Scroll to:
Interferon system in typical chronic and atypically occurring chronic active herpes virus infections
https://doi.org/10.21886/2219-8075-2023-14-4-58-65
Abstract
Objective: to study the characteristics of the functioning of the IFN system, the presence of autoantibodies to INFα in patients suffering from atypical chronic active herpesvirus infections (ACA-HVI) in comparison with patients with a typical course of chronic herpesvirus infections (CHVI).
Materials and methods: under our supervision were 485 patients of both sexes aged 23 to 70 years, suffering from chronic herpes virus infections, of which 335 patients suffered from AHA-HVI and 150 people suffered from CHVI. The comparison group was 250 conditionally healthy individuals (CG). The complex of the study included methods for detecting herpesviruses: serodiagnostics, PCR-RT. The IFN system (spontaneous and induced production, serum concentration) was tested by ELISA. The study was approved by the ethics board and informed consent was obtained from all patients.
Results: the incidence of various mono-mixed herpesvirus infections in patients with ACA-HVI (mono – 26,6% and mixed – 73.4%) and CHVI (mono – 23.1% and mixed – 76.9%) was determined, with EBV dominance in patients of both groups. Serum IFNα deficiency was detected in 100% of cases in both groups, and IFNα in 67% in ACA-HVI and 57% in CHVI. At the same time, significant differences were found between the ACA-HVI and HGVI groups in the level of IFNα reduction: 10 and 5 times, respectively, and for IFNγ – 2.0 and 2.6 times, respectively. The induced IFNα production decreased by 89.1% in ACA-HVI and 47.2% in CHVI. A decrease in induced IFNγ production is characteristic of 50% of patients in both groups. At the same time, the level of induced production of IFN α in patients with ACA-HVI was 9 times lower than in the control group and 4.75 times lower than in the group of patients with CHVI. And the level of induced IFNγ production was 2 times lower compared to CHVI and the control group.
Conclusions: when assessing the state of the IFN system in patients with various chronic herpes virus infections, significant differences were revealed. Thus, the most pronounced manifestations of interferonopathy, consisting in a significant decrease in serum IFNα and IFNγ and defects in induced IFN production of both types, are observed statistically significantly more often in the group of patients with an atypical course of the disease than in the group of patients with a typical course of CHVI. The most pronounced disorders in the IFN system and the lack of recovery of IFNα and IFNγ levels in the interrelational period cause atypicity of the course and active viral replication in patients with ACA-HVI.
For citations:
Khalturina E.O., Nesterova I.V., Malinovskaya V.V., Myandiev M.S. Interferon system in typical chronic and atypically occurring chronic active herpes virus infections. Medical Herald of the South of Russia. 2023;14(4):58-65. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-4-58-65
Introduction
It is known that an imbalance of the interferon (IFN) system is the cornerstone of the immunopathogenesis of atypical chronic active herpesvirus infections (ACA-HVI). It is often associated not only with congenital, genetically determined defects, so-called inborn errors of immunity, but also with acquired dysregulation of the IFN system at different levels: receptor, molecular, and at the level of the nuclear signal transmitter1,2 [1–5]. Type I IFNs play an important role in the development and modulation of the antiviral immune response by the induction of IFN-stimulated genes (ISGs) through the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signaling pathways [6][7]. In addition to the JAK-STAT-dependent pathway (canonical pathway), type I IFN can also activate signaling through STAT-independent pathways, so-called non-canonical activation through MAPK-mitogen-activated protein kinases and PI3K-phosphatidylinositol 3-kinase [7]. There are numerous cellular and molecular regulatory mechanisms of the IFN system that coordinate post-translational modification of signaling molecules and epigenetic modification of gene expression programs. They are the two important mechanisms for regulating IFN signaling that are critical for the development of IFN-mediated immune responses. It has been shown that the intervention of viruses, in particular viruses of the Herpesviridae family, frustrates the functional activity of the IFN system that underlies the development of immunopathogenesis of both infectious diseases and autoinflammatory syndromes and processes, through aberrant activation of inflammatory reactions or inadequate control over the course of viral infection [8– 10]. Thus, the adequacy of the IFN system's response to viral stimuli determines the efficacy of the immune response, facilitating viral clearance.
That is why the investigation of the functioning features of the interferon system and the associated to them special aspects of diagnosing these disorders are so relevant in modern medicine.
