Scroll to:
Comparative effectiveness of different methods of prescribing rosuvastatin and ezetimibe in combination with PCSK9 inhibitors
https://doi.org/10.21886/2219-8075-2024-15-2-81-89
Abstract
Objective: comparative evaluation of the effectiveness of combined lipid-lowering therapy with rosuvastatin and ezetimibe in fixed and separate combinations with PCSK9 inhibitors in patients with very high cardiovascular risk.
Materials and methods: 65 study participants were divided into 2 groups depending on the method of combined lipid-lowering therapy and were observed for 5 months on 6 visits. The effectiveness of the hypolipidemic response in each of the studied groups was evaluated.
Results: in the group with separate administration of lipid-lowering drugs, the target values of LDL cholesterol reached 50% of patients, the level of LDL cholesterol during 5 months of treatment decreased by 38.22% from 2.25 [1.82; 2.47] mmol/l to 1.39 [1.21; 1.59] mmol/l (p <0.001). In the group with a fixed combination of rosuvastatin and ezetimibe, 61.29% of the study participants reached the target ranges of LDL cholesterol, the concentration of LDL cholesterol decreased by 47.46% from 2.36 [1.92; 2.57] mmol/l to 1.24 [1.18; 1.56] mmol/l (p <0.001). Logistic regression analysis showed a significant association of females with a higher risk of not reaching the target ranges of LDL cholesterol (χ2<0.001; OR 0.13 95 % CI 0.04-0.39; p<0.001).
Conclusion: the use of a fixed combination of rosuvastatin and ezetimibe in combination with PCSK9 inhibitors in patients with very high cardiovascular risk makes it possible to reduce the concentration of LDL cholesterol by 9.24% more intensively relative to the drug regimen with separate administration of drugs.
For citations:
Kuznetsov A.A., Mal G.S., Saraev I.A. Comparative effectiveness of different methods of prescribing rosuvastatin and ezetimibe in combination with PCSK9 inhibitors. Medical Herald of the South of Russia. 2024;15(2):81-89. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-2-81-89
Introduction
Despite significant progress in diagnostics and treatment of cardiovascular diseases (CVDs), diseases of the circulatory system have long occupied leading positions in the mortality structure of the population of Russia [1]. Over the past 20 years, it has definitely been possible to reduce this indicator by almost two times (from 927 to 570 cases per 100,000 population)1, but cardiovascular pathology still poses a significant danger, reducing the duration and worsening the quality of life. It should also be noted that, according to forecasts of the World Health Organization, the situation will not improve over the next 30 years2.
During numerous population studies [2], the results of which are consistent with the results of the Russian epidemiological study ESSE-RF [3], it has been shown that the most common modifiable risk factor for CVDs is dyslipidemia, which was detected in more than half of the population of Russia, regardless of age. The epidemiological multicenter study INTERHEART demonstrated that lipid metabolism disorders made the greatest contribution to the development of the first myocardial infarction [4]. The CVD pathogenesis is based on atherosclerosis processes, the formation and course of which directly depend on the concentration of atherogenic lipoproteins in the blood plasma. According to the current clinical guidelines for the diagnosis and correction of lipid metabolism disorders of the VIII revision of 2023, the target ranges of low-density lipoprotein cholesterol (LDL-C) depend on the category of relative cardiovascular risk (CVR) [5].
To date, significant progress has been made in controlling LDL-C levels; however, as shown by the results of the DYSIS and DA VINCI studies [6–8], a significant proportion of patients with very high CVR did not achieve the required target LDL-C values. For more than 40 years, the first line of pharmacotherapy for lipid metabolism disorders has been 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors — statins, and one of the most potent drugs in this group is rosuvastatin. Its efficacy and safety were proven during the GALAXY program, which included 18 multicenter randomized trials [9]. The use of rosuvastatin at a maximum dose of 40 mg/day allows reducing LDL-C levels by 50%, but, as shown by real clinical practice in the EUROASPIRE IV and EUROASPIRE V surveys, this is not enough to achieve target concentrations in patients with dyslipidemia [10][11].
If statin monotherapy is ineffective, it is recommended to add ezetimibe, a selective inhibitor of intestinal cholesterol absorption, to the treatment [5]. Lipid-lowering therapy combined with ezetimibe can further reduce LDL-C concentrations by 21–27% [12]. However, adding a second drug to the treatment can reduce patient adherence to the prescribed treatment regimen. In order to increase the level of compliance in patients receiving combined lipid-lowering therapy, it is possible to consider the use of drugs with a fixed combination of various drugs. In order to compare the efficiency of the two methods of prescribing lipid-lowering therapy, a retrospective cohort study was conducted in 2019 to investigate the trend in prescribing statins and ezetimibe as a fixed combination or individual tablets, which showed that prescribing rosuvastatin and ezetimibe as a fixed combination was 9% more efficient in reducing LDL-C levels [13].
