Scroll to:
Using the method of artificial neural networks for integration into the decision support system as a tool for optimizing outpatient management of patients with chronic obstructive pulmonary disease
https://doi.org/10.21886/2219-8075-2024-15-1-126-140
Abstract
Objective: to evaluate the possibility of using artificial neural networks for integration into the medical decision support system as an optimization of outpatient management of patients with COPD.
Materials and methods: a dynamic followup of 150 patients with chronic obstructive pulmonary disease, registered at the dispensary for the underlying disease, who completed the outpatient stage of pulmonary rehabilitation after a moderate exacerbation was carried out. The material of the study was a universal questionnaire of 69 indicators, including anamnesis, clinic, laboratory and instrumental diagnostics. A four-layer neural network has been created: the first two layers — 69 neurons, the third layer — 34 neurons and the last layer — 3 neurons.
Results: the software was used in the Java programming language using the Encog 3.4 module.
Conclusion: the use of the capabilities of artificial neural networks for integration into the medical decision support system in the outpatient management of patients with chronic obstructive pulmonary disease has shown high specificity. The predictive model is implemented in the form of a computer program: "The program for predicting an unfavorable outcome, the development of cardiovascular complications and the effectiveness of rehabilitation measures in patients with chronic obstructive pulmonary disease (CardioRisk)" and was introduced into the work of outpatient polyclinic institutions in Rostov-on-Don.
Keywords
For citations:
Tayutina T.V., Shlyk S.V., Vodopyanov A.S., Kazaryan T.M. Using the method of artificial neural networks for integration into the decision support system as a tool for optimizing outpatient management of patients with chronic obstructive pulmonary disease. Medical Herald of the South of Russia. 2024;15(1):126-140. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-126-140
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, causing approximately 2.8 million deaths annually, according to the World Health Organization (WHO) [1]. The objective of optimizing outpatient management for COPD patients is to slow disease progression and prevent cardiovascular complications, while also improving the patient's social adaptation through outpatient rehabilitation [2].
The Clinical Decision Support System (CDSS) is software designed to collect and analyze information to assist in clinical decision-making during examination, diagnosis, and treatment to reduce medical errors and improve healthcare delivery [3][4].
Since many clinicians are currently focused on evidence-based medicine, these systems are considered as medical technologies that potentially contribute to influencing a patient's condition. However, it is crucial that they are scientifically sound, effective, and safe [3][4].
Artificial neural networks (ANNs) are an integral part of intelligent systems that emulate or simulate a clinician's thinking. They consist of interconnected units called artificial neurons that interact to identify solutions to specific problems. ANNs learn from analyzing examples to find a solution rather than following a set of instructions for completing a specific task [5–7].
The integration of ANNs in predicting individual risk of unfavorable outcomes of underlying diseases, cardiovascular complications, and the effectiveness of outpatient rehabilitation for COPD patients will enhance the effectiveness of outpatient management.
The study aimed to assess the feasibility of integrating ANNs into the CDSS to optimize outpatient management of COPD patients.
Materials and Methods
The study included 150 COPD outpatients followed up at Rostov-on-Don City Polyclinic No. 4 and Rostov-on-Don City Polyclinic No. 1. The median age of COPD patients was 61.0 [ 58.9, 62.4; 95% CI] years. The study group included 110 (73.3%) males and 40 (26.7%) females, with 30.0 pack-years [ 19.9, 25.1; 95% CI] of smoking history.
The median duration of the underlying disease was 8.0 [ 4.7; 5.9; 95% CI] years. The median follow-up time was 5.0 [ 3.8, 4.7; 95% CI] years.
The diagnosis of COPD was based on the 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) with a comprehensive assessment of the symptoms, medical history, physical examination, and spirometry (using a standardized procedure to assess post-bronchodilator forced expiratory volume in one second [ FEV1], FEV1 to forced vital capacity [ FEV1/FVC] ratio after inhaling 400 µg salbutamol). Post-bronchodilator FEV1/FVC <0.70 was considered a major criterion for COPD [8].
Participants eligible for the study included males and females over 18 years of age who had been diagnosed with COPD at least 12 months prior to the study and had provided written informed consent. Individuals with COPD exacerbation at the time of the study, confirmed occupational exposures, indications for long-term oxygen therapy, BMI less than 18.5 kg/m², a malignant tumor of any site and stage (including in the past medical history), concomitant lung diseases such as lung tumor, interstitial lung disease, tuberculosis, sarcoidosis, bronchial asthma, bronchiolitis obliterans, and bronchiectasis, or previous lung volume reduction surgery were excluded. Individual limitations to adequate participation in outpatient pulmonary rehabilitation programs, including physical therapy and physical rehabilitation, were considered. These limitations included grade III respiratory failure, decompensated heart failure, acute musculoskeletal disorders, or those with severely limited range of motion. Prior to the study, the ethics approval was obtained from the Local Ethics Committee of the Rostov State Medical University founded by the Russian Ministry of Health.
Since ANNs are trained by examining examples, the 12-month follow-up included patients who were under the care of their local general practitioners and underwent outpatient pulmonary rehabilitation following a moderate COPD exacerbation without hospitalization. The outpatient physical rehabilitation began on day 25 of COPD exacerbation, after all significant symptoms had improved with basic therapy. The first stage, lasting 10 days, consisted of supervised daily therapeutic exercises and controlled walking. The patients walked 150 meters twice daily at a speed of 3 km/h with a step rate of 75 steps/min. During the second 14-day stage, the patients had physical therapy four times a week at home. Additionally, they were engaged in controlled walking for a distance of 300 meters daily and climbed one flight of stairs twice daily at 3 km/h with a step rate of 75 steps/min.
All patients underwent a comprehensive evaluation of their clinical symptoms, as well as structural and functional bronchopulmonary and cardiovascular disorders prior to and after the outpatient rehabilitation. It included spirometry, resting and stress pulse oximetry, electrocardiography, heart ultrasound to evaluate the right and left heart, and chest computed tomography. Additionally, an exercise tolerance score and estimated 4-year survival (BODE index) were measured. Patients with COPD and one or more cardiovascular risk factors, such as hypercholesterolemia, obesity, and elevated blood pressure, underwent color Doppler ultrasonography of the brachiocephalic arteries and ankle-brachial index measurement for early detection of systemic and peripheral atherosclerosis.
During the follow-up, a high risk of an unfavorable outcome of the underlying disease was defined as an increase in the estimated 4-year survival (BODE index) that included the severity of airway obstruction, a decrease in body mass index, severity of dyspnea and exercise tolerance based on a 6-minute walk test [1].
The outpatient rehabilitation effectiveness was evaluated by assessing dyspnea severity (using the mMRC [Modified Medical Research Council] Dyspnea Scale), the impact of COPD on the patient's quality of life (using the COPD Assessment Test [CAT]), FEV1/FVC ratio, and exercise tolerance (using the 6-minute walk test). Blood oxygen saturation was measured by pulse oximetry before and after exercise to detect desaturation indicative of systemic hypoxemia. Systemic hypoxemia was defined as a decrease in the blood oxygen saturation of 4% or more, measured by pulse oximetry on the 6-minute walk test after subtracting the saturation index after the exercise from the value before the test. If the blood oxygen saturation falls under 88%, it is also considered suggestive of systemic hypoxemia. Changes in the parameters were scored as follows: 4 points for improvement by more than 25%, 3 points for 15–25% improvement, 2 points for 5–15% improvement, 1 point for 0–5% improvement, and 0 points for no changes. The percentage change was calculated using the formulas:
if x2>x1, (x2/x1–1)·100%,
if x2<x1, (x1/x2–1)·100%,
where x1 is the value before rehabilitation, and x2 is the value after rehabilitation.
