this regard, it is interesting that HIV infection required not only a certain load of this virus in the mother’s blood in the second third of the gestational process but also a certain level of coinfecting hepatitis C virus in the blood in the later stages of pregnancy.
In this study, the risk of HCV infection in a child, having the TT and AT genotype of the rs2430561 allele of the IFNγ gene, was identified against the background of the HIV viral load above 950 copies/ml in the second trimester, as well as the HCV viral load above 975,000 copies/ml in the second trimester and above 360,000 copies/ml in the third trimester.
Assessing the prognostic significance of the single nucleotide polymorphism of the rs2617160 allelic variant of the NKG2D gene, it should be noted that if it is confirmed at the level of population studies, these data deserve wide implementation in clinical practice. This will make it possible, when identifying the AA and AT genotypes of the rs2617160 allele of the NKG2D gene in a woman of reproductive age with HIV/HCV co-infection, to classify her as a risk group for the possibility of a child becoming infected with either HIV or HIV/HCV. In this aspect, immunological signs of risk could be the absolute number of CD3+CD4+ lymphocytes and the value of IRI in the blood of a pregnant woman. Indicatively, the number of CD3+CD4+ lymphocytes less than 290 cells·109/l and the IRI less than 0.27 in the second trimester of pregnancy create a threat of HIV infection to the child, while the number of CD3+CD4+ lymphocytes less than 320 cells·109/l and the IRI less than 0.43 in third trimester attest the possibility for a child to become infected with HIV/HCV. The data obtained are original and promising from the standpoint of determining this risk group not only during pregnancy but also at the stage of its planning, focusing on the results of determining the single nucleotide polymorphism of the allelic variant rs2617160 of the NKG2D gene.
Discussion
The data obtained attest to the prognostic significance of single-nucleotide polymorphisms of the NKG2D gene, which partly reflects the level of the activation processes of natural killer cells in pregnant women coinfected with HIV/HCV. The results largely confirm the working hypothesis that the features of this important population of innate immune lymphocytes, which play a significant role in the formation of the placenta as an interface in the mother-fetus system, give an idea of the state of the barrier for the penetration of viral pathogens into the child’s body. In addition, the results demonstrate the importance for the infection of a child of such indicators in the blood of a pregnant woman as the viral loads of HIV and HCV, and the level of reduction in the number of CD3+CD4+ lymphocytes, which are predominantly impaired by HIV.
A number of the noted phenomena require interpretation, which is not entirely easy to do on the basis of the contradictory data available in the scientific literature. This is especially true when a child is infected with HIV or HCV against the background of an increase in the viral load of both pathogens simultaneously.
At present, there is information available that HIV significantly increases the vertical transmission rate of HCV. Evidence of the effect of HCV on the progression of HIV infection is less convincing. In particular, there are certain studies, showing that coinfection with HCV reduces the efficacy of combination antiretroviral therapy [25]. Since in this study, pregnant women received antiretroviral therapy, which may be one of the explanations for the established fact of a child infection with HIV when the HCV viral load increased.
The statement about the role of an increase in the viral load of HIV, along with HCV, for the infection of a child with the latter pathogen is largely contradictory. In particular, the risk of vertical transmission of HCV is believed to be associated with high maternal HCV RNA levels, but not with HIV load [26]. However, there is evidence that the incidence of hepatitis C virus transmission from women coinfected with HIV/HCV is reduced when the HIV viral load is well controlled [27]. All this information was obtained by researchers without regard to the polymorphism of the IFNγ gene in infected children, which is yet more proof of the significance of data revealed in this study and their prospects for further investigation.