When target ranges of LDL-C are not achieved, along with blockers of absorption and production of cholesterol in the liver, it is recommended to add genetically engineered lipid-lowering drugs, monoclonal antibodies, i.e. inhibitors of the plasma proprotein convertase subtilisin-kexin type 9 (PCSK9) protein which allow achieving target values of LDL-C in more than 90% of patients [14]. However, to date, the use of this group of drugs is limited due to their high cost and low availability in the regions. At the same time, there are no data in the scientific literature on the efficiency of the use of PCSK9 inhibitors as part of triple combined lipid-lowering therapy with various methods of prescribing statins and ezetimibe.
The study was aimed at comparing the efficiency of lipid-lowering therapy combined with rosuvastatin and ezetimibe in fixed and separate combinations with PCSK9 inhibitors in patients with very high CVR.
Materials and methods
The comparative prospective open-label study included 65 patients with very high CVR requiring intensive lipid-lowering therapy (37 men and 28 women) aged 40 to 79 years; the average age of the participants was 61.11 ± 10.55 years. The study was conducted in 2022–2023 at the cardiology department of the State Budgetary Healthcare Institution of the Moscow Region "Moscow Regional Hospital named after Prof. V.N. Rozanov". The study protocol was approved by the local ethics committee of the Kursk State Medical University of the Ministry of Health of the Russian Federation (protocol No. 3 dated March 16, 2020), complied with the principles set forth in the Helsinki Declaration of the World Medical Association “Ethical Principles for Medical Research Involving Human Subjects” and the requirements of good clinical practice. Participation in the study was voluntary, all patients signed informed consent.
Inclusion criteria for patients in this study were as follows: primary dyslipidemia, failure to achieve target LDL-C ranges recommended by current clinical guidelines for the diagnosis and correction of lipid metabolism disorders of the VIII revision [5] during 8 weeks of combined lipid-lowering therapy with a high-intensity dose of rosuvastatin and ezetimibe. Exclusion criteria for patients were as follows: individual intolerance to any component of the triple lipid-lowering therapy, terminal forms of chronic heart failure (according to Vasilenko-Strazhesko), and grade 3 obesity. The withdrawal criterion was the patient's refusal to continue the study.
Eight weeks before the start of the study, all participants were divided into two groups depending on the method of receiving combined lipid-lowering therapy. The first group included 46 people who were prescribed rosuvastatin at a dose of 40 mg/day and ezetimibe 10 mg/day in two tablets, and the second group included 49 patients who were prescribed a fixed combination of rosuvastatin and ezetimibe at a dose of 40 + 10 mg/day in one tablet (ZENON, Sanofi-aventis group JSC, France). During treatment with a combination of rosuvastatin and ezetimibe in the first group, 12 people reached the target LDL-C values in the first group, and in the second group – 18 patients, and therefore they were excluded from further study. PCSK9 inhibitors were prescribed to 34 patients in the separate rosuvastatin/ezetimibe group and to 31 patients in the fixed-dose combination of lipid-lowering drugs group. All study participants were prescribed alirocumab (PRALUENT, Sanofi-aventis Group JSC, France) at a dose of 150 mg according to the standard regimen: 1 subcutaneous injection every 14 days, followed by monitoring the effect of the lipid-lowering response during the next five visits once a month (the median observation period after the start of treatment with PCSK9 inhibitors was five months). At the time of screening, study participants in both groups were comparable in terms of gender, age, major risk factors, background and concomitant diseases, as well as lipid spectrum parameters (Table 1).
Таблица / Table 1
Клиническая характеристика исследуемых групп на момент скрининга
Clinical characteristics of the studied groups at the time of screening
|
Показатели Indicators |
Все пациенты All patients (n=65) |
Раздельный приём Separate reception (n=34) |
Фиксированная комбинация Fixed combination (n=31) |
p |
|
Возраст, годы Age, years |
61,11±10,55 |
60,5 [ 52,25; 70,5] |
62 [ 55; 70] |
0,56 |
|
Мужчины / женщины Men / women |
37/28 |
20/14 |
17/14 |
0,94 |
|
Ишемическая болезнь сердца, n (%) Coronary heart disease, n (%) |
62 (95,38) |
32 (94,12) |
30 (96,77) |
0,93 |
|
Артериальная гипертензия, n (%) Arterial hypertension, n (%) |
64 (98,46) |
33 (97,06) |
31 (100) |
0,96 |
|
Курение, n (%) Smoking, n (%) |
45 (69,23) |
21 (61,76) |
24 (77,42) |
0,27 |
|
Отягощённая наследственность по ССЗ, n (%) Burdened heredity by CVD, n (%) |
22 (33,85) |
9 (26,47) |
13 (41,94) |
0,29 |
|
Сахарный диабет 2 типа, n (%) Type 2 diabetes mellitus, n (%) |
17 (26,15) |
10 (29,41) |
7 (22,58) |
0,73 |
|
Ожирение 1–2 степени, n (%) Obesity of 1–2 degrees, n (%) |
26 (40) |
12 (35,29) |
14 (45,16) |
0,58 |
|
ОХ, ммоль/л TCh, mmol/l |
5,03±0,45 |
4,95 [ 4,6; 5,36] |
5,12 [ 4,68; 5,46] |
0,49 |
|
ХС ЛПНП, ммоль/л LDL cholesterol, mmol/l |
2,24±0,43 |
2,25 [ 1,82; 2,47] |
2,36 [ 1,92; 2,57] |
0,78 |
|
ХС ЛПВП, ммоль/л HDL cholesterol, mmol/l |
1,1±0,13 |
1,17 [ 0,98; 1,22] |
1,08 [ 1,02; 1,2] |
0,47 |
|
ТГ, ммоль/л TG, mmol/l |
2,26±0,39 |
2,2 [ 1,92; 2,57] |
2,32 [ 2,03; 2,6] |
0,41 |
Примечание: ССЗ — сердечно-сосудистые заболевания; ОХ — общий холестерин; ХС ЛПНП — холестерин липопротеидов низкой плотности; ХС ЛПВП — холестерин липопротеидов высокой плотности; ТГ — триглицериды; p — достоверность различий между группами.