Next, the total score was determined and the mean value was calculated using the formula:
(mMRC(change score) + CAT(change score) + FEV1/FVC (change score) + 6-minute walk test (change score))/4.
For patients who remained desaturated (a decrease in blood oxygen saturation of 4% on the 6-minute walk test or a decrease in blood oxygen saturation after exercise below 88%) after outpatient rehabilitation, one point was subtracted (-1) from the mean score. The outpatient rehabilitation effectiveness was then scored with 0–1 points for poor rehabilitation and 2 or more points for effective rehabilitation with clinical improvement.
A general 69-item questionnaire was presented. The list included functions that involve both continuous and categorical variables with different units of measurement and responses. Each clinical case contained variables that were grouped into 15 categories, including medical history, physical examinations, laboratory tests, and instrumental examinations (Table 1).
Таблица / Table 1
Категории оцениваемых показателей в качестве вероятно значимых при построении ANN
Categories of estimated indicators as probably significant in the construction of ANN
|
Категория/ Category |
Оцениваемые показатели / Estimated indicators |
|
Общие данные / General data |
Мужчина - параметр оценивался как 1 (положительно) или 0 (отрицательно) / The male parameter was evaluated as 1 (positive) or 0 (negative) Среднее кровяное давление / Average blood pressure |
|
Параметр «Женщина» оценивался как 1 (положительно) или 0 (отрицательно) / The female parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Возраст (лет) / Age (years) |
|
|
Индекс курильщика (пачка-лет) / Smoker's index (pack-years) |
|
|
Частота сердечных сокращений / Heart rate |
|
|
Частота дыхания / Respiratory rate |
|
|
Антропометрические данные / Anthropometric data |
Рост (см) / Height (cm) |
|
Вес (кг) / Weight (kg) |
|
|
Индекс массы тела (кг/м²) / Body mass index (kg/m²) |
|
|
Среднее артериальное давление (АД ср=ДАД+((ДАД-САД)/2) / Average blood pressure (BP mid=DBP+((DBP-SBP)/2) |
|
|
Клинические проявления основного заболевания / Clinical manifestations of the underlying disease |
Клинический тип (А, В, E): параметр оценивался как 1 (положительно) или 0 (отрицательно) / Clinical type (A, B, E): the parameter was evaluated as 1 (positive) or 0 (negative) |
|
Выраженность одышки (баллы по шкале mMRS) / Severity of shortness of breath (MMRs scores) |
|
|
Качество жизни (баллы по шкале CAT) / Quality of life (CAT scores) |
|
|
Количество обострений (за год) / Number of exacerbations (per year) |
|
|
Переносимость физической нагрузки (баллы по шкале Borge) / Exercise tolerance (Borge scores) |
|
|
Индекс BODE (баллы) / BODE Index (points) |
|
|
Индекс SCORE (%) / SCORE index (%) |
|
|
Тест шестиминутной ходьбы (м) / Six-minute walk test (m) |
|
|
Сатурация крови кислородом SpО2, % / Blood oxygen saturation SPO2, % |
|
|
Функция внешнего дыхания / The function of external respiration |
Форсированнаяжизненнаяёмкостьлёгких ФЖЕЛ (%) / Accelerated vital capacity of the lungs FVC (%) |
|
Объем форсированного выдоха за 1 секунду ОФВ1 (%) / Volume of forced exhalation in 1 second FEV1 (%) |
|
|
Индекс Тиффно ОФВ1/ФЖЕЛ (%) / Tiffno Index FEV1/FVC (%) |
|
|
Мгновенная объёмная скорость на уровне 25 % от ФЖЕЛ - МОС25 (%) / Instantaneous volumetric velocity at the level of 25% of FVC - MEF25 (%) |
|
|
Мгновенная объёмная скорость на уровне 50 % от ФЖЕЛ МОС50 (%) / Instantaneous volumetric velocity at the level of 50% of FVC MEF50 (%) |
|
|
Мгновенная объёмная скорость на уровне 75 % от ФЖЕЛ МОС75 (%) / Instantaneous volumetric velocity at the level of 75% of FVC MEF75 (%) |
|
|
Рентгенологический признак по данным спиральной компьютерной томографии высокого разрешения / X-ray sign according to high-resolution spiral computed tomography |
Признаки диффузного бронхита: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Signs of diffuse bronchitis: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
Признаки буллезной и диффузной эмфиземы: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Signs of bullous and diffuse emphysema: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Сочетание бронхита и эмфиземы лёгких: параметр оценивался как 1 (положительно) или 0 (отрицательно) / The combination of bronchitis and emphysema of the lungs: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Маркеры атеросклеротического поражения сосудов / Markers of atherosclerotic vascular lesion |
Толщина интима-медиа (по данным ультразвукового исследования брахиоцефальных артерий), (см) / Intima-media thickness (according to ultrasound examination of brachiocephalic arteries), (cm) |
|
Лодыжечно-плечевой индекс / Ankle-shoulder index |
|
|
Показатели гемодинамики и структурно-функционального состояния правого и левого желудочка / Indicators of hemodynamics and structural and functional state of the right and left ventricles |
Толщина стенки правого желудочка (мм) / Wall thickness of the right ventricle (mm) |
|
Левое предсердие (мм) / Left atrium (mm) |
|
|
Систолическое давления в лёгочной артерии СДЛА (мм рт. ст) / Systolic pressure in the pulmonary artery SPLA (mmHg) |
|
|
Фракция выброса левого желудочка ФВ ЛЖ (%) / Left ventricular ejection fraction LVF (%) |
|
|
Е/А на трикуспидальном клапане / E/A on the tricuspid valve |
|
|
Показатели электрофизиологического состояния сердечно-сосудистой системы / Indicators of the electrophysiological state of the cardiovascular system |
Индекс вариабельности сердечного ритма SDNN / Heart Rate Variability Index SDNN |
|
Циркадный индекс ЦИ / Circadian Index CI |
|
|
Фибрилляция предсердий: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Atrial fibrillation: parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Желудочковая экстрасистолия: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Ventricular extrasystole: parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Наджелудочковая экстрасистолия: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Supraventricular extrasystole: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Депрессия сегмента ST, по данным ЭКГ: параметр оценивался как 1 (положительно) или 0 (отрицательно) / ST segment depression according to ECG data: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Показатели общего анализа крови / Indicators of the general blood test |
Гемоглобин (г/л) / Hemoglobin (g/l) |
|
Эритроциты,10¹²/л / Red blood cells,10¹²/l |
|
|
Лейкоциты, 10⁹/л / White blood cells, 10⁹/l |
|
|
Скорость оседания эритроцитов (мм/ч) / Erythrocyte sedimentation rate (mm/h) |
|
|
Биохимические показатели крови / Biochemical parameters of blood |
С-реактивный белок (г/л) / C-reactive protein (g/l) |
|
Фибриноген (г/л) / Fibrinogen (g/l) |
|
|
Глюкоза (ммоль/л) / Glucose (mmol/l) |
|
|
Креатинин (мкмоль/л) / Creatinine (mmol/l) |
|
|
Электролиты крови/ Blood electrolytes |
К+ (ммоль/л) / K+ (mmol/L) |
|
Na+ (ммоль/л) / Na+ (mmol/L) |
|
|
Показатели липидограммы / Lipidogram indicators |
Общий холестерин (ммоль/л) / Total cholesterol (mmol/l) |
|
Триглицериды (ммоль/л) / Triglycerides (mmol/l) |
|