The purpose of the study was to examine the features of the functioning of the IFN system, revealing autoantibodies to INFα in patients suffering from atypical chronic active herpesvirus infections (ACA-HVI) and comparing to patients with the typical course of chronic herpesvirus infections (TCHVI).
Materials and methods
The study included 485 patients of both sexes, aged from 23 to 70 years and suffering from chronic herpesvirus infections. Among them, 335 patients had an atypical course of a chronic viral process (ACA-HVI) and 150 patients suffered from chronic herpesvirus infections of a typical course (TCHVI). The criteria for the atypical course of HVI were the recurrent or persistent recurrent course of HSV1, HSV2 with a relapse rate of 5 to 20 episodes per year and a relapse duration of 5 to 12 days, recurrent ARVIs from 5 to 24 times a year and their duration of over 7 days (5–10 days) with a duration of clinical well-being from 3 to 6 months. In addition, recurrent acute respiratory viral infections were often complicated by the addition of bacterial infections of the respiratory tract (acute bronchitis, acute pneumonia, etc.) and ENT organs (acute sinusitis, acute purulent adenoiditis, etc.). The dominant clinical manifestations in patients in this group were chronic fatigue syndrome and various cognitive disorders. At typical CHVI (TCHVI), the frequency of relapses of HSV1 and HSV2 does not exceed 2–4 times a year, while inducers of exacerbations are the effects of stressors, injuries, severe somatic diseases in the acute stage, surgical interventions, sudden changes in climate, temperature and time zones, etc. Relapses of TCHVI are uncomplicated, and their duration does not exceed 3–5 days. The comparison group consisted of 250 apparently healthy individuals, comparable in gender and age to HVI patients.
In addition to traditional research methods (history collection, physical examination, general clinical examinations), in order to verify the diagnosis of herpesvirus infections, serodiagnostic methods were used including the determination of antibodies of the IgM and IgG classes to Epstein-Barr virus (EBV) viral capsid antigen (VCA), IgG to Epstein-Barr virus nuclear antigen (EBNA), IgG to human herpes virus 6 (HHV6), IgM and IgG to cytomegalovirus (CMV), IgM and IgG to herpes simplex virus (HSV) of types 1/2 using ELISA test systems of NPO "Diagnostic Systems" (Russia), molecular genetic research methods including detection of the genome of herpes viruses in biomaterials, such as blood, saliva, urine, scrapings from the tonsils and posterior pharyngeal wall, by PCR-Real time test systems "AmpliSens" (Russia)). For a comprehensive assessment of the functional state of the interferon system, investigation was carried out on the spontaneous and induced production of IFNα and IFNγ after their induction in vitro by standard inducers including Newcastle disease virus (NDV) and phytohemagglutinin (PHA), respectively; the concentrations of serum IFNα and IFNγ and determination of the level of anti- IFNα Ig G were carried out by ELISA using test systems (ZAO Vector-Best, Novosibirsk; LLC Cytokin, St. Petersburg).
The study was approved by the ethics committee. According to the Helsinki Declaration of the World Medical Association; informed consent was obtained from every patient to participate in the study and to process personal data.
For statistical processing of the obtained data, Microsoft Excel computer software was used. The results were presented as the median (upper and lower quartiles) Me [Q1;Q3], and the Mann-Whitney and Wilcoxon tests were determined. Differences were considered significant at p<0.05.
Results
In patients suffering from TCHVI and ACA-HVI, the frequency of occurrence of various mono- and mixed herpesvirus infections was determined by the PCR-RT method. The results of determining the etiological structure of those features are presented in Figures 1 and 2.