Note: CVD — cardiovascular diseases; TCh — total cholesterol; LDL cholesterol — low-density lipoprotein cholesterol; HDL cholesterol — high-density lipoprotein cholesterol; TG — triglycerides; p-values — significant differences between respective groups.
Blood samples for biochemical testing were taken once from the cubital vein in the morning on an empty stomach 12 hours after eating. The analysis was performed on an automatic biochemical analyzer Mindray BS-120 (China). The assessed lipid metabolism indicators included the levels of total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG).
Statistical processing of the data obtained in the study was carried out using the computer program SPSS 23.0 (IBM, USA). The normality of distribution was tested using the Lilliefors-corrected Kolmogorov-Smirnov test and the Shapiro-Wilk test (if the number of subjects in the groups was less than 50). With a normal distribution, quantitative indicators were presented as M ± SD, where M was the arithmetic mean, SD was the standard deviation; for indicators with a distribution different from normal – as a median and interquartile range (Me [Q1; Q3]). For indicators characterizing qualitative features, the absolute number and/or relative value in percent were indicated. In order to assess comparability between the two study groups for quantitative indicators, the Mann-Whitney criteria were used; for dependent samples, the Wilcoxon test and the Student’s t-test were used to assess the hypolipidemic response among all study participants. Differences were considered statistically significant at p<0.05. Comparative analysis of qualitative parameters was performed using Pearson's chi-squared (χ2) or Fisher's exact test. In order to assess the influence of some features on treatment outcomes, the odds ratio (OR) was determined using the standard method, and the 95% confidence interval (CI) was indicated.
Results
At the time of initiation of alirocumab treatment, all patients were comparable in terms of lipid profile parameters (Fig. 1) and major comorbidities. No acute cardiovascular events or lethal outcomes were recorded in patients during the study. Before initiation of alirocumab treatment, all patients had received dual lipid-lowering therapy with rosuvastatin and ezetimibe for 8 weeks and had not achieved the target LDL-C values recommended by the current clinical guidelines for the diagnosis and correction of lipid metabolism disorders of the VIII revision [5]. Prior to this, 44.62% (n=29) of the participants in this study had previously taken statins, 16.92% (n=11) of them had taken them in high-intensity doses, and 4.62% (n=3) of patients had previously taken ezetimibe at a standard dose of 10 mg/day.
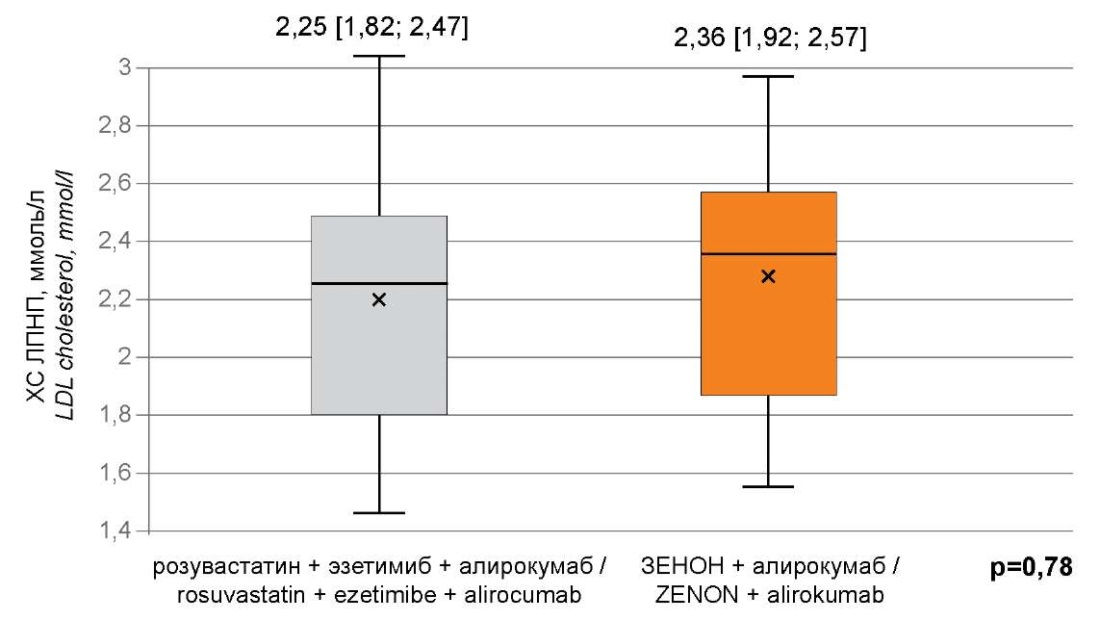
Рисунок 1. Концентрация ХС ЛПНП в исследуемых группах
на момент начала исследования.