|
Липопротеины очень низкой плотности-холестерин (ммоль/л) / Very low density lipoproteins-cholesterol (mmol/l) |
|
|
Липопротеины высокой плотности-холестерин (ммоль/л) / High-density lipoproteins-cholesterol (mmol/l) |
|
|
Показатели свертывающей системы / Indicators of the coagulation system |
Протромбиновое время (сек.) / Prothrombin time (sec.) |
|
МНО (у.е.) / INR (cu.) |
|
|
Показатели общего анализа мокроты / Indicators of the general sputum analysis |
Нейтрофилы (кл./в поле зрения) / Neutrophils (cl./in the field of view) |
|
Эозинофилы (кл./в поле зрения) / Eosinophils (cl./in the field of view) |
|
|
Наличие сопутствующей патологии / The presence of concomitant pathology |
Артериальная гипертензия: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Arterial hypertension: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
Постинфарктный кардиосклероз: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Postinfarction cardiosclerosis: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Сахарный диабет: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Diabetes mellitus: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Острый коронарный синдром в анамнезе: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Acute coronary syndrome in the anamnesis, the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Острое нарушение мозгового кровообращения в анамнезе: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Acute cerebrovascular accident in the anamnesis: the parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Хроническая сердечная недостаточность: параметр оценивался как 1 (положительно) или 0 (отрицательно) / Chronic heart failure parameter was evaluated as 1 (positive) or 0 (negative) |
|
|
Ожирение: параметр оценивался как 1 (положительно) или 0 (отрицательно) / The obesity parameter was evaluated as 1 (positive) or 0 (negative) |
To achieve the study objective, prognostic values were predicted by interpreting multivariate observations. The process of network training aimed to select a setup that enabled a set of input nodes (an input vector) to produce the required set of output nodes (a target vector). The training comprised a training set of input vectors, each paired with a matched target vector (a training set). The training utilized a backpropagation of error algorithm, and the neural network was specifically structured as a multi-layered network, as illustrated in Figure 1.
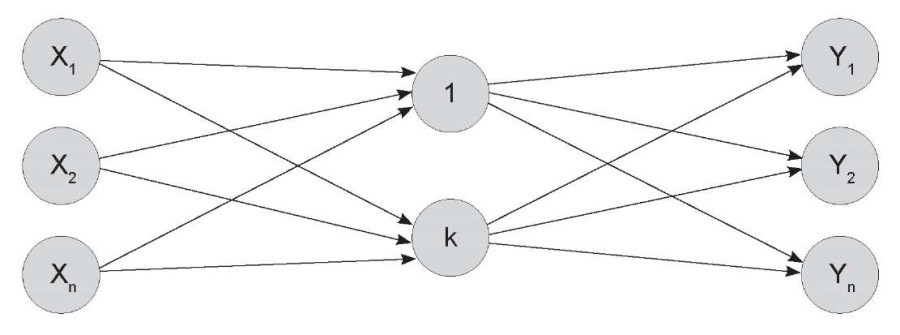
Figure 1. An image of a multilayer neural network.
The number of layers between the input and output layers
and the number of neurons in these layers may vary depending on the task at hand.
Рисунок 1. Изображение многослойной нейронной сети.
Число слоев между входными и выходными слоями и количество нейронов в этих слоях
может быть различным в зависимости от поставленной задачи.
The neural network was trained by feeding vectors from the training set into the input node. The network output value was compared to the reference, and the error was calculated. Following this, the backpropagation process was initiated, which involved adjusting the weighting coefficients of the connections resulting in the target layer. The connections were then adjusted one layer closer to the input node of the network, up to the input layer. The training process was successfully completed when the network accurately converted input data to output data, effectively approximating an unknown function.
A prognostic model was constructed in the Java-based software using the Encog 3.4 framework [9]. Statistical analyses were performed with the Statistica 10.0 software. The parameters selected were presented as a mean ± standard deviation, and 95% confidence intervals were calculated for operating characteristics. The size of the analyzed sample was represented as n.
Results
A neural network consisting of four layers was constructed. The first two layers contained 69 neurons each, corresponding to the number of input values. The third layer had 34 neurons, and the final (output) layer had 3 neurons, corresponding to the number of output values. The internal dimensions of a four-layer neural network were determined using the node growth method. This method involves gradually increasing the model complexity measured by the number of neurons in the intermediate layer to compensate for the error generated by the smaller model. The optimal number of intermediate neurons was determined by analyzing the rate of variation in recognition error as a function of the number of neurons used at this stage.
The initial stage involved normalizing the baseline data (all values from 0 to 1). Therefore, the minimum and maximum values were calculated for each parameter, and the following formula was used:
N = (V – Min) / (Max – Min),
where N is the normalized value; V is the baseline value; Max is the maximum value; Min is the minimum value.
Then, 20 patients were randomized from the sample to the control group. The neural network was trained using the remaining 129 patients. The neural network was tested on 20 control patients after the training. Each output parameter was ranked from 0.0 to 1.0 with a higher value indicating a higher probability ratio for a positive outcome, i.e. 0–0.3 for negative, 0.31–0.7 for doubtful, and >0.7 for positive. Table 2 presents the results of neural network testing in the control group.
Таблица / Table 2
Результаты проверки нейронной сети на контрольной группе
The results of the neural network test on the control group
|
№ Пациента / Patient's No. |
Фактические данные / Actual data |
Рассчитанные данные / Calculated data |
||||
|
Неблагоприятный исход основного заболевания / Unfavorable outcome of the underlying disease Unfavorable |
Риск развития сердечно-сосудистых осложнений / Risk of cardiovascular complications |
Эффективность реабилитационных мероприятий / Effectiveness of rehabilitation measures |
Неблагоприятный исход основного заболевания / Unfavorable outcome of the underlying disease Unfavorable |
Риск развития сердечно-сосудистых осложнений / Risk of cardiovascular complications |
Эффективность реабилитационных мероприятий / Effectiveness of rehabilitation measures |
|
|
1 |
1 |
1 |
1 |
1 |
1 |
0,21 |
|
12 |
0 |
0 |
1 |
0,01 |
0.01 |
1 |
|
24 |
1 |
1 |
0 |
1 |
1 |
0,01 |
|
38 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
53 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
61 |
1 |
1 |
0 |
1 |
1 |
0,01 |
|
67 |
1 |
1 |
0 |
1 |
1 |
0,01 |
|
73 |
1 |
1 |
1 |
1 |
1 |
1 |
|
79 |
1 |
1 |
0 |
1 |
1 |
0,01 |
|
25 |
0 |
1 |
1 |
1 |
1 |
0,01 |
|
89 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
95 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
102 |
1 |
1 |
0 |
1 |
1 |
0,01 |
|
107 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
109 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
117 |
1 |
1 |
0 |
1 |
1 |
0,01 |
|
121 |
1 |
1 |
1 |
1 |
1 |
0,01 |
|
130 |
0 |
1 |
1 |
0,01 |
1 |
1 |
|
141 |
0 |
1 |
1 |
0,01 |
0,01 |
1 |
|
148 |
0 |
1 |
1 |
0,01 |
1 |
1 |
Примечание: ошибочные значения (несовпадение фактического
и рассчитанного результата) выделены цветом.
