Scroll to:
Correlations of cytokines, regulating the synthesis of structural macromolecules of connective tissue, in undifferentiated connective tissue dysplasia in adolescent girls
https://doi.org/10.21886/2219-8075-2023-14-3-92-100
Abstract
Objective: to study the correlations of serum levels of cytokines, regulating the synthesis of structural macromolecules of connective tissue, in undifferentiated connective tissue dysplasia in adolescent girls with menstrual cycle disorders. Materials and methods: 176 adolescent girls aged 11 to 17 years with menstrual cycle disorders (MCD) and undifferentiated connective tissue dysplasia (UCTD) and 69 healthy girls of the same age were examined. Levels of cytokines IL-1β, IL-4, IL-6, IL-10, IL-17A, TNF-α, OPG, RANKL were studied in serum samples. Results: a significant increase in serum IL-6 and RANKL (p<0,01), as well as a tendency (p<0,1) to increase OPG production was found in adolescent girls with UCTD with MCD. In UCTD with NMC, correlations between the studied cytokines are much more common than in the control group (p=0,01). The most pronounced in adolescent girls with UCTD are positive correlations (moderate strength) between the concentrations of three cytokines IL-6, RANKL and OPG. Conclusions: The data obtained reflect the mechanisms of cytokine dysregulation of metabolism and remodeling of connective tissue and indicate the development of a weakly expressed systemic inflammatory process in UCTD with MCD in adolescent girls. The results of the study can be used to develop effective individualized schemes of therapeutic and preventive measures.
For citations:
Reznichenko N.A., Zoloto E.V., Maylyan E.A., Lesnichenko D.A., Nemsadze I.G., Prilutskii А.S., Bagriy A.E., Trunova O.A., Prochorov E.V. Correlations of cytokines, regulating the synthesis of structural macromolecules of connective tissue, in undifferentiated connective tissue dysplasia in adolescent girls. Medical Herald of the South of Russia. 2023;14(3):92-100. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-3-92-100
Introduction
The diversity of clinical manifestations and polysystemic lesions in connective tissue dysplasia (CTD) and its high prevalence among young people allows the authors to consider this disease as an urgent medical and social problem. In recent studies, many pathogenetic and phenotypic aspects of CTD were clarified, and approaches to diagnosis, treatment, and prevention of individual forms of dysplastic syndromes were developed [1][2]. Currently, it is accepted to divide СTD into 2 groups: differentiated and undifferentiated. Undifferentiated forms of CTD (UCTD) include a genetically heterogeneous group of nosologic forms associated with the development of multiple chronic diseases. The set of their clinical features does not fit into any of the hereditary monogenic diseases [3].
The high relevance of CTD in adolescence should be noted. Currently, adolescent girls with UCTD are considered to be at risk for reproductive system dysfunction [4–6]. Hormonal disorders accompanying the course of UCTD contribute to the formation of menstrual cycle disorders (MCDs) in these individuals, which include early menarche, uterine bleeding, and hypomenstrual syndrome [7].
It should be pointed out that the peculiarities of the regulatory link of the immune system in CTD remain understudied and do not lose their relevance. At the same time, one of the many effects of cytokines on target tissues and organs is the regulation of the synthesis of structural macromolecules of connective tissue. In particular, this is mediated through the effect on the source cells of connective tissue fibers. For example, tumor necrosis factor-α (TNF-α) has antifibrotic activity by triggering the down-regulation of the transforming growth factor-β type II receptor in human skin fibroblasts, which leads to desensitization of human skin fibroblasts to this factor [8]. In addition, it was shown that skin and lung fibroblasts in the presence of TNF-α demonstrated an antifibrotic response, which included the reduction of type I collagen synthesis [9]. Interleukin (IL)-1α induces proinflammatory responses of fibroblasts and suppresses the production of extracellular matrix (collagen, fibronectin, periostin), as well as the ability of cells to repair fibrillar collagen I [10]. IL-6 leads to decreased cell proliferation, enhanced apoptosis, and differentiation of primary human scleral fibroblasts, playing a role in sclera remodeling in myopic eyes [11]. It should be noted that myopia is one of the phenotypes of UCTD [12]. On the other hand, there is evidence that IL-6 along with transforming growth factor-β can increase the expression levels of type I collagen and fibronectin by myofibroblasts, thus playing a profibrotic role [13]. In turn, several studies have shown that IL-4 is the main profibrotic cytokine that stimulates collagen synthesis by fibroblasts. In response to the in vitro stimulation of human skin fibroblasts with interleukin-4, an increase in the levels of collagen types I and III and fibronectin was observed [14]. Significantly higher levels of IL-4 were found in skin biopsy samples, dermal fibroblast cultures, and serum from patients with systemic sclerosis [15]. In cutaneous wound healing, IL-4 promotes chemotaxis and proliferation of fibroblasts, differentiation of myofibroblasts, and production of collagen and extracellular matrix macromolecules [16]. IL-10, having no direct effect on fibroblasts, induces M2 macrophages to secrete factors that enhance fibroblast proliferation [17]. IL-17A also acts as a profibrotic cytokine and plays a key role in the fibrotic process of various organs such as the lung, kidney, heart, and skin [13]. Finally, the cytokine system RANK/RANKL/OPG (nuclear factor receptor activator κB/nuclear factor receptor activator ligand κB/osteoprotegerin) regulates bone remodeling as a specialized form of connective tissue by affecting the function of osteoclasts [18].