Figure 1. The concentration of LDL cholesterol in the study groups
at the time of the start of the study.
Примечание: ХС ЛПНП — холестерин липопротеидов низкой плотности;
p — значение критерия Манна-Уитни.
Note: LDL cholesterol — low-density lipoprotein cholesterol;
p-values — the value of the Mann-Whitney criterion.
During the observation period, 50% of patients (n=17) in the group with separate administration of rosuvastatin and ezetimibe and 61.29% of patients (n=19) in the group with a fixed combination of these drugs achieved the target LDL-C ranges. The concentration of LDL-C among all study participants during five months of triple lipid-lowering therapy with rosuvastatin, ezetimibe, and alirocumab decreased by 38.39% from 2.24±0.43 mmol/L to 1.38±0.31 mmol/L (p<0.001) (Fig. 2). Herewith, in the group of patients with separate administration of rosuvastatin and ezetimibe, a less pronounced lipid-lowering effect was observed for all lipid profile indicators. The LDL-C level in patients in this group decreased by 38.22% from 2.25 [ 1.82; 2.47] mmol/L to 1.39 [ 1.21; 1.59] mmol/L (p<0.001), TC decreased by 29.49% from 4.95 [ 4.6; 5.36] mmol/L to 3.49 [ 3.32; 3.72] mmol/L (p<0.001), the HDL-C level increased by 2.56% from 1.17 [ 0.98; 1.21] mmol/L to 1.2 [ 1.01; 1.25] mmol/L (p<0.001), TG decreased by 18.64% from 2.2 [ 1.92; 2.57] mmol/L to 1.79 [ 1.48; 1.95] mmol/L (p<0.001). In contrast, in the group with the fixed combination of rosuvastatin and ezetimibe, there was a more efficient decrease in the concentration of LDL-C in observed patients by 47.46% from 2.36 [ 1.92; 2.57] mmol/L to 1.24 [ 1.18; 1.56] mmol/L (p<0.001), TC decreased by 37.5% from 5.12 [ 4.68; 5.46] mmol/L to 3.2 [ 2.97; 3.37] mmol/L (p<0.001), the level of HDL-C increased by 6.48% from 1.08 [ 1.02; 1.2] mmol/L to 1.15 [ 1.06; 1.26] mmol/L (p<0.001), TG decreased by 25.43% from 2.32 [ 2.03; 2.6] mmol/L to 1.73 [ 1.44; 2.03] mmol/L (p<0.001) (Fig. 3). The dynamics of changes in lipid spectrum parameters by visits are presented in Table 2.
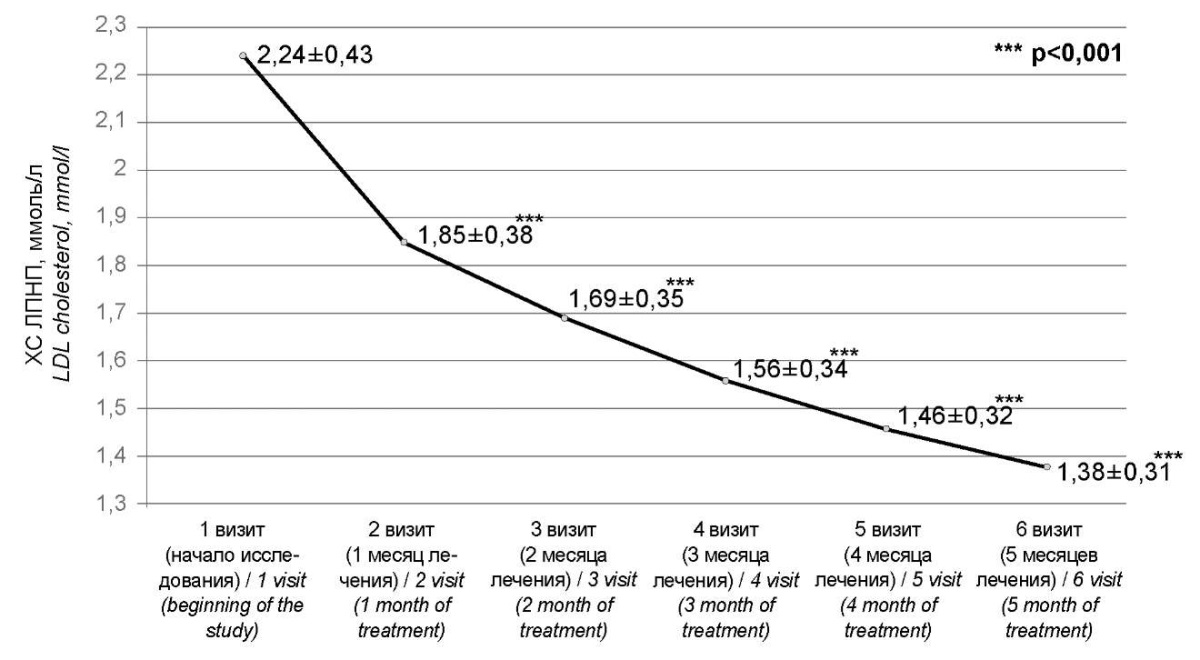
Рисунок 2. Динамика изменения ХС ЛПНП среди всех участников исследования.