Note: erroneous values (discrepancy between the actual
and calculated result) are highlighted in color.
To assess the probability of an unfavorable outcome of the underlying disease and the risk of cardiovascular complications in COPD patients, the agreement of calculated and actual values was reported for 19 of 20 cases, with a specificity of 95%. When evaluating the effectiveness of outpatient rehabilitation, it was found that in 17 of 20 cases, there was agreement, with a specificity of 85%.
To minimize the number of parameters in the input vector, the input neurons with the lowest weighting coefficients were sequentially disconnected after training the neural network. The stopping criterion was based on the quality of recognition of the training sample. Specifically, the removal of uninformative parameters did not significantly deteriorate the quality of recognition.
The analysis showed that the following parameters proved to be uninformative in predicting a positive (Table 3) and negative (Table 4) output vector (therefore, they were excluded from further analysis): the patient's age, tricuspid E/A, left ventricular ejection fraction, blood glucose, blood creatinine, K+, Na+, ventricular and supraventricular extrasystoles, SpO2, hemoglobin, international normalized ratio, and prothrombin time.
Таблица / Table 3
Значения показателей входного вектора и положительного выходного вектора
при определении неинформативных показателей в ходе обучения нейронной сети
The values of the indicators of the input vector and the positive output vector
when determining uninformative indicators during neural network training
|
Входной вектор / Выходной вектор Input vector / Output vector |
Среднее +/- ДИ (95%) Average +/- CI (95%) |
Ме |
25–75 перцентиль 25–75 percentile |
n |
δ |
|
Возраст / Исход благоприятный Age / Outcome favorable |
60,72 [ 59,6;61,8] |
62 |
53–69 |
84 |
10,629 |
|
Возраст / Риск осложнений есть Age / The presence of the risk of complications |
61,86 [ 61,0;62,7] |
62 |
57–69 |
139 |
9,764 |
|
Возраст / Эффективность мероприятий Age / Effectiveness of events |
60,54 [ 59,5;61,6] |
60 |
54–68 |
95 |
10,379 |
|
E/A на трикуспидальном клапане / Исход благоприятный E/A on the tricuspid valve / The outcome is favorable |
0,9 [ 0,8;0,9] |
0,83 |
0,69–1,05 |
84 |
0,323 |
|
E/A на трикуспидальном клапане / Риск осложнений есть E/A on the tricuspid valve / The presence of the risk of complications |
0,92 [ 0,8;0,9] |
0,85 |
0,72–1,09 |
139 |
0,296 |
|
E/A на трикуспидальном клапане / Эффективность мероприятий E/A on the tricuspid valve / Effectiveness of measures |
0,95 [ 0,92;0,97] |
0,9 |
0,78–1,1 |
95 |
0,257 |
|
Фракция выброса левого желудочка / Исход благоприятный Left Ventricular ejection fraction / Favorable outcome |
58,91 [ 58,2;59,6] |
60 |
56–64 |
84 |
6,795 |
|
Фракция выброса левого желудочка / Риск осложнений есть Left ventricular ejection fraction / The presence of the risk of complications |
58,81 [ 58,2;59,4] |
61 |
55–64 |
139 |
7,073 |
|
Фракция выброса левого желудочка / Эффективность мероприятий Left ventricular ejection fraction / Effectiveness of measures |
59,78 [ 59,1;60,5] |
62 |
55–65 |
95 |
7,033 |
|
Глюкоза крови / Исход благоприятный Blood glucose / The outcome is favorable |
5,48 [ 5,2;5,7] |
5 |
4,2–6 |
84 |
2,283 |
|
Глюкоза крови / Риск осложнений есть Blood glucose / The presence of the risk of complications |
5,89 [ 5,3;6,5] |
5 |
4–6 |
139 |
6,808 |
|
Глюкоза крови / Эффективность мероприятий Blood glucose / Effectiveness of measures |
6,19 [ 5,4;7,02] |
5 |
4–6 |
95 |
8,112 |
|
Креатинин крови / Исход благоприятный Blood Creatinine / The outcome is favorable |
105,06 [ 102,1;108,05] |
100 |
89–123 |
84 |
27,371 |
|
Креатинин крови / Риск осложнений есть Blood Creatinine / The presence of the risk of complications |
105,64 [ 103,3;108,02] |
101 |
87–123 |
139 |
28,107 |
|
Креатинин крови / Эффективность мероприятий Blood Creatinine / Effectiveness of measures |
102,93 [ 100,05;105,8] |
94 |
84–116 |
95 |
28,065 |
|
К+ / Исход благоприятный K+ / The outcome is favorable |
4 [ 3,8;4,1] |
3,8 |
3,3–4,8 |
84 |
1,086 |
|
К+ / Риск осложнений есть K+ / The presence of the risk of complications |
3,96 [ 3,9;4,05] |
3,8 |
3,3–4,8 |
139 |
1,109 |
|
К+ / Эффективность мероприятий K+ / Effectiveness of measures |
3,99 [ 3,9;4,1] |
3,8 |
3,3–4,8 |
95 |
1,116 |
|
Na+ / Исход благоприятный Na+ / The outcome is favorable |
143,22 [ 142,06;4,4] |
144 |
139–147 |
84 |
10,556 |
|
Na+ / Риск осложнений есть Na+ / The presence of the risk of complications |
142,52 [ 141,5;143,5] |
143 |
137–147 |
139 |
11,603 |
|
Na+ / Эффективность мероприятий Na+ / Effectiveness of measures |
141,04 [ 139,9;142,2] |
141 |
136–147 |
95 |
11,226 |
|
Желудочковая экстрасистолия / Исход благоприятный Ventricular extrasystole / The outcome is favorable |
0,04 [ 0,02;0,06] |
0 |
0–0 |
84 |
0,213 |
|
Желудочковая экстрасистолия / Риск осложнений есть Ventricular extrasystole / The presence of the risk of complications |
0,08 [ 0,06;0,1] |
0 |
0–0 |
139 |
0,281 |
|
Желудочковая экстрасистолия / Эффективность мероприятий Ventricular extrasystole / Effectiveness of measures |
0,08 [ 0,05;0,11] |
0 |
0–0 |
95 |
0,278 |
|
Наджелудочковая экстрасистолия / Исход благоприятный Supraventricular extrasystole / The outcome is favorable |
0,09 [ 0,06;0,1] |
0 |
0–0 |
84 |
0,294 |
|
Наджелудочковая экстрасистолия / Риск осложнений есть Supraventricular extrasystole / The presence of the risk of complications |
0,07 [ 0,05;0,09] |
0 |
0–0 |
139 |
0,258 |
|
Наджелудочковая экстрасистолия / Эффективность мероприятий Supraventricular extrasystole / Effectiveness of measures |
0,05 [ 0,03;0,07] |
0 |
0–0 |
95 |
0,223 |
|