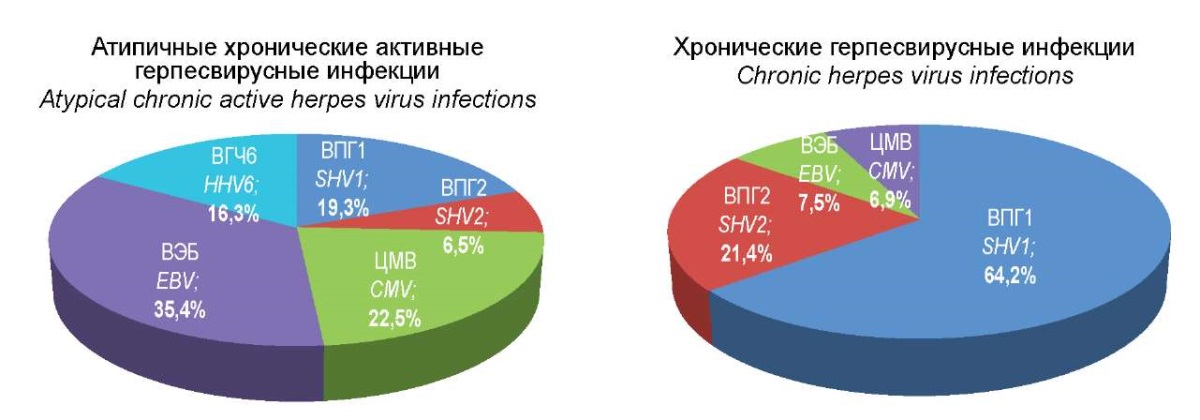
Figure 1. Etiological structure of mono-herpesvirus infections.
Analysis of the data obtained demonstrated that in the group of patients with ACA-GVI, 26.6% of patients suffered from monoinfections; 35.4% of them were patients with EBV infection; 22.5% were patients with CMV; 19.3% of monoinfections in the etiological structure were HSV type 1, 16.5% were HHV6 and 6.5% were HSV type 2, respectively (Figure 1).
In the meantime, in the group of patients with TCHVI, the etiological structure of HVI was completely different, although the total proportion of monoinfections in this group was no different from the group with ACA-HVI and amounted to 23.1%. The proportion of HSV type 1 was 62.4%, HSV type 2 – 21.4%, EBV and CMV – 7.5% and 6.9%, respectively, while the HHV6 type genome was not detected.
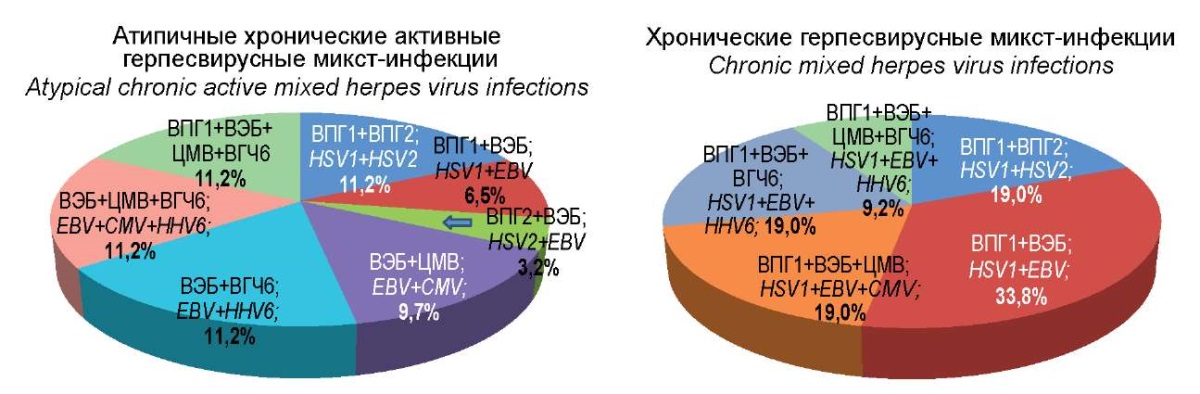
Figure 2. Etiological structure of herpes virus mixed infections.
When comparing the etiological structure of mixed herpesvirus infections in the group of patients with ACA-HVI and the comparison group of CHVI with a typical course, significant differences were also revealed.
Indicatively, the combination of HSV1 + HSV2 in TCHVI was recorded 1.7 times more often, while the combination of HSV1 + EBV was found 5.2 times more often than in ACA-HVI. Thus, the group of patients with TCHVI had significant differences from the group of patients with ACA-HVI in the frequency of occurrence of these two combinations of herpes viruses. Other variants of the combination of two types of herpes viruses, including HSV2, CMV, and HHV6, were found only in the group of patients with ACA-HVI with different frequencies of occurrence. With regard to the simultaneous registration of three pathogens, the finding showed that the combination of EBV + CMV + HHV6 was revealed only in the group of patients with ACA-HVI (11.2%), while the combination of HSV1 + EBV + CMV or HHV6 was determined only in patients of the comparison group and amounted to 19%. Finally, the combination of all four detectable herpesviruses occurred in both groups with approximately the same frequency, namely 11.2% and 9.2%, respectively.