Figure 2. Dynamics of changes in LDL cholesterol among all study participants.
Примечание: ХС ЛПНП — холестерин липопротеидов низкой плотности;
p — значение критерия Стьюдента для зависимых выборок.
Note: LDL cholesterol — low-density lipoprotein cholesterol;
p-values — the value of the Student's criterion for dependent samples.
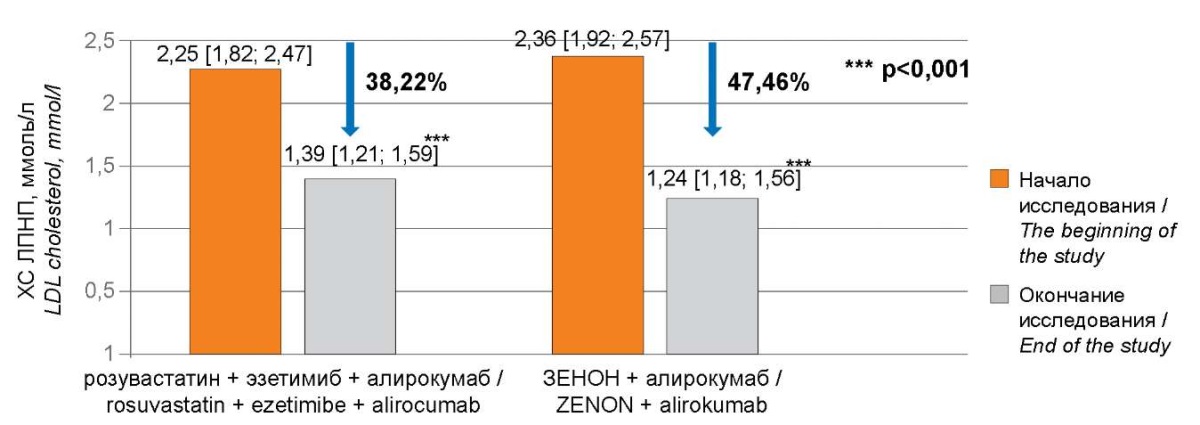
Рисунок 3. Снижение ХС ЛПНП в исследуемых группах.
Figure 3. Reduction of LDL cholesterol in the study groups.
Примечание: ХС ЛПНП — холестерин липопротеидов низкой плотности;
p — значение критерия Вилкоксона.
Note: LDL cholesterol — low-density lipoprotein cholesterol;
p-values — the value of the Wilcoxon criterion.
Таблица / Table 2
Динамика изменения показателей липидного спектра
в рассматриваемых группах в процессе проведения исследования
Dynamics of changes in lipid spectrum indicators
in the groups under consideration during the study
|
Показатели Indicators |
1 визит (скрининг) 1 visit (screening) |
2 визит (1 месяц лечения) 2 visit (1 month of treatment) |
3 визит (2 месяца лечения) ) 3 visit (2 month of treatment) |
4 визит (3 месяца лечения)) 4 visit (3 month of treatment) |
5 визит (4 месяца лечения)) 5 visit (4 month of treatment) |
6 визит (5 месяцев лечения)) 6 visit (5 month of treatment) |
||||||
|
Значение Meaning |
Значение Meaning |
p |
Значение Meaning |
p |
Значение Meaning |
p |
Значение Meaning |
p |
Значение Meaning |
p |
||
|
ОХ, ммоль/л TCh, mmol/l |
Не фикс Not a fix |
4,95 [ 4,6; 5,36] |
4,39 [ 4,23; 4,8] |
<0,001 |
4,13 [ 4,04; 4,31] |
<0,001 |
3,9 [ 3,81; 4,03] |
<0,001 |
3,61 [ 3,52; 3,81] |
<0,001 |
3,49 [ 3,32; 3,72] |
<0,001 |
|
Фикс Fix |
5,12 [ 4,68; 5,46] |
4,48 [ 4,22; 4,83] |
<0,001 |
4,08 [ 3,98; 4,21] |
<0,001 |
3,78 [ 3,59; 3,94] |
<0,001 |
3,45 [ 3,19; 3,63] |
<0,001 |
3,2 [ 2,97; 3,37] |
<0,001 |
|
|
ХС ЛПНП, ммоль/л LDL cholesterol, mmol/l |
Не фикс Not a fix |
2,25 [ 1,82; 2,47] |
1,88 [ 1,48; 2,14] |
<0,001 |
1,69 [ 1,37; 1,97] |
<0,001 |
1,55 [ 1,33; 1,77] |
<0,001 |
1,48 [ 1,27; 1,66] |
<0,001 |
1,39 [ 1,21; 1,59] |
<0,001 |
|
Фикс Fix |
2,36 [ 1,92; 2,57] |
1,81 [ 1,55; 2,13] |
<0,001 |
1,63 [ 1,42; 1,93] |
<0,001 |
1,42 [ 1,32; 1,78] |
<0,001 |
1,31 [ 1,22; 1,66] |
<0,001 |
1,24 [ 1,18; 1,56] |
<0,001 |
|
|
ХС ЛПВП, ммоль/л HDL cholesterol, mmol/l |
Не фикс Not a fix |
1,17 [ 0,98; 1,21] |
1,17 [ 0,99; 1,23] |
<0,001 |
1,16 [ 1; 1,23] |
<0,001 |
1,17 [ 0,99; 1,23] |
<0,001 |
1,18 [ 1,05; 1,23] |
<0,001 |
1,2 [ 1,01; 1,25] |
<0,001 |
|
Фикс fix |
1,08 [ 1,02; 1,2] |
1,1 [ 1,01; 1,22] |
<0,001 |
1,11 [ 1,02; 1,23] |
<0,001 |
1,13 [ 1,04; 1,25] |
<0,001 |
1,13 [ 1,05; 1,25] |
<0,001 |
1,15 [ 1,06; 1,26] |
<0,001 |
|
|
ТГ, ммоль/л TG, mmol/l |
Не фикс Not a fix |
2,2 [ 1,92; 2,57] |
2,02 [ 1,81; 2,38] |
<0,001 |
1,91 [ 1,72; 2,28] |
<0,001 |
1,87 [ 1,64; 2,2] |
<0,001 |
1,83 [ 1,58; 2,07] |
<0,001 |
1,79 [ 1,48; 1,95] |
<0,001 |
|
Фикс Fix |
2,32 [ 2,03; 2,6] |
2,16 [ 1,87; 2,46] |
<0,001 |
2,08 [ 1,73; 2,31] |
<0,001 |
2,01 [ 1,64; 2,23] |
<0,001 |
1,89 [ 1,57; 2,17] |
<0,001 |
1,73 [ 1,44; 2,03] |
<0,001 |
|
Примечание: ОХ — общий холестерин; ХС ЛПНП — холестерин липопротеидов низкой плотности; ХС ЛПВП — холестерин липопротеидов высокой плотности; ТГ — триглицериды; p — достоверность различий между группами.
Note: TCh — total cholesterol; LDL cholesterol — low-density lipoprotein cholesterol; HDL cholesterol — high-density lipoprotein cholesterol; TG — triglycerides; p-values — significant differences between respective groups.