There are works devoted to the cytokine status in CTD in individuals of different sexes and ages, confirming one of the key roles of cytokine regulation in normal collagen metabolism and bone mineralization [19][20]. Nevertheless, the authors did not find any data on the relationship between the synthesis of various cytokines regulating the synthesis of structural macromolecules of connective tissue in UCTD and MCD in adolescent girls in the available publications.
The aim of the study was to investigate the correlations between the serum levels of cytokines regulating the synthesis of structural macromolecules of connective tissue in adolescent girls with UCTD and MCD.
Materials and Methods
The work was carried out at Donetsk National Medical University. A total of 176 adolescent girls with MCD combined with UCTD aged from 11 to 17 years old (mean value – 14.0±0.15 years old) were examined and constituted the comparison group. The control group included 69 adolescent girls of similar age without UCTD and MCD (mean value – 14.0±0.25 years old).
The criteria of Milkovska-Dimitrova were used to diagnose UCTD. The diagnosis was made by two major criteria (joint hypermobility, arthralgia for more than 3 months of non-inflammatory and non-traumatic nature), or one major and two minor criteria. Minor criteria included a marfanoid phenotype, recurrent dislocations in one joint or dislocation/subluxation of more than 1 joint, and characteristic skin changes (tissue paper sign, stretchy skin, thin skin). The studied patients had menstrual disorders, including early onset of menarche, hypomenstrual syndrome, symptoms of dysmenorrhea, and pubertal uterine bleeding.
The study excluded individuals with endocrine and metabolic disorders (thyroiditis, diabetes mellitus, etc.), chronic liver and kidney diseases, hematologic and psychiatric diseases, neoplastic conditions, autoimmune pathology (systemic connective tissue diseases, rheumatism, etc.), chronic inflammatory diseases, and individuals taking anti-inflammatory and immunosuppressive drugs (glucocorticosteroid drugs, etc.) or characterized by advance or delayed sexual development.
Peripheral blood serum was used as biological material for laboratory studies; it was collected in the morning hours using vacuum systems for intravenous blood sampling. The levels of IL-1β, -4, -6, -10, TNF-α (Vector-Best, Russian Federation, Novosibirsk region, Koltsovo), interleukin 17A (eBiosciences, San Diego, CA, USA), osteoprotegerin – OPG, and nuclear factor κB receptor activator ligand – RANKL (Biomedica Medizinprodukte, GmbH & Co KG, A-1210 Wien) in serum samples were analyzed using enzyme immunoassay test systems.
Statistical analysis of data was performed using the MedStat and MedCalc® Statistical Software version 20 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021). At the initial stage of mathematical processing of quantitative signs, variation series were evaluated for the normality of data distribution using the χ2 test. Taking into account the fact that for most of the studied indicators, the distribution was different from normal, the median (Me) and interquartile range (Q1-Q3) were calculated. Pairwise comparisons between the centers of independent samples were performed using the Mann-Whitney U-test. A comparison of the occurrence rate of a feature in two groups was performed using Fisher’s angular transformation with the Yates correction. The strength of the relationship between the indicators was determined using the Spearman rank correlation coefficient (rs). The closeness of the relationship was assessed using the Cheddock scale, considering values of the coefficient less than 0.3 as a sign of weak strength of the relationship; values greater than 0.3 but less than 0.5 – as a sign of moderate strength of the relationship; values greater than 0.5 but less than 0.7 – as a sign of marked strength of the relationship; values greater than 0.7 but less than 0.9 – as a sign of high strength of the relationship; and values of 0.9 or more – as a sign of very high strength of the relationship [21].