Thus, in the group of patients with ACA-HVI, the identified characteristic features of the etiological structure consisted in a higher frequency of occurrence of EBV, CMV, and HHV6 with dominating EBV, while in mixed herpesvirus infections, they were related to a greater variety of combinations of herpesviruses of different types.
The investigation of the interferon status in patients with ACA-HVI was carried out by analyzing the content of IFN α and γ in the blood serum of patients in comparison with groups of TCHVI patients and apparently healthy people (Table 1, Figure 3).
Table 1
Status of serum interferons in patients with ACA-HVI
versus patients with CHVI and the control group
|
Interferons |
Median [ minimum; maximum] |
p1 p2 p3 |
||
|
ACA-HVI (n=335) |
CHVI (n = 150) |
Control (n=250) |
||
|
IFNα, pg/ml |
20.8 [ 9.0; 32.3] |
29.9 [ 23.9; 42.6] |
31.0 [ 28.2; 46.4] |
<0.001* <0.001* 0.002* |
|
IFNγ, pg/ml |
29.5 [ 10.3; 46.1] |
53.6 [ 40.3; 122.3] |
51.8 [ 40.7; 82.1] |
<0.001* 0.006* 0.885 |
Note: n — the number of examined persons in each group;
p1 — probability of differences in the ACA-HVI group and control group;
p2 — probability of differences in the CHVI and control groups;
p3 — probability of differences in ACA-HVI and CHVI;
* — reliability of Mann-Whitney differences at p < 0.05.
In the group of patients with ACA-GVI, a significant decrease in the serum concentration of both IFNα and IFNγ was revealed. A similar trend was found in the group of patients with TCHVI. Concurrently, the differences between the two groups in the level of the reduction in IFNα were significant and very pronounced, approximately 10 and 5 times less in the respective groups. For IFNγ, the degree of reduction was not so pronounced, namely, a decrease was 2 and 2.6 times, respectively; and no significant differences were found between the groups.
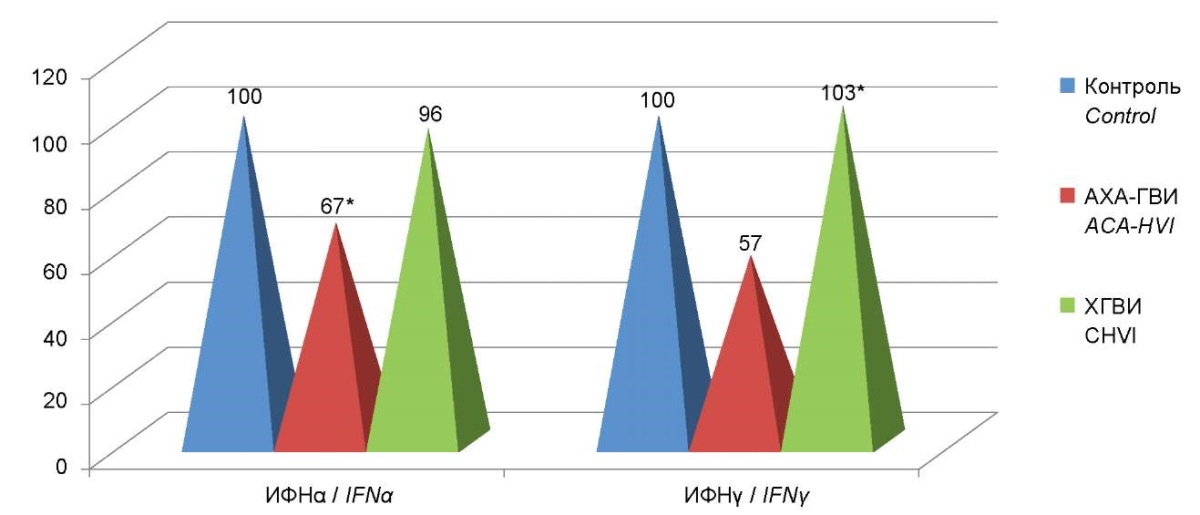
Figure 3. Percent deviation from the serum interferon control
in the ACA-HVI group compared to patients with CHVI.
It is of interest to note that there were no significant correlations between the levels of interferons and the revealing anti-interferon antibodies in the blood serum of patients in both groups, that is, a mechanism involving autoantibodies was not detected.