Discussion
To date, a strong evidence base is provided for the concept of the efficiency of using “polypills” with fixed combinations of drugs. According to the results of such trials as UMPIRE [15], IMPACT [16], and CNIC [17], patients taking multicomponent drugs demonstrate a significant CVR reduction against the background of increased adherence to treatment. Herewith, despite the potential benefit and the presence of a certain evidence base, the lack of randomized clinical trials of multicomponent drugs limits the further implementation of this approach in clinical practice. Nevertheless, the last decade has been marked by the creation of many medications with fixed combinations of drugs, including those aimed at achieving target lipoprotein values.
The present study showed an increase in the efficiency of triple lipid-lowering therapy with a fixed combination of rosuvastatin and ezetimibe by 9.24% compared to the group of patients with separate administration of the drugs, which was consistent with the results of a retrospective analysis of two methods of prescribing lipid-lowering therapy conducted in 2019, where the efficiency of the fixed method of prescribing rosuvastatin and ezetimibe was 9% higher than the separate regimen of taking the drugs [13], and the results of the Multicenter Randomized Study of Rosuvastatin and Ezetimibe (MRS-ROSE), which demonstrated a more effective reduction in LDL-C levels with the fixed use of rosuvastatin and ezetimibe [18]. This study specifically used the combination of two methods of prescribing rosuvastatin and ezetimibe with alirocumab.
The results of the present study were consistent with the results of previous studies on the use of triple lipid-lowering therapy with statin, ezetimibe, and alirocumab; however, in previous studies, atorvastatin was used at the maximum tolerated dose of 40–80 mg/day [19–21].
During five months of combined lipid-lowering therapy, 55.38% of study participants achieved target LDL-C ranges, which was not consistent with the results of the FOURIER trial, where during triple combination lipid-lowering therapy, 97% of study participants had achieved target LDL-C values, which might be associated with longer follow-up of patients (2.2 years) and less ambitious target LDL-C limits (<1.8 mmol/L) [22].
The present study demonstrated that the achieved hypolipidemic effect one month after adding alirocumab to the treatment was maintained throughout the study, which might indicate the stability of the studied parameters and might show that there was no need for constant monitoring of lipid spectrum parameters in individuals with dyslipidemia, which was consistent with the results of the OSLER-1 trial, which observed patients receiving PCSK9 inhibitors for 48 months. The study identified the absence of a “fading effect” during the long-term use of PCSK9 inhibitors, which was most likely due to the lack of immunogenicity in this group of drugs [23].
It has been proven that female patients had a higher risk of not achieving target LDL-C values, which was inconsistent with a study conducted in 2020 at the Central Clinical Hospital of the Russian Academy of Sciences, where men had a higher chance of not achieving target LDL-C limits (χ2=0.004; OR 0.157; 95% CI 0.043–0.572), which might be associated with the lower statistical power of this study (49 patients were observed for 10 weeks) [24].