SpО2 / Исход благоприятный SpО2 / The outcome is favorable |
92,63 [ 92,3; 92,95] |
93 |
91–95 |
84 |
3,011 |
|
SpО2 / Риск осложнений есть SpО2 / The presence of the risk of complications |
92,99 [ 92,7;93,3] |
93 |
92–95 |
139 |
3,092 |
|
SpО2 / Эффективность мероприятий SpО2 / Effectiveness of measures |
93,45 [ 93,1;93,8] |
93 |
92–96 |
95 |
3,162 |
|
Hb /Исход благоприятный Hb / The outcome is favorable |
142,2[ 140,07;144,33] |
147 |
130–158 |
84 |
19,542 |
|
Hb / Риск осложнений есть Hb / The presence of the risk of complications |
140,56[ 138,98;142,14] |
144 |
126–156 |
139 |
18,631 |
|
Hb / Эффективность мероприятий Hb / Effectiveness of measures |
139,56[ 137,91;141,21] |
143 |
125–152 |
95 |
16,057 |
|
МНО / Исход благоприятный INR / The outcome is favorable |
0,93[ 0,91;0,95] |
0,9 |
0,8–1,1 |
84 |
0,212 |
|
МНО / Риск осложнений есть INR / The presence of the risk of complications |
0,9[ 0,88;0,92] |
0,9 |
0,78–1,0 |
139 |
0,196 |
|
МНО / Эффективность мероприятий INR / Effectiveness of measures |
0,9[ 0,88;0,92] |
0,9 |
0,78–1,0 |
95 |
0,181 |
|
Протромбиновое время / Исход благоприятный Prothrombin time / Favorable outcome |
12,85 [ 12,53;13,17] |
12,7 |
11–15 |
84 |
2,973 |
|
Протромбиновое время / Риск осложнений есть Prothrombin time / The presence of the risk of complications |
12,67[ 12,41;12,93] |
12,5 |
11–15 |
139 |
3,022 |
|
Протромбиновое время / Эффективность мероприятий Prothrombin time / Effectiveness of measures |
12,75[ 12,44;13,06] |
12,7 |
11–15 |
95 |
3,041 |
Таблица / Table 4
Значения показателей входного вектора и отрицательного выходного вектора
при определении неинформативных показателей в ходе обучения нейронной сети
The values of the indicators of the input vector and the negative output vector
when determining uninformative indicators during neural network training
|
Входной вектор / Выходной вектор Input vector / Output vector |
Среднее +/- ДИ (95%) Average +/- CI (95%) |
Ме |
25–75 перцентиль 25–75 percentile |
n |
δ |
|
Возраст / Исход неблагоприятный Age / Outcome unfavorable |
60,56 [ 59,22;61,9] |
60 |
54–68 |
65 |
10,822 |
|
Возраст / Риска осложнений нет Age / No risk of complications |
43,9 [ 41;46,8] |
45 |
33–53 |
10 |
9,17 |
|
Возраст / Неэффективность мероприятий Age / Inefficiency of measures |
60,85 [ 59,32;62,39] |
62 |
55–69 |
54 |
11,277 |
|
E/A на ТК / Исход неблагоприятный E/A on TV / The outcome is unfavorable |
0,94 [ 0,91;0,97] |
0,9 |
0,8–1,1 |
65 |
0,232 |
|
E/A на ТК / Риска осложнений нет E/A on TV / There is no risk of complications |
0,97 [ 0,94;0,99] |
0,95 |
0,87–1,1 |
10 |
9,274 |
|
E/A на ТК / Неэффективность мероприятий E/A on TV / Inefficiency of measures |
0,88 [ 0,84;0,93] |
0,805 |
0,65–1,02 |
54 |
0,329 |
|
ФВ ЛЖ / Исход неблагоприятный EF LV / Unfavorable outcome |
60,05 [ 59,1;61] |
62 |
55–66 |
65 |
7,657 |
|
ФВ ЛЖ / Риска осложнений нет EF LV / There is no risk of complications |
67,7 [ 67,02;68,38] |
67,5 |
66–70 |
10 |
2,147 |
|
ФВ ЛЖ / Неэффективность мероприятий EF LV / Inefficiency of measures |
58,75 [ 57,74;59,77] |
60,5 |
57–64 |
54 |
7,456 |
|
Глюкоза крови / Исход неблагоприятный Blood glucose / Unfavorable outcome |
6,24 [ 5,05;7,43] |
4,8 |
4–6 |
65 |
9,608 |
|
Глюкоза крови / Риска осложнений нет Blood glucose / There is no risk of complications |
4,7 [ 4,57;4,84] |
4,705 |
4,25 – 5,2 |
10 |
0,428 |
|
Глюкоза крови / Неэффективность мероприятий Blood glucose / Inefficiency of measures |
5,15 [ 4,91;5,39] |
4,85 |
4,1–5,7 |
54 |
1,773 |
|
Креатинин крови / Исход неблагоприятный Blood Creatinine / Unfavorable outcome |
102,06 [ 98,46;105,66] |
93 |
83–119 |
65 |
29,052 |
|
Креатинин крови / Риска осложнений нет Blood Creatinine / There is no risk of complications |
77,52 [ 74,48;80,56] |
76 |
70–89 |
10 |
9,616 |
|
Креатинин крови / Неэффективность мероприятий Blood Creatinine / Inefficiency of measures |
105,2 [ 101,36;109,05] |
102,5 |
89–123 |
54 |
28,257 |
|
К+ / Исход неблагоприятный K+ / The outcome is unfavorable |
4,04 [ 3,9;4,18] |
4,1 |
3,3–4,8 |
65 |
1,11 |
|
К+ / Риска осложнений нет K+ / There is no risk of complications |
4.74 [ 4,58;4,91] |
4,75 |
4,2–5,2 |
10 |
0,522 |
|
К+ / Неэффективность мероприятий K+ / Inefficiency of measures |
4,06 [ 3,92;4,21] |
4,2 |
3,3–4,8 |
54 |
1,062 |
|
Na+ / Исход неблагоприятный Na+ / Unfavorable outcome |
141,48 [ 139,98;142,98] |
141 |
135–147,8 |
65 |
12,094 |
|
Na+ / Риска осложнений нет Na+ / There is no risk of complications |
141,69 [ 140,01;143,3] |
141,5 |
139–148 |
10 |
5,1 |
|
Na+/ Неэффективность мероприятий Na+ / Inefficiency of measures |
144,96 [ 143,47;146,45] |
145 |
140–149 |
54 |
10,954 |
|
Желудочковая экстрасистолия / Исход неблагоприятный Ventricular extrasystole / Unfavorable outcome |
0,13 [ 0,09;0,17] |
0 |
0–0 |
65 |
0,345 |
|
Желудочковая экстрасистолия / Риска осложнений нет Ventricular extrasystole / There is no risk of complications |
0,1 [ 0,01;0,195] |
0 |
0–0 |
10 |
0,3 |
|
Желудочковая экстрасистолия / Неэффективность мероприятий Ventricular extrasystole / Ineffectiveness of measures |
0,09 [ 0,05;0,13] |
0 |
0–0 |
54 |
0,289 |
|
Наджелудочковая экстрасистолия / Исход неблагоприятный Supraventricular extrasystole / Unfavorable outcome |
0,04 [ 0,01;0,07] |
0 |
0–0 |
65 |
0,21 |
|
Наджелудочковая экстрасистолия / Риска осложнений нет Supraventricular extrasystole / There is no risk of complications |
0,1 [ 0,01;0,195] |
0 |
0–0 |
10 |
0,3 |
|
Наджелудочковая экстрасистолия / Эффективность мероприятий Supraventricular extrasystole / Effectiveness of measures |
0,11 [ 0,07;05] |
0 |
0–0 |
54 |
0,314 |
|
SpО2 / Исход неблагоприятный SpО2 / The outcome is unfavorable |
93,87 [ 93,49;94,25] |
94 |
93–96 |
65 |
3,041 |
|
SpО2/ Риска осложнений нет SpО2/ There is no risk of complications |