When determining the diagnostic significance of IFN α and γ levels in the blood sera of patients from the examined groups and healthy people (Figures 4 and 5), the isolation of the 95% confidence intervals of IFN levels was clearly shown in patients with ACA-GVI in the range of low values. In particular, for IFNα it was < 23.5 pg/ml, and for IFNγ, it was <37 pg/ml. The diagnostic significance of these deviations for ACA-HVI in both cases was very high (AUC were 0.888 and 0.865, respectively).
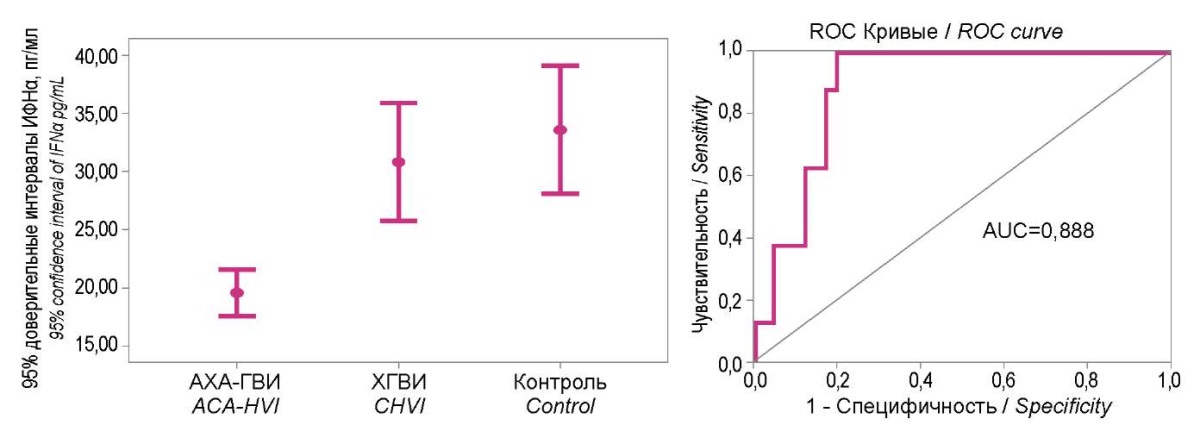
Figure 4. 95% confidence intervals of the interferon α level
in study groups and their diagnostic significance ROC analysis.
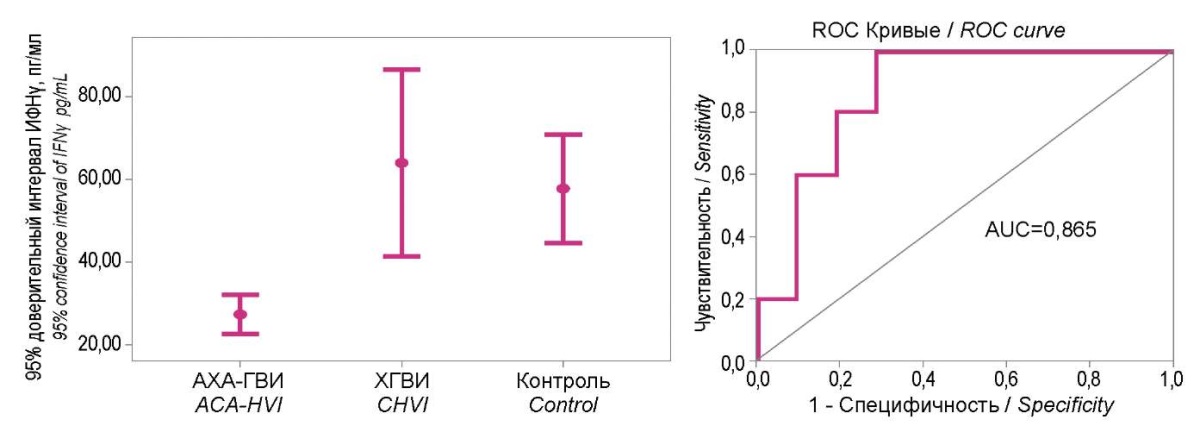
Figure 5. 95% confidence intervals of the interferon γ level
in study groups and their diagnostic significance ROC analysis.