Therefore, the use of a fixed combination of rosuvastatin at the maximum tolerated dose and ezetimibe along with PCSK9 inhibitors was associated with a more efficient reduction in lipoprotein levels compared to triple hypolipidemic therapy with free combinations of drugs.
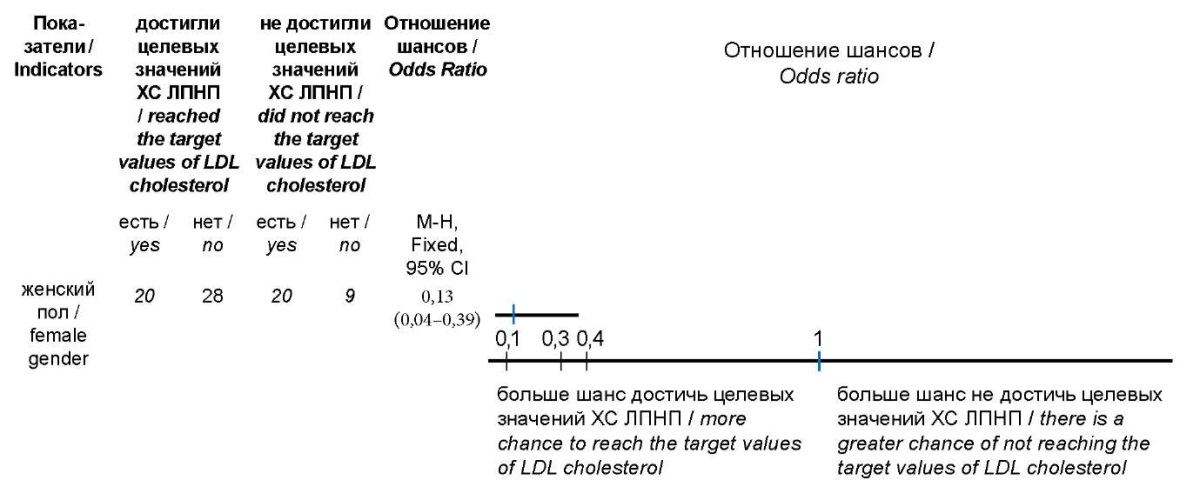
Рисунок 4. Отношение шансов показателей,
ассоциированных с более высоким риском
недостижения целевых концентраций ХС ЛПНП.
Figure 4. The ratio of the odds of indicators associated with a higher risk
of not reaching the target concentrations of LDL cholesterol.
Примечание: ХС ЛПНП — холестерин липопротеидов низкой плотности.
Note: LDL cholesterol — low-density lipoprotein cholesterol.
Conclusion
According to the results of this study, the use of a fixed combination of rosuvastatin and ezetimibe in triple combined lipid-lowering therapy with PCSK9 inhibitors in patients with very high CVR allows for a 9.24% more intensive reduction in LDL-C concentration compared to a drug regimen with separate administration of the drugs.
It seems appropriate to opt for the administration of lipid-lowering agents in the form of fixed combinations, especially in patients with low adherence to therapy.
1. https://www.gks.ru/folder/12781 (accessed: 09.09.2023).
2. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed: 09.09.2023)
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982-3021. Erratum in: J Am Coll Cardiol. 2021;77(15):1958-1959. https://doi.org/10.1016/j.jacc.2020.11.010.
2. Watts GF, Catapano AL, Masana L, Zambon A, Pirillo A, Tokgözoğlu L. Hypercholesterolemia and cardiovascular disease: Focus on high cardiovascular risk patients. Atheroscler Suppl. 2020;42:e30-e34. https://doi.org/10.1016/j.atherosclerosissup.2021.01.006
3. Boytsov S.A., Drapkina O.M., Shlyakhto E.V., Konradi A.O., Balanova Yu.A., et al. Epidemiology of Cardiovascular Diseases and their Risk Factors in Regions of Russian Federation (ESSE-RF) study. Ten years later. Cardiovascular Therapy and Prevention. 2021;20(5):3007. (In Russ.) https://doi.org/10.15829/1728-8800-2021-3007
4. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-52. https://doi.org/10.1016/S0140-6736(04)17018-9
5. Ezhov M.V., Kukharchuk V.V., Sergienko I.V., Alieva A.S., Antsiferov M.B., et al. Disorders of lipid metabolism. Clinical Guidelines 2023. Russian Journal of Cardiology. 2023;28(5):5471. (In Russ.) https://doi.org/10.15829/1560-4071-2023-5471
6. Gitt AK, Lautsch D, Ferrières J, De Ferrari GM, Vyas A, et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: Results from the Dyslipidemia International Study II. Atherosclerosis. 2017;266:158-166. https://doi.org/10.1016/j.atherosclerosis.2017.08.013
7. Gitt AK, Lautsch D, Ferrieres J, Kastelein J, Drexel H, et al. Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis. 2016;255:200-209. https://doi.org/10.1016/j.atherosclerosis.2016.09.004
8. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279-1289. https://doi.org/10.1093/eurjpc/zwaa047
9. Schuster H. The GALAXY Program: an update on studies investigating efficacy and tolerability of rosuvastatin for reducing cardiovascular risk. Expert Rev Cardiovasc Ther. 2007;5(2):177-193. https://doi.org/10.1586/14779072.5.2.177
10. Kotseva K, Wood D, De Bacquer D, De Backer G, Rydén L, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23(6):636-48. https://doi.org/10.1177/2047487315569401
11. De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Ž, et al. Management of dyslipidaemia in patients with coronary heart disease: Results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis. 2019;285:135-146. https://doi.org/10.1016/j.atherosclerosis.2019.03.014
12. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. Erratum in: Eur Heart J. 2020;41(44):4255. https://doi.org/10.1093/eurheartj/ehz455.