Data on the prognostic value of the NKG2D gene polymorphism in pregnant women coinfected with HIV/HCV for infection of the child are more certain, especially in terms of the identified correspondence between this polymorphism and the level of reduction in the amount of CD3+CD4+ lymphocytes in the blood. There is scientific information about the role of a decrease in the number of these cells (below 350) in the vertical transmission of the pathogen [28][29]. It is debatable why, if a pregnant woman with HIV/HCV co-infection has a polymorphism of this gene, the child can be infected with HIV/HCV or only HIV, but not HCV. Meanwhile, when analyzing immunological data without their connection with the polymorphism of the NKG2D gene, it was noted that infection of a child with HCV was possible under the condition of a decrease in cytotoxic T-lymphocytes (CD3+CD8+) in a pregnant woman. The authors did not find available information that explains this phenomenon. One can assume that control of HCV is carried out both by the antiviral adaptive immune response with CTLs and by the innate immunity with natural killer cells. A decrease in the amount or functional activity of one of these cellular elements disrupts their ratio, which entails a diminution in control over HCV reproduction.
Conclusions
- Determination of the single nucleotide polymorphism of the IFNγ gene in the allelic variant rs2430561 in a pregnant woman has no prognostic significance.
- Single nucleotide polymorphism of the IFNγ gene on the allelic variant rs2430561 when registering the TT and AT genotypes in a child is combined with the possibility of infection under certain conditions. First, HIV/HCV transmission is possible if the mother’s HIV viral load is at least 2,530 copies/ml throughout the pregnancy, while the HCV viral load exceeds 1,450,000 copies/ml in the first trimester and 120,000 copies/ml in the third trimester. Second, HIV transmission is possible if the mother's HIV viral load exceeds 950 copies/ml in the second trimester of pregnancy, and the HCV viral load exceeds 120,000 copies/ml in the third trimester. Third, HCV transmission is possible if in the second trimester, the mother's HIV viral load is above 950 copies/ml and the HCV viral load is more than 975,000 copies/ml; while in the third trimester, the HCV viral load exceeds 360,000 copies/ml.
- Registration of the AA and AT genotypes of the single nucleotide polymorphism of the allelic variant rs2617160 of the NKG2D gene in a pregnant woman allows her to be classified as a risk for the possibility of the child becoming infected with either HIV or HIV/HCV.
- Registration of the AA and AT genotypes of the single nucleotide polymorphism of the allelic variant rs2617160 of the NKG2D gene in a pregnant woman is associated with intrauterine infection of the child under certain conditions. First, HIV transmission is possible if the absolute number of CD3+CD4+ lymphocytes in the mother is less than 290 cells·109/l, and the immunoregulatory index is less than 0.27 in the second trimester of pregnancy. Second, HIV/HCV transmission is possible if the absolute number of CD3+CD4+ lymphocytes in the mother is less than 320 cells·109/l, and the immunoregulatory index is less than 0.43 in the third trimester of pregnancy.
- The polymorphism of the IFNγ and NKG2D genes affects the mechanisms of development of the immune response and the level of viral load at co-infection with HIV and HCV in a pregnant woman, which determines the risk of perinatal infection of the child.
References
1. Abiodun OE, Adebimpe O, Ndako JA, Oludoun O, Aladeitan B, Adeniyi M. Mathematical modeling of HIV-HCV coinfection model: Impact of parameters on reproduction number. F1000Res. 2022;11:1153. https://doi.org/10.12688/f1000research.124555.2
2. Yastrebova E.B., Gutova L.V. Children born with HIV: developmental problems and opportunities for a healthy life. HIV Infection and Immunosuppressive Disorders. 2016;8(4):94. (In Russ.)
3. Baroncelli S, Pirillo MF, Amici R, Tamburrini E, Genovese O, et al. HCV-HIV coinfected pregnant women: data from a multicentre study in Italy. Infection. 2016;44(2):235-42. https://doi.org/10.1007/s15010-015-0852-0
4. Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797-808. https://doi.org/10.1016/S1473-3099(15)00485-5
5. Cimpoca B, Panaitescu AM, Gica N, Veduta A, Ciobanu A. Risk of vertical transmission of chronic viral infections after invasive prenatal procedures. Ginekol Pol. 2022. Epub ahead of print. PMID: 35072256. https://doi.org/10.5603/GP.a2021.0196
6. Dieye NL, Varol M, Zorich SC, Millen AE, Yu KOA, Gómez-Duarte OG. Retrospective analysis of vertical Hepatitis C exposure and infection in children in Western New York. BMC Gastroenterol. 2023;23(1):242. https://doi.org/10.1186/s12876-023-02871-8
7. Oboskalova T.A., Prokhorova O.V., Vorontsova A.V. HIV infection and pregnancy: a textbook. Ekaterinburg: USMU; 2019. (in Russ.).