Considering the identified features of changes in the concentrations of interferons of both types in the blood serum of patients with ACA-HVI, a comparative assessment of the content of IFN of both types in the serum of patients suffering from mono and mixed ACA-HVI was conducted. A significant decrease in the level of serum interferon α by 1.7 times was revealed in both groups of patients. The determined findings were as follows: for mono-HVI it was 18.94 (13.2; 28.1), for mixed-HVI it was 20.89 (18.7; 21.9) pg/ml vs. 31.05 (29.2; 35.0) pg/ml in the comparison group (p<0.05). The level of serum IFNγ in patients with mono-HVI was 31.89 (20.83; 39.44) pg/ml and 40.0 (23.4;40.0) pg/ml in the group of patients with mixed-HVI, which statistically differs them from the indicators of the comparison group (p˂0.05). There were no statistically significant differences in the level of serum IFNγ between the groups of patients with mono- and mixed-ACA-HVI (Figure 6).
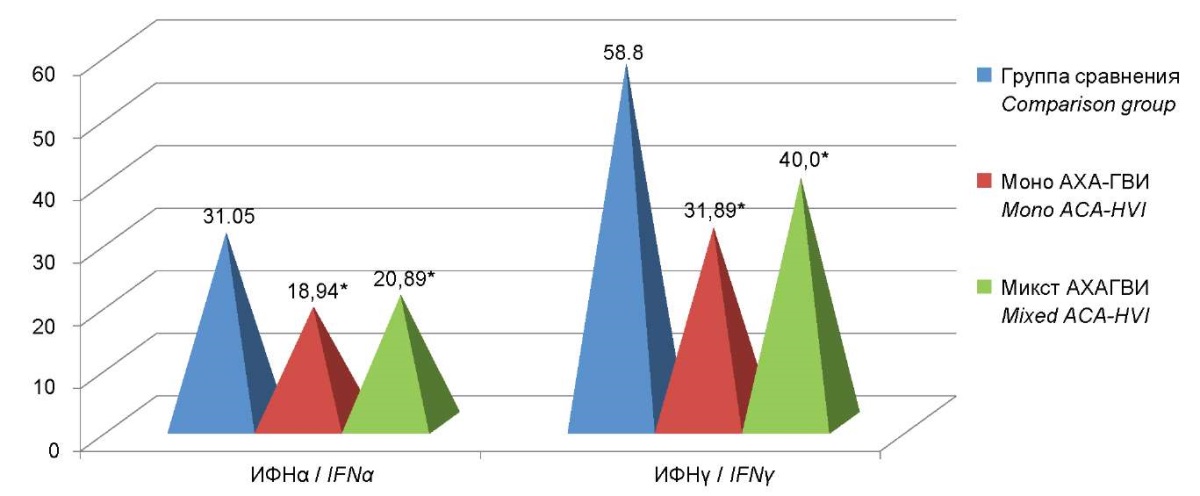
Figure 6. The levels of serum interferon in patients with mono and mixed-ACA-HVI.
Note: * — statistically significant differences compared to the comparison group (p<0.05).
An investigation of the features of the induced production of IFN α and γ revealed a decrease in the production of induced interferon α in 100% of patients with ACA-HVI, while a decrease in the induced production of interferon γ was recorded only in 48% of patients in this group.
The results obtained demonstrate that in patients with ACA-HVI and TCHVI, the levels of induced production of IFNα and IFNγ were significantly different from the indicators in the control group. Moreover, pronounced significant differences were revealed between the two groups themselves. The level of induced IFN α production in patients with ACA-HVI was 9 times lower than in the control group, and 4.75 times lower than in the group of patients with TCHVI. Furthermore, the level of induced IFNγ production was 2 times lower than that of the control group and TCHVI (Figure 7).