95,7 [ 95,23;96,17] |
95,5 |
94–98 |
10 |
1,487 |
|
SpО2 / Неэффективность мероприятий SpО2 / Inefficiency of measures |
92,68 [ 92,29;93,07] |
93 |
91–95 |
54 |
2,886 |
|
Hb / Исход неблагоприятный Hb / The outcome is unfavorable |
137,84 [ 135,83;139,85] |
141 |
123–152 |
65 |
16,187 |
|
Hb / Риска осложнений нет Hb / There is no risk of complications |
136,6 [ 132,84;140,36] |
133,5 |
126–151 |
10 |
11,884 |
|
Hb / Неэффективность мероприятий Hb / Inefficiency of measures |
141,59 [ 138,65;144,53] |
149 |
128–158 |
54 |
21,591 |
|
МНО / Исход неблагоприятный INR / Unfavorable outcome |
0,89 [ 0,87;0,91] |
0,89 |
0,75–1 |
65 |
0,175 |
|
МНО / Риска осложнений нет INR / There is no risk of complications |
1,06 [ 1,01;1,11] |
1,01 |
0,96–1,31 |
10 |
0,154 |
|
МНО / Неэффективность мероприятий INR / Inefficiency of measures |
0,94 [ 0,91;0,97] |
0,9 |
0,8–1,1 |
54 |
0,222 |
|
Протромбиновое время / Исход неблагоприятный Prothrombin time / Unfavorable outcome |
12,58 [ 12,19;12,97] |
12,7 |
10,2–15 |
65 |
3,139 |
|
Протромбиновое время / Риска осложнений нет Prothrombin time / There is no risk of complications |
13,57 [ 12,53;14,61] |
13,44 |
10,2–18,3 |
10 |
3,299 |
|
Протромбиновое время / Неэффективность мероприятий Prothrombin time / Inefficiency of measures |
12,7 [ 12,28;13,12] |
12,44 |
10,7–15 |
54 |
3,064 |
After eliminating the uninformative parameters, the neural network was reduced from 69 to 55 neurons in the input layer (by the number of input parameters). The "reduced" neural network was retrained and tested on test data.
Таблица / Table 5
Результаты проверки нейронной сети после сокращения на контрольной группе
Results of neural network verification after reduction in the control group
|
№ Пациента / Patient's No. |
Фактические данные / Actual data |
Рассчитанные данные / Calculated data |
||||
|
Неблагоприятный исход основного заболевания / Unfavorable outcome of the underlying disease |
Риск развития сердечно-сосудистых осложнений / Risk of cardiovascular complications |
Эффективность реабилитационных мероприятий / Effectiveness of rehabilitation measures |
Неблагоприятный исход основного заболевания / Unfavorable outcome of the underlying disease |
Риск развития сердечно-сосудистых осложнений / Risk of cardiovascular complications |
Эффективность реабилитационных мероприятий / Effectiveness of rehabilitation measures |
|
|
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
12 |
0 |
0 |
1 |
0.01 |
0.01 |
1 |
|
24 |
1 |
1 |
0 |
1 |
1 |
0.01 |
|
38 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
53 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
61 |
1 |
1 |
0 |
1 |
1 |
0.01 |
|
67 |
1 |
1 |
0 |
1 |
1 |
0.01 |
|
73 |
1 |
1 |
1 |
1 |
1 |
0.01 |
|
79 |
1 |
1 |
0 |
1 |
1 |
0.01 |
|
83 |
0 |
1 |
1 |
1 |
1 |
0.01 |
|
89 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
95 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
102 |
1 |
1 |
0 |
1 |
1 |
0.01 |
|
107 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
109 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
117 |
1 |
1 |
0 |
1 |
1 |
0.01 |
|
121 |
1 |
1 |
1 |
1 |
1 |
1 |
|
130 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
141 |
0 |
1 |
1 |
0.01 |
1 |
1 |
|
148 |
0 |
1 |
1 |
0.01 |
1 |
1 |
Примечание: ошибочные значения (несовпадение фактического
и рассчитанного результата) выделены цветом.
Note: erroneous values (discrepancy between the actual
and calculated result) are highlighted in color.
A comparative analysis demonstrated that using a reduced set of parameters improved prediction accuracy. The risk of an unfavorable outcome of the underlying disease was calculated with 95% specificity in 19 of 20 cases. The probability of cardiovascular complications was correctly predicted in all cases with 100% specificity. The rehabilitation effectiveness was predicted in 18 cases, compared to 17 cases previously, with 90% specificity.
Discussion
Russian literature provides evidence of the use of artificial intelligence in diagnosing respiratory diseases through analyzing audio recordings of human breathing [10]. However, the neural network model described in the publication can only provide a preliminary diagnosis for several conditions, including COPD, bronchial asthma, pneumonia, bronchiectasis, bronchiolitis, and upper and lower respiratory tract infections, with an accuracy of 87%. In the foreign literature, there are anecdotal data on using neural network models in patients with COPD to categorize [11] the type of medical care provided and to predict the patient's level of self-management [12]. The sensitivity of these models has been reported to vary between 82% and 97%.
The prognostic accuracy obtained in this study was found to be generally comparable to that of other authors who have constructed ANN-based prognostic models for other diseases. Thus, Cheng et al. [13] used an ANN to predict the prognosis of ischemic stroke patients with an accuracy ranging from 79% to 95%. Chung et al. [14] obtained comparable data by developing a neural network to predict the risk of intracerebral hemorrhage and death in stroke patients receiving a plasminogen activator, achieving an accuracy of 91–95%. Another neural network predicting the outcome of a stroke provided approximately 80% accuracy [9].
Currently, there is no evidence to support the use of ANNs for integration into the CDSS in the outpatient management of COPD patients. Moreover, this study presented the first ANN-based prognostic model with three target vectors (the risk of an unfavorable outcome of the underlying disease, the risk of cardiovascular complications, and the rehabilitation effectiveness). The model provided increased sensitivity.