Differences were considered statistically significant at p<0.05.
Results
It was found that the serum content of most cytokines in the comparison group did not differ (p>0.05) from similar parameters of healthy individuals; and for IL-1β, it amounted to 2.15 (1.40–2.85) pg/mL, for IL-4 – 1.95 (1.30–2.65) pg/mL, for IL-10 – 3.10 (2.10–4.25) pg/mL, for IL-17A – 1.90 (1.10–2.80) pg/mL, and for TNF-α – 0.00 (0.00–0.30) pg/mL. At the same time, individuals with UCTD and MCD were characterized by a significant increase in the levels of IL-6 (1.60 (0.90–2.40) pg/ml vs. 1.05 (0.40–1.55) pg/ml in the control group; p=0.002) and RANKL (3.20 (2.25–4.25) pg/ml vs. 2.50 (1.55–3.20) pg/ml in the control group; p=0.003). Also in the comparison group, the authors registered a tendency (p=0.064) to the elevation of the osteoprotegerin content to values of 81.10 (62.75–100.30) pg/mL in comparison with 78.15 (53.35–78.85) pg/mL in healthy individuals.
The study of correlations between cytokine levels revealed significant positive associations both in the group of healthy individuals and in the group of adolescent girls with UCTD and MCD. In particular, in the control group, two direct correlations of weak strength out of 29 possible correlations were registered. TNF-α content correlated with OPG concentration (rs=0.247, p=0.041), and the IL-6 level correlated with IL-17A concentration (rs=0.263, p=0.029). In turn, in the comparison group, the authors registered 11 significant correlations of weak and moderate strength between the content of various cytokines, which was significantly more frequent (p=0.010) than in the control group. The values of Spearman rank correlation coefficients of weak strength between the content of cytokines in the group of adolescent girls with UCTD are presented in the Table.
Table
Indicators of Spearman's rank correlation coefficients
between the values of cytokines,
regulating the synthesis of structural macromolecules of connective tissue,
in adolescent girls with undifferentiated connective tissue dysplasia
and menstrual cycle disorders (n=176)
|
Parameter |
IL-6 |
TNF-α |
OPG |
|
IL-1β |
rs=0.175; p=0.0201 |
rs=0.160; p=0.0338 |
|
|
IL-6 |
rs=0.155; p=0.0404 |
||
|
IL-10 |
rs=–0.159; p=0.0348 |
||
|
IL-17A |
rs=0.179; p=0.0172 |
rs=0.255; p=0.0006 |
|
|
RANKL |
rs=0.293; p=0.0001 |
||
|
OPG |
rs=0.184; p=0.0161 |
Note: the table shows only statistically significant (p<0.05) values
of correlation coefficients.
The most pronounced (of moderate strength) were the correlations of IL-6 content with RANKL and OPG concentrations, as well as RANKL and OPG contents with each other. Thus, the value of the Spearman rank correlation coefficient in assessing the strength of the relationship between IL-6 and RANKL levels (Figure 1) was 0.324 (p<0.0001).
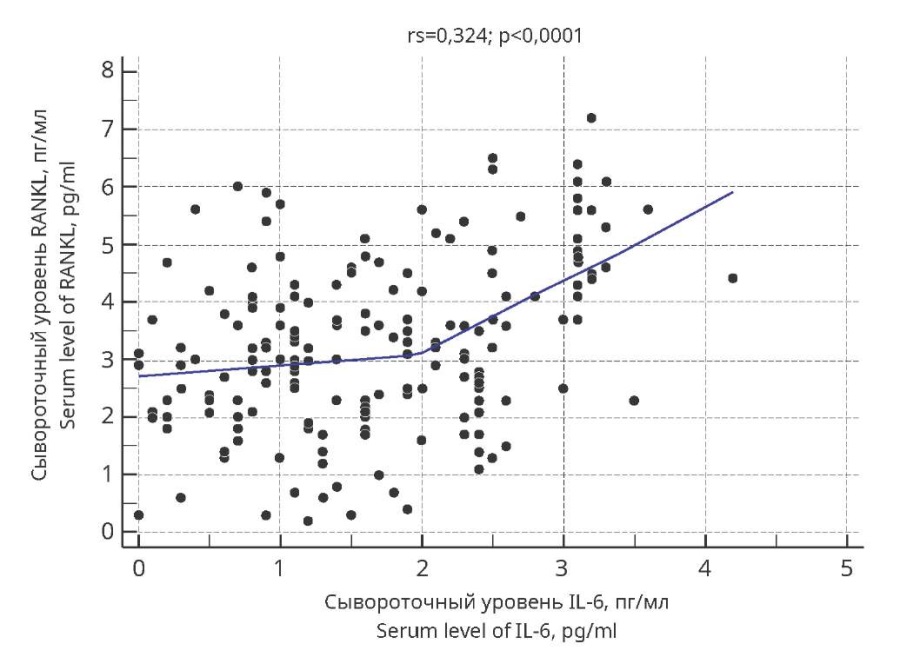
Figure 1. Scattering diagrams of Spearman’s rank correlation
between IL-6 and RANKL serum concentrations
in adolescent girls with undifferentiated connective tissue dysplasia
and menstrual cycle disorders.