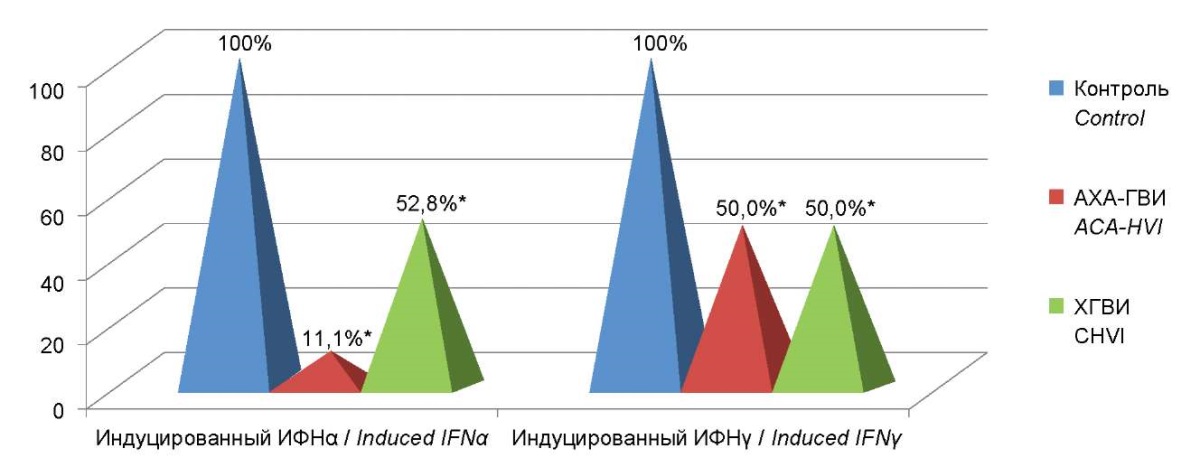
Figure 7. Percentage deviations from control of levels
of production of induced interferons α and γ
in the group of patients with ACA-HVI
compared to the comparison group and control group.
Note: * — statistically significant differences
with respect to the comparison group (p<0.05).
Discussion
Thus, in patients with ACA-HVI, various defects in the functioning of the interferon system were revealed – interferonopathies, including a significant decrease in the induced production of interferons of both types and a pronounced decrease in serum IFNα in relation to correspondent indicators in patients with TCHVI and in a group of apparently healthy individuals. They cause an inferior response of the immune system to the viral infectious process and a decrease in the efficacy of antiviral immune defense. One can assume that the identified disturbances in the interferon system may be caused by hereditary changes in interferon genes, as well as be acquired. It has been convincingly proven that herpes viruses are capable of exerting a negative suppressive effect on the interferon system using various strategies of suppression and antigenic mimicry, which is one of the bases for the immunopathogenesis of the atypical chronic active course of this infection.
Furthermore, the most pronounced manifestations of interferonopathy, consisting in a significant decrease in serum IFNα and IFNγ and defects in the induced production of IFN of both types, were revealed significantly more often in the group of patients with an atypical course of the disease than in the group of patients with a typical course of CHVI. It is important to note that in the inter-relapse period in the group of patients with TCHVI, the functioning of the IFN system was restored and the levels of IFNα and IFNγ reached the levels in apparently healthy individuals. Meanwhile, patients with ACA-HVI had the most pronounced impairments in the IFN system. In particular, IFNα and IFNγ levels were initially lower than in patients with a typical course of herpesvirus infection; and outside the period of exacerbation, they did not reach the values relatively equal to apparently healthy individuals. That is associated with a more serious damage to the IFN system and determines the atypical course of this infection and active viral replication in patients of this group.
Conclusions
The frequency of occurrence of various mono- and mixed herpesvirus infections in patients with ACA-HVI (mono 26.6%; mixed 73.4%) and TCHVI (mono 23.1%; mixed 76.9%) was determined. The dominance of EBV in various combinations of mixed herpesvirus infections in patients of both groups was revealed.
In all patients suffering from chronic herpesvirus infections of typical and atypical courses, various defects in the functioning of the interferon system were identified – interferonopathies of varying degrees of manifestation, including defects in the induced production of interferons of both types, and a marked decrease in serum IFNα.
In the blood serum of patients with ACA-GVI and TCHVI, this study revealed a statistically significant, pronounced decrease in the concentrations of both IFNα (in 100% of cases in both groups) and IFNγ (in 67% and 57%, respectively). Moreover, significant differences were revealed between the ACA-HVI and TCHVI groups in the manifestation of the decrease in the IFNα level, accompanied by a drop in concentration by 10 and 5 times, respectively. For IFNγ, the degree of reduction was significantly less and consisted of 2 and 2.6 times, respectively. No significant differences were found between the groups.
It was found that 89.1% of patients with ACA-HVI had a statistically significant, pronounced decrease in induced IFNα production by 9 times compared to the control group and 4.75 times compared to the group of patients with TCHVI. Meanwhile, a decrease in the induced production of IFNγ was revealed in 50% of patients both in the groups of patients with ACA-HVI and TCHVI compared with the group of apparently healthy individuals, which causes an inferior response of the immune system to the viral infectious process and a decrease in the efficacy of antiviral immune defense.