13. Catapano AL, Vrablik M, Karpov Y, Berthou B, Loy M, Baccara-Dinet M. A Phase 3 Randomized Controlled Trial to Evaluate Efficacy and Safety of New-Formulation Zenon (Rosuvastatin/Ezetimibe Fixed-Dose Combination) in Primary Hypercholesterolemia Inadequately Controlled by Statins. J Cardiovasc Pharmacol Ther. 2022;27:10742484221138284. https://doi.org/10.1177/10742484221138284
14. Kuznetsov A.A., Mal. G.S. Secondary prevention of coronary heart disease and PCSK9 inhibitors. Therapy. 2021;2:105-111. (in Russ.) https://doi.org/10.18565/therapy.2021.2.105-111
15. Thom S, Poulter N, Field J, Patel A, Prabhakaran D, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310(9):918-929. Erratum in: JAMA. 2013;310(14):1507. https://doi.org/10.1001/jama.2013.277064 .
16. Selak V, Elley CR, Bullen C, Crengle S, Wadham A, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ. 2014;348:g3318. https://doi.org/10.1136/bmj.g3318
17. Castellano JM, Sanz G, Peñalvo JL, Bansilal S, Fernández-Ortiz A, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64(20):2071-2082. https://doi.org/10.1016/j.jacc.2014.08.021
18. Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe). Cardiovasc Ther. 2016;34(5):371-382. https://doi.org/10.1111/1755-5922.12213
19. Mal G.S., Kuznetsov A.A. Coronary heart disease and chronic kidney disease: the possibilities of PCSK9 inhibitors in the achievement of atherogenic lipoproteins target values. Innovative Medicine of Kuban. 2022;(2):14-21. (In Russ.) https://doi.org/10.35401/2541-9897-2022-25-2-14-21
20. Kuznetsov A.A., Mal. G.S. The dynamics of atherogenic lipoproteins and estrogens during the management of dyslipidemia with PCSK9 inhibitors in patients with various comorbidities. International Journal of Heart and Vascular Diseases. 2022;10(35):23-32. (In Russ.) eLIBRARY ID: 49725100 EDN: BIAEWL
21. Kuznetsov A.A., Mal G.S. Application possibilities of PCSK9 inhibitors in patients with coronary heart disease in combination with type 2 diabetes mellitus. Regional blood circulation and microcirculation. 2022;21(2):16-25. (In Russ.) https://doi.org/10.24884/1682-6655-2022-21-2-16-25
22. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713-1722. https://doi.org/10.1056/NEJMoa1615664
23. Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, et al. Long-term Low-Density Lipoprotein Cholesterol-Lowering Efficacy, Persistence, and Safety of Evolocumab in Treatment of Hypercholesterolemia: Results Up to 4 Years From the Open-Label OSLER-1 Extension Study. JAMA Cardiol. 2017;2(6):598-607. https://doi.org/10.1001/jamacardio.2017.0747
24. Nikitin A.E., Averin E.E., Rozhkov D.E., Sozykin A.V., Procenko G.A. Alirocumab Administration Experience to Achieve Low Density Lipoprotein Cholesterol Target Levels in Secondary Prevention of Cardiovascular Disease. Rational Pharmacotherapy in Cardiology. 2020;16(1):33-39. (In Russ.) https://doi.org/10.20996/1819-6446-2020-02-06
About the Authors
A. A. KuznetsovRussian Federation
Andrei A. Kuznetsov - cardiologist of the cardiology department.
Moscow region
Competing Interests:
Authors declares no conflict of interest
G. S. Mal
Russian Federation
Galina S. Mal - Dr. Sci. (Med.), Professor, Head of the Department of Pharmacology.
Kursk
Competing Interests:
Authors declares no conflict of interest
I. A. Saraev
Russian Federation
Igor A. Saraev - Dr. Sci. (Med.), Professor of the Department of Internal Diseases No. 2.
Kursk
Competing Interests:
Authors declares no conflict of interest
Review
For citations:
Kuznetsov A.A., Mal G.S., Saraev I.A. Comparative effectiveness of different methods of prescribing rosuvastatin and ezetimibe in combination with PCSK9 inhibitors. Medical Herald of the South of Russia. 2024;15(2):81-89. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-2-81-89







