8. Niauri D.A., Kolobov A.V., Tsinzerling V.A., Gzgzyan A.M., Dzhemlikhanova L.K., et al. The placenta as the epidemic factor of vertical HIV transmission risk in conditions of comorbidity. HIV Infection and Immunosuppressive Disorders. 2016;8(4):7-16. (In Russ.) https://doi.org/10.22328/2077-9828-2016-8-4-7-16
9. Milligan C, Slyker JA, Overbaugh J. The Role of Immune Responses in HIV Mother-to-Child Transmission. Adv Virus Res. 2018;100:19-40. https://doi.org/10.1016/bs.aivir.2017.10.001
10. Stuart JD, Salinas E, Grakoui A. Immune system control of hepatitis C virus infection. Curr Opin Virol. 2021;46:36-44. https://doi.org/10.1016/j.coviro.2020.10.002
11. Blackard JT, Sherman KE. Drugs of Abuse and Their Impact on Viral Pathogenesis. Viruses. 2021;13(12):2387. https://doi.org/10.3390/v13122387
12. Vacca P, Vitale C, Munari E, Cassatella MA, Mingari MC, Moretta L. Human Innate Lymphoid Cells: Their Functional and Cellular Interactions in Decidua. Front Immunol. 2018;9:1897. https://doi.org/10.3389/fimmu.2018.01897
13. Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563(7731):347-353. https://doi.org/10.1038/s41586-018-0698-6
14. Zhang L, Wang HR, Shao YZ, Yan M, Fu T, et al. [Effect of IFN-γ and IL-12 expressions on intrauterine transmission in HBsAg-positive parturientsin late pregnancy]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(9):1077-1083. (In Chinese). https://doi.org/10.3760/cma.j.issn.0254-6450.2019.09.011
15. Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, et al. Trained Memory of Human Uterine NK Cells Enhances Their Function in Subsequent Pregnancies. Immunity. 2018;48(5):951-962.e5. https://doi.org/10.1016/j.immuni.2018.03.030
16. Khamatova A.A., Chebotareva T.A., Balmasova I.P Tissueresident natural killer cells: features of functioning in the uterus and decidual membrane. Immunologiya. 2021;42(5):574-580. (in Russian) https://doi.org/10.33029/0206-4952-2021-42-5-574-580
17. Mariaselvam CM, Tamouza R, Krishnamoorthy R, Charron D, Misra DP, et al. Association of NKG2D gene variants with susceptibility and severity of rheumatoid arthritis. Clin Exp Immunol. 2017;187(3):369-375. https://doi.org/10.1111/cei.12891
18. Ma J, Guo X, Wu X, Li J, Zhu X, et al. Association of NKG2D genetic polymorphism with susceptibility to chronic hepatitis B in a Han Chinese population. J Med Virol. 2010;82(9):1501-7. https://doi.org/10.1002/jmv.21855
19. Schena FP, Cerullo G, Torres DD, Scolari F, Foramitti M, et al. Role of interferon-gamma gene polymorphisms in susceptibility to IgA nephropathy: a family-based association study. Eur J Hum Genet. 2006;14(4):488-96. https://doi.org/10.1038/sj.ejhg.5201591
20. Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Hum Immunol. 2000;61(9):863-6. https://doi.org/10.1016/s0198-8859(00)00167-1
21. Sarangi S, Nahak SK, Padhi S, Pradhan B, Nayak N, et al. Interferon-gamma (IFN-γ) intronic variant (rs2430561) is a risk factor for systemic lupus erythematosus: Observation from a meta-analysis. Lupus. 2023;32(2):284-294. https://doi.org/10.1177/09612033221146700