It was shown that in patients with ACA-HVI and TCHVI, Ig G autoantibodies to IFNα were not detected in the blood serum, which excludes the autoimmune genesis of serum IFNα deficiency.
1. Isakova V.A., Isakov D.V., Arkhipova E.I. Human herpesvirus infections. Guideline for doctors. St. Petersburg; 2015
2. Nesterova I.V., Kovaleva S.V., Chudilova G.A., Khalturina E.O., Malinovskaya V.V. Congenital and acquired interferonopathies associated with atypical viral infections and COVID-19 (monograph). St. Petersburg: Publishing house "Dialogue"; 2022.
References
1. Dyudyun A.D., Polyon N.M., Nagorny O.Ye. Herpesviral infection. The clinical and immunological features. A clinical lecture. Dermatovenerologiya. Kosmetologiya. Seksopatologiya. 2015;(3-4):119–125. (In Russ.) eLIBRARY ID: 26697236
2. Goreiko T.V., Kalinina N.M., Drygina L.V. The modern conceptions about immunopathogenesis of infection caused by the Epstein–Barr virus. Russian Journal of Infection and Immunity. 2014;1(2):121-130. https://doi.org/10.15789/2220-7619-2011-2-121-130
3. Novikovа I.A., Romanivа O.A. Features of cytokine production in patients with recurrent herpetic infection. Medical Immunology (Russia). 2013;15(6):571-576. (In Russ.) https://doi.org/10.15789/1563-0625-2013-6-571-576
4. Blaszczyk K, Nowicka H, Kostyrko K, Antonczyk A, Wesoly J, Bluyssen HA. The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses. Cytokine Growth Factor Rev. 2016;29:71-81. https://doi.org/10.1016/j.cytogfr.2016.02.010
5. Samarajiwa SA, Forster S, Auchettl K, Hertzog PJ. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 2009;37(Database issue):D852-7. https://doi.org/10.1093/nar/gkn732
6. Chen K, Liu J, Cao X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J Autoimmun. 2017;83:1-11. https://doi.org/10.1016/j.jaut.2017.03.008
7. Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, et al. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1(4):eaah6817. https://doi.org/10.1126/sciimmunol.aah6817
8. Li W, Lv S, Liu G, Lu N, Jiang Y, et al. Epstein-Barr virus DNA seropositivity links distinct tumoral heterogeneity and immune landscape in nasopharyngeal carcinoma. Front Immunol. 2023;14:1124066. https://doi.org/10.3389/fimmu.2023.1124066
9. Crow MK, Ronnblom L. Type I interferons in host defence and inflammatory diseases. Lupus Sci Med. 2019;6(1):e000336. https://doi.org/10.1136/lupus-2019-000336
10. Xia C, Anderson P, Hahm B. Viral dedication to vigorous destruction of interferon receptors. Virology. 2018;522:19-26. https://doi.org/10.1016/j.virol.2018.06.017
About the Authors
E. O. KhalturinaRussian Federation
Evgeniya O. Khalturina – Cand. Sci. (Med.), Associate Professo Associated Professor of Microbiology, Virology and Immunology Department named after A.A.Vorobiev
Moscow
Competing Interests:
Authors declares no conflict of interest
I. V. Nesterova
Russian Federation
Irina V. Nesterova – Dr. Sci. (Med.), Professor, Professor of the Department of Clinical Immunology
Moscow
Competing Interests:
Authors declares no conflict of interest
V. V. Malinovskaya
Russian Federation
Valentina V. Malinovskaya – Cand. Sci. (Bio.), Professor, Head of the Laboratory of Ontogenesis and Correction of the Interferon System
Moscow
Competing Interests:
Authors declares no conflict of interest
M. S. Myandiev
Russian Federation
Maurice S. Myandiev – Cand. Sci. (Med.), Assistant, Department of Propaedeutics of Dental Diseases
Moscow
Competing Interests:
Authors declares no conflict of interest
Review
For citations:
Khalturina E.O., Nesterova I.V., Malinovskaya V.V., Myandiev M.S. Interferon system in typical chronic and atypically occurring chronic active herpes virus infections. Medical Herald of the South of Russia. 2023;14(4):58-65. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-4-58-65







