Similarly, the authors recorded a moderately strong positive correlation between IL-6 content and OPG concentration (Figure 2). The value of the Spearman rank correlation coefficient was 0.341 (p<0.0001).
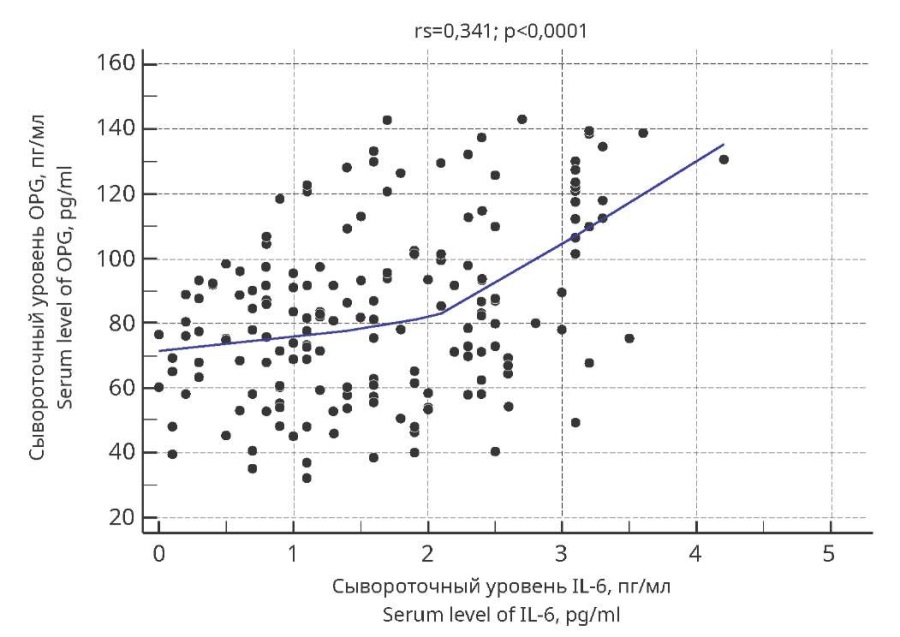
Figure 2. Scattering diagrams of Spearman’s rank correlation
between IL-6 and OPG serum concentrations
in adolescent girls with undifferentiated connective tissue dysplasia
and menstrual cycle disorders.
In turn, RANKL concentration positively correlated with OPG concentration (Figure 3). The value of the Spearman rank correlation coefficient was 0.320 (p<0.0001).