22. Areeshi MY, Mandal RK, Dar SA, Jawed A, Wahid M, et al. IFN-γ +874 A>T (rs2430561) gene polymorphism and risk of pulmonary tuberculosis: a meta-analysis. Arch Med Sci. 2019;17(1):177-188. https://doi.org/10.5114/aoms.2019.88481
23. Dondeti MF, Abdelkhalek MS, El-Din Elezawy HM, Alsanie WF, Raafat BM, et al. Association between interferon-gamma (IFN-γ) gene polymorphisms (+874A/T and +2109A/G), and susceptibility to hepatitis B viral infection (HBV). J Appl Biomed. 2022;20(1):37-43. https://doi.org/10.32725/jab.2022.001
24. Börekçi G, Çetinkaya A, Kandemir Ö, Bekalp Yılmaz İ, Orekici Temel G, Aras N. TNF-α, IL-12A, IL-12B ve IFN-γ Gen Polimorfizmleri ile Kronik Hepatit C Arasındaki İlişkinin Araştırılması [Investigation of the Relationship Between TNF-α, IL-12A, IL-12B and IFN-γ Gene Polymorphisms and Chronic Hepatitis C]. Mikrobiyol Bul. 2020;54(2):279-290. (In Turkish). https://doi.org/10.5578/mb.69332
25. Puoti M, Prestini K, Putzolu V, Zanini B, Baiguera C, et al. HIV/HCV co-infection: natural history. J Biol Regul Homeost Agents. 2003;17(2):144-6. PMID: 14518713.
26. Ngo-Giang-Huong N, Jourdain G, Sirirungsi W, Decker L, Khamduang W, et al. Human immunodeficiency virushepatitis C virus co-infection in pregnant women and perinatal transmission to infants in Thailand. Int J Infect Dis. 2010;14(7):e602-7. https://doi.org/10.1016/j.ijid.2009.09.002
27. Checa Cabot CA, Stoszek SK, Quarleri J, Losso MH, Ivalo S, et al. Mother-to-Child Transmission of Hepatitis C Virus (HCV) Among HIV/HCV-Coinfected Women. J Pediatric Infect Dis Soc. 2013;2(2):126-35. https://doi.org/10.1093/jpids/pis091
28. Wu M, Yan Y, Zou S, Wu S, Feng L, et al. Adverse pregnancy outcomes among pregnant women living with HIV in Hubei province, China: prevalence and risk factors. AIDS Care. 2023;35(3):351-358. https://doi.org/10.1080/09540121.2022.2039358
29. Tariku MK. Determinants of mother to child HIV transmission (HIV MTCT); a case control study in governmental health centers of East Gojjam Zone, Northwest Ethiopia, 2019. Reprod Health. 2022;19(1):195. https://doi.org/10.1186/s12978-022-01501-y
About the Authors
A. A. KhamatovaRussian Federation
Agunda A. Khamatova – pediatrician of the outpatient pediatric department
Moscow
Competing Interests:
Authors declares no conflict of interest
I. P. Balmasova
Russian Federation
Irina P. Balmasova – Dr. Sci. (Med.), Professor, leading researcher of the Laboratory of Molecular Biological
Research of the Research Medical Dental Institute
Moscow
Competing Interests:
Authors declares no conflict of interest
T. A. Chebotareva
Russian Federation
Tatyana A. Chebotareva – Dr. Sci. (Med.), Professor, professor of Pediatric Infectious Diseases Department
Moscow
Competing Interests:
Authors declares no conflict of interest
Review
For citations:
Khamatova A.A., Balmasova I.P., Chebotareva T.A. Polymorphisms of interferon γ and NKG2D receptor genes in predicting vertical transmission of HIV/HCV coinfection. Medical Herald of the South of Russia. 2023;14(4):44-57. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-4-44-57







