The developed neural network mathematical model has been introduced as a software program, Program for Predicting an Unfavorable Outcome, Cardiovascular Complications and Rehabilitation Effectiveness in Patients with Chronic Obstructive Pulmonary Disease (CardioRisk) (State Registration Certificate No. RU2023666935, issued on August 8, 2023) and implemented at Rostov-on-Don City Polyclinic No. 4 and Rostov-on-Don City Polyclinic No. 1. The program offers a quantitative evaluation of the overall risk of an unfavorable outcome of the underlying disease, cardiovascular complications and the estimated effectiveness of the outpatient rehabilitation at different confidence levels on two scales.
A case report demonstrating the use of the Program for Predicting an Unfavorable Outcome, Cardiovascular Complications and Rehabilitation Effectiveness in Patients with Chronic Obstructive Pulmonary Disease (CardioRisk) (State Registration Certificate No. RU2023666935, issued on August 8, 2023) is presented below.
Case Report. The 55-year-old female patient K. was diagnosed with GOLD 3 COPD (severe bronchial obstruction), non-acute at presentation, chronic bronchitis phenotype, Group E (mMRC 3, CAT 26, 3 exacerbations per year), complicated with grade II respiratory failure.
At presentation, the patient reported experiencing shortness of breath during mild physical activity, cough with thick mucopurulent sputum, and heart palpitations. The patient reported suffering from the disease for 15 years. In the past year, she experienced 3 exacerbations, one of which required hospitalization. The patient was a non-smoker.
The patient's height was 1.71 m, weight was 72 kg, and BMI was 24.6 kg/m². The skin appeared to have normal color and moisture, with mild cyanosis. The patient's vital signs were recorded, including a respiratory rate of 19 breaths per minute, a heart rate of 101 beats per minute, and a blood pressure of 130/90 mmHg. The physical examination indicated a barrel chest and bandbox resonance. Auscultation of the lungs showed vesicular breathing and scattered dry rales throughout the lungs, which increased with forced exhalation. The Kurlov's liver size was 12×10×9 cm. The abdomen was soft and painless on palpation. No visible swelling was observed. The ancle-brachial index was 0.6. The six-minute walk distance (6MWD) was 378 m.
The spirometry was as follows: FEV1, 42% of normal values; FVC, 61% normal values; FEV1/FVC, 59% of normal values; maximal expiratory flows after 25 (MEF25), 50 (MEF50), and 75 (MEF75) percent of FVC, 24%, 19% and 19% of normal values respectively. The spiral computed tomography of the chest showed pneumofibrosis, signs of bronchitis, and single bullae of the left upper lobe. The ultrasonography of the brachiocephalic arteries revealed an intima-media thickness (IMT) of 1.0, with no significant stenoses observed. A pulmonary artery systolic pressure of 22 mmHg, right ventricular paries thickness of 5.0 mm, E/A of 0.80, left atrial size of 37 mm, left ventricular ejection fraction of 67% were reported by echocardiography. Holter electrocardiography: sinus rhythm (max 146, min 46); circadian index 1.52; mean daytime heart rate 89 beats per minute; mean night-time heart rate 87 beats per minute; no pauses longer than 2,000 msec; normal atrioventricular conduction; PQ 121-200 ms; QT 315 ms with the maximum heart rate and 410 ms with the minimum heart rate; ectopic activity: supraventricular arrhythmia 10,120, ventricular arrhythmia 599, mixed-type extrasystoles 4, SDNN 43.
Hematology: red blood cells 3.5×10¹²/L, hemoglobin 100 g/L, white blood cells 6.2×10⁹/L, eosionophils 5%, band neutrophils 5%, segmented neutrophils 50%, lymphocytes 37%, monocytes 3%, platelets 257×10⁹/L, and erythrocyte sedimentation rate 5 mm/h. Blood chemistry: glucose 5.2 mmol/L, total cholesterol 7.1 mmol/L, low-density lipoprotein cholesterol 3.8 mmol/L, high-density lipoprotein cholesterol 0.45 mmol/L, triglycerides 2.0 mmol/L, C-reactive protein 6.0 g/L, and fibrinogen 6.3 g/L. The sputum culture showed mucopurulent, viscous, gray sputum, with 30 white blood cells in the field of view and 3 eosinophils in the field of view. The program included all data for the relevant categories, including the existing concomitant disease (Figure 2).
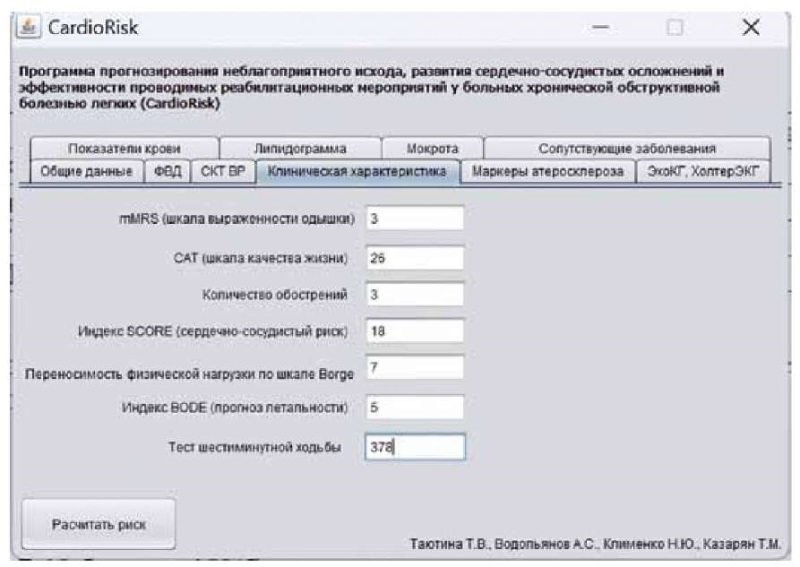
Figure 2. An example of data entry
into the use of the Cardio Risk program for patients with COPD.
Рисунок 2. Пример ввода данных
при использовании программы CardioRisk для пациентов с ХОБЛ.
Concomitant medication (over the past year): tiotropium bromide 18 µg once daily, phenoterol hydrobromide 50 µg + ipratropium bromide 20 µg as needed.
Based on the program calculations, the probability of an unfavorable outcome of the underlying disease was 1% (Figure 3) with the risk of cardiovascular complications estimated to be 100%, while the estimated effectiveness of outpatient rehabilitation was also 100%.
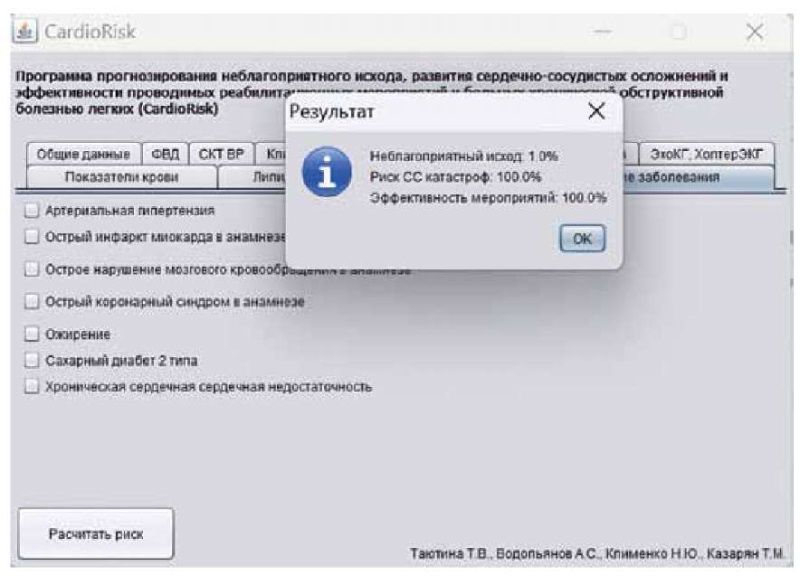
Figure 3. An example of calculating the predicted risks
in using the Cardio Risk program for patients with COPD.