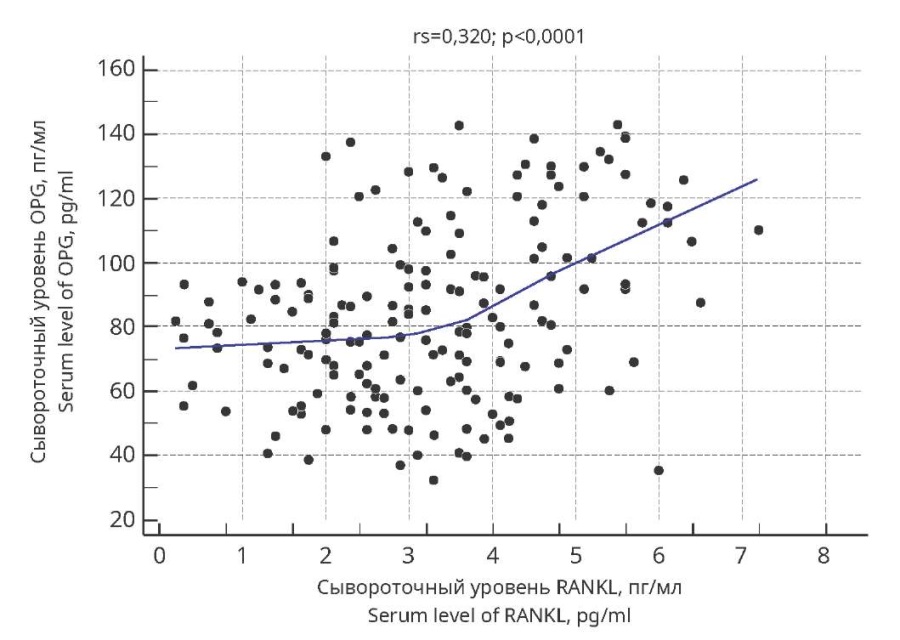
Figure 3. Scattering diagrams of Spearman’s rank correlation
between RANKL and OPG serum concentrations
in adolescent girls with undifferentiated connective tissue dysplasia
and menstrual cycle disorders.
Discussion
The performed studies allowed the authors to establish the associations of cytokine synthesis regulating the synthesis of structural macromolecules of connective tissue at UCTD in adolescent girls with MCD, which were more frequent in this group of patients compared to healthy individuals of similar sex and age (p=0.01). In these individuals, a significant increase in serum IL-6 and RANKL (p<0.01), as well as a tendency (p<0.1) to increase the level of OPG in 11 cases, were associated with correlations between the levels of a number of studied cytokines (p<0.05 – p<0.0001). The most pronounced correlations (of moderate strength) were registered between serum levels of three cytokines – IL-6, RANKL, and OPG. Thus, the values of serum IL-6 concentration had direct correlations with the values of serum concentrations of RANKL and OPG (p<0.0001). It is known that RANKL is a key factor in the differentiation and activation of osteoclasts, and OPG is known as an osteoclast inhibitory factor that binds RANKL [18][22]. It should be noted that the authors found a significant direct relationship between RANKL and OPG levels (p<0.0001).
The obtained results are consistent with the current understanding of the importance of the immune system, including regulators of fibroblast and osteoclast function, in the development of UCTD. It is known that bone is a specialized form of connective tissue, which is mineralized and includes about 28% of type I collagen and 5% of non-collagen matrix proteins [23]. In turn, the development of CTD is based on qualitative and quantitative changes in the production and assembly of collagen and elastin, synthesis of immature collagen, and defects in the structure of connective tissue fibers [24].
It should be noted that the presence of UCTD in adolescent girls with monthly cycle disorders is accompanied by the risk of osteopenic syndrome [25]. In addition, few studies indicate the presence of an imbalance in the cytokine system RANK/RANKL/OPG in pediatric patients with UCTD, which is accompanied by the activation of osteoclastogenesis and enhanced bone resorption [26].
It can be assumed that in response to the increase in RANKL production, a compensatory increase in OPG production develops, aimed ultimately at leveling the resorptive effect of this ligand and preventing excessive activation of osteoclasts. Elevated values of IL-6 content may indicate the development of a compensatory reaction, which is aimed at stimulation of collagen synthesis and acceleration of fibroblast maturation, which to some extent, contributes to preventing the development of pronounced dysplastic changes in bone tissue [27].
At the same time, a parallel increase in IL-6 levels with RANKL and OPG may also indicate the development of mild inflammation, which is exerted by a number of mechanisms. First, UCTD is accompanied by impaired lipid peroxidation with the development of oxidative stress, which provokes an inflammatory response [28]. Secondly, UCTD increases the risk of inflammatory processes in various systems of the body as a result of disruption of the structure of the connective tissue framework [29][30]. Together, these mechanisms result in an increase in the synthesis of various proinflammatory and anti-inflammatory cytokines.
Conclusion
It was found that in adolescent girls with UCTD and menstrual disorders, serum concentrations of IL-6 (p=0.002) and RANKL (p=0.003) were elevated. These changes were accompanied by an increase in the number of correlations between the studied cytokines from 2 to 11 out of 29 possible (p=0.010). The most pronounced correlations (of moderate strength) were registered between serum levels of IL-6, RANKL, and OPG. The obtained data reflect the mechanisms of cytokine dysregulation of metabolism and remodeling of connective tissue and indicate the development of a weakly expressed systemic inflammatory process in adolescent girls with UCTD and MCD. The results of the study can be used to develop effective individualized schemes of therapeutic and preventive measures.