Рисунок 3. Пример расчёта прогнозируемых рисков
при использовании программы CardioRisk для пациентов с ХОБЛ.
The available prognostic data suggest that treatment adjustment may be necessary, taking into account the comprehensive evaluation of the individual's risk of cardiovascular complications, which are among the most significant risk factors for COPD patients. When developing an individual rehabilitation program, it is important to consider the potential benefits of outpatient rehabilitation, which may include remote control elements.
Conclusion
The analysis of the prognostic model for unfavorable outcomes of the underlying disease, cardiovascular complications, and rehabilitation effectiveness in COPD patients using the ANN has not identified any limitations that are specific to standard methods. The neural network can be useful in modeling parameters, especially when there are no linear relationships between the factors affecting the object of analysis and response parameters. This approach is particularly valuable when dealing with unstable distributions and groups that have objects with zero variance. In situations where information about the system is incomplete, the three-endpoint prediction has been found to be highly specific.
The Program for Predicting an Unfavorable Outcome, Cardiovascular Complications and Rehabilitation Effectiveness in Patients with Chronic Obstructive Pulmonary Disease (CardioRisk) (State Registration Certificate No. RU2023666935, issued on August 8, 2023) will provide the outpatient management of COPD patients by local general practitioners through a comprehensive assessment of the individual's overall risk of an unfavorable outcome of the underlying disease and cardiovascular complications as the most significant risk factors for COPD patients. It is advised that the obtained data be taken into consideration when developing an individual outpatient rehabilitation program, which should include a preliminary assessment of its effectiveness. However, the CDSS is informative software that serves as a second opinion in clinical practice. Clinicians may or may not take it into account when making their own decisions.
References
1. CHuchalin A.G., ed. Pul'monologiya. Natsional'noe rukovodstvo. Kratkoe izdanie. Moscow: GEHOTAR-Media; 2015. (In Russ.)
2. Tayutina T.V. An integrated approach to the implementation of the stage of pulmonary rehabilitation of patients with chronic obstructive pulmonary disease: the importance of lifestyle modification. Klinitsist = The Clinician. 2023;17(1):28–38. (In Russ.). https://doi.org/10.17650/1818-8338-2023-17-1-К689
3. Varghese J, Kleine M, Gessner SI, Sandmann S, Dugas M. Effects of computerized decision support system implementations on patient outcomes in inpatient care: a systematic review. J Am Med Inform Assoc. 2018;25(5):593-602. https://doi.org/10.1093/jamia/ocx100
4. Tomaselli Muensterman E, Tisdale JE. Predictive Analytics for Identification of Patients at Risk for QT Interval Prolongation: A Systematic Review. Pharmacotherapy. 2018;38(8):813-821. https://doi.org/10.1002/phar.2146
5. Guan M, Cho S, Petro R, Zhang W, Pasche B, Topaloglu U. Natural language processing and recurrent network models for identifying genomic mutation-associated cancer treatment change from patient progress notes. JAMIA Open. 2019;2(1):139-149. https://doi.org/10.1093/jamiaopen/ooy061
6. Nagarajan, R., Thirunavukarasu, R. A neuro-fuzzy based healthcare framework for disease analysis and prediction. Multimed Tools Appl. 2022;81:11737–11753. https://doi.org/10.1007/s11042-022-12369-2
7. Sharma, A., Banerjee, P.S., Sharma, A., Yadav, A. A French to English Language Translator Using Recurrent Neural Network with Attention Mechanism. In: Nath, V., Mandal, J., eds. Nanoelectronics, Circuits and Communication Systems. NCCS 2018. Lecture Notes in Electrical Engineering.; vol 642. Springer, Singapore; 2020. https://doi.org/10.1007/978-981-15-2854-5_38
8. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of chronic obstructive pulmonary disease: 2022 Report. 2021.
9. Heaton, J. Encog: Library of Interchangeable Machine Learning Models for Java and C. Journal of Machine Learning Research. 2015;16:1243-1247.
10. Katermina T.S., Sibagatulin A.F. Application of Artificial Intelligence Methods to the Task of Diagnosing Respiratory Diseases. Computational nanotechnology. 2022;9(2):92-103. https://doi.org/10.33693/2313-223X-2022-9-2-92-103
11. Swaminathan S, Qirko K, Smith T, Corcoran E, Wysham NG, et al. A machine learning approach to triaging patients with chronic obstructive pulmonary disease. PLoS One. 2017;12(11):e0188532. https://doi.org/10.1371/journal.pone.0188532
12. Bugajski A, Lengerich A, Koerner R, Szalacha L. Utilizing an Artificial Neural Network to Predict Self-Management in Patients With Chronic Obstructive Pulmonary Disease: An Exploratory Analysis. J Nurs Scholarsh. 2021;53(1):16-24. https://doi.org/10.1111/jnu.12618
13. Chen M, Li H, Fan H, Dillman JR, Wang H, et al. ConCeptCNN: A novel multi-filter convolutional neural network for the prediction of neurodevelopmental disorders using brain connectome. Med Phys. 2022;49(5):3171-3184. https://doi.org/10.1002/mp.15545
14. Chung CC, Chan L, Bamodu OA, Hong CT, Chiu HW. Artificial neural network based prediction of postthrombolysis intracerebral hemorrhage and death. Sci Rep. 2020;10(1):20501. https://doi.org/10.1038/s41598-020-77546-5
About the Authors
T. V. TayutinaRussian Federation
Tatiana V. Tayutina, Cand. Sci. (Med.), Associate Professor of the Department of Therapy with a course of Polyclinic Therapy
Rostov-on-Don
S. V. Shlyk
Sergey V. Shlyk, Dr. Sci. (Med.), Professor, Rector, Head of the Department of Therapy with a course of Polyclinic Therapy
Rostov-on-Don
A. S. Vodopyanov
Alexey S. Vodopyanov, Leading Researcher at the Laboratory of Molecular Biology of Natural Focal and Zoonotic Infections
Rostov-on-Don
T. M. Kazaryan
Tatiana M. Kazarian, 5th year student of the Faculty of Medical Prevention, laboratory assis-tant of the Department of Therapy with a course of Polyclinic Therapy
Rostov-on-Don
Review
For citations:
Tayutina T.V., Shlyk S.V., Vodopyanov A.S., Kazaryan T.M. Using the method of artificial neural networks for integration into the decision support system as a tool for optimizing outpatient management of patients with chronic obstructive pulmonary disease. Medical Herald of the South of Russia. 2024;15(1):126-140. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-126-140







