References
1. Yagoda A.V., Gladkikh N.N., Gladkikh L.N. The specifics of adhesion function of endothelium in various clinical variants of primary mitral valve prolapse. Cardiovascular Therapy and Prevention. 2016;15(1):45-50. (In Russ.) https://doi.org/10.15829/1728-8800-2016-1-45-50
2. Yakovlev V.M., Martynov A.I., Yagoda A.V. Kliniko-visualnaya diagnostika klapannyh sindromov i podklapannyh anomaliy rasvitiya nasledstvennoy soyedinitelnotkannoy displasii serdca. Stavropol: StGMU; 2014. (in Russ.).
3. Kadurina T.I., Gorbunova V.N. Displazija soedinitelnoj tkani. Rukovodstvo dlja vrachej. Sankt-Peterburg: ELBI-SPb; 2009. (in Russ.).
4. Duma S.N., Shcherbakova L.V. Pain Syndromes in Patients with Undifferentiated Connective Tissue Disease and Approaches to their Correction. Medical Сare. 2019;3:23-33. (in Russ.). https://doi.org/10.24411/2071-5315-2019-12137
5. Lutsenko Yu.A., Cherkasov N.S., Davydova O.V., Ledyaev M.Ya., Makukhina L.P. Clinical and Instrumental Assessment of Forms and Syndromes Undifferentiated Connective Tissue Dysplasia in Children. Bulletin of the Volgograd State Medical University. 2019;3(71):58-61. (in Russ.). https://doi.org/10.19163/1994-9480-2019-3(71)-58-61
6. Pope MK, Ratajska A, Johnsen H, Rypdal KB, Sejersted Y, Paus B. Diagnostics of Hereditary Connective Tissue Disorders by Genetic Next-Generation Sequencing. Genet Test Mol Biomarkers. 2019;23(11):783-790. https://doi.org/10.1089/gtmb.2019.0064
7. Antunes M, Scirè CA, Talarico R, Alexander T, Avcin T, et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open. 2019;4(Suppl 1):e000786. https://doi.org/10.1136/rmdopen-2018-000786
8. Yamane K, Ihn H, Asano Y, Jinnin M, Tamaki K. Antagonistic effects of TNF-alpha on TGF-beta signaling through down-regulation of TGF-beta receptor type II in human dermal fibroblasts. J Immunol. 2003;171(7):3855-62. https://doi.org/10.4049/jimmunol.171.7.3855
9. Gonzalez Rodriguez A, Schroeder ME, Grim JC, Walker CJ, Speckl KF, et al. Tumor necrosis factor-α promotes and exacerbates calcification in heart valve myofibroblast populations. FASEB J. 2021;35(3):e21382. https://doi.org/10.1096/fj.202002013RR
10. Osei ET, B Mostaço-Guidolin L, Hsieh A, Warner SM, Al-Fouadi M, et al. Epithelial-interleukin-1 inhibits collagen formation by airway fibroblasts: Implications for asthma. Sci Rep. 2020;10(1):8721. https://doi.org/10.1038/s41598-020-65567-z
11. Liu L, Zhou W, Fan Y, Zhang L, Liu S, et al. Effect of Interleukin 6 on Scleral Fibroblast Proliferation, Differentiation, and Apoptosis Involved in Myopic Scleral Remodeling. Ophthalmic Res. 2022;65(5):529-539. https://doi.org/10.1159/000524502
12. Yuan J, Wu S, Wang Y, Pan S, Wang P, Cheng L. Inflammatory cytokines in highly myopic eyes. Sci Rep. 2019;9(1):3517. https://doi.org/10.1038/s41598-019-39652-x
13. Wei L, Abraham D, Ong V. The Yin and Yang of IL-17 in Systemic Sclerosis. Front Immunol. 2022;13:885609. https://doi.org/10.3389/fimmu.2022.885609
14. Gillery P, Fertin C, Nicolas JF, Chastang F, Kalis B, et al. Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures. Potential role in fibrosis. FEBS Lett. 1992;302(3):231-4. https://doi.org/10.1016/0014-5793(92)80448-p
15. Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24(2):328-32. PMID: 9034992.
16. Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. 2013;1832(7):1049-60. https://doi.org/10.1016/j.bbadis.2012.09.014
17. Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112(3):33. https://doi.org/10.1007/s00395-017-0622-5
18. Ignatenko G.A., Nemsadze I.G., Mirovich E.D., Churilov A.V., Maylyan E.A., et al. The role of cytokines in bone remodeling and the pathogenesis of postmenopausal osteoporosis. Medical Herald of the South of Russia. 2020;11(2):6-18. (In Russ.) https://doi.org/10.21886/2219-8075-2020-11-2-6-18
19. Hofbauer LC, Kühne CA, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact. 2004;4(3):268-75. PMID: 15615494.
20. Yagoda A.V., Gladkikh N.N Condition of Autoimmunity to Collagen and Cytokine Structure in Patients with Mitral Valve Prolapse. Medical news of North Caucasus. 2008;2:30-33. (in Russ.). eLIBRARY ID: 15287240
21. Zhukova A.A., Minets M.L. Biometriya: posobie. V 3 ch. Ch. 3. Korrelyatsiya i regressiya. Minsk: BGU; 2021. (In Russ.)
22. Maylyan E.A., Reznichenko N.A., Ignatenko G.A. Cytokines serum levels in postmenopausal osteoporosis. Crimean Journal of Experimental and Clinical Medicine. 2018;8(1):36-42. (In Russ.) eLIBRARY ID: 35310840
23. Vammi S, Bukyya JL, Ck AA, Tejasvi MLA, Pokala A, et al. Genetic Disorders of Bone or Osteodystrophies of Jaws-A Review. Glob Med Genet. 2021;8(2):41-50. https://doi.org/10.1055/s-0041-1724105
24. Ben Salha M., Repina N.B. Clinical diagnostics of undifferentiated connective tissue dysplasia. I.P. Pavlov Russian Medical Biological Herald. 2016;24(4):164-172. (In Russ.) https://doi.org/10.23888/PAVLOVJ20164164-172
25. Chaika A.V., Zoloto E.V. Features of Bone Mineral Density in Adolescent Girls with Undifferentiated Connective Tissue Dysplasia and Menstrual Disorders. Torsuyevskiye Chteniya: Scientific and Practical Journal on Dermatology, Venereology and Cosmetology. 2018;4(22):27-31. (in Russ.). eLIBRARY ID: 36931680
26. Pavlov S.B., Pavlova G.B. Study the role of intercellular mediators in the metabolism of connected tissue in children with cardiomyopathy and osteopeny. Journal of Education, Health and Sport. 2016;6(9):902-916. https://doi.org/10.5281/zenodo.163528
27. Choy E., Rose-John S. Interleukin-6 as a multifunctional regulator: inflammation, immune response, and fibrosis. J Scleroderma Relat. 2017;2:S1–5. https://doi.org/10.5301/jsrd.5000265
28. Smirnova T.L., Gerasimova L.I. Specific Clinical Features of Undifferentiated Connective Tissue Dysplasia Syndrome. Doctor.Ru. 2018;8(152):40-44. https://doi.org/10.31550/1727-2378-2018-152-8-40-44
29. Nechaeva G.I., Martynov A.I. Guidelines of the russian scientific medical society of internal medicine on the diagnosis, treatment and rehabilitation of patients with the connective tissue dysplasia (first edition). Medical News of North Caucasus. 2018;13(1–2):137-209 (in Russ.). https://doi.org/10.14300/mnnc.2018.13037
30. Moskovenko N.V., Beznoshchenko G.B., Andryukhin M.I. The hormonal status of female patients of reproductive age with chronic cystitis and undifferentiated connective tissue dysplasia. Doctor.Ru. 2017;7(136):46-50 (in Russ.). eLIBRARY ID: 29824953
About the Authors
N. A. ReznichenkoРоссия
Natalia A. Reznichenko - Dr. Sci. (Med.), Professor, professor of the Department of obstetrics, gynecology and perinatology No 1, Institute «Medical Academy named after S.I. Georgievsky» V.I. Vernadsky Crimean Federal University.
Simferopol
Competing Interests:
The author declare that there is no conflict of interests
E. V. Zoloto
Россия
Elena V. Zoloto - Dr. Sci. (Med.), associate Professor, director of the Research Institute of Reproductive health of children, adolescents and youth, assoc. prof of the Department of obstetrics, gynecology, perinatology, pediatric and adolescent gynecology, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
E. A. Maylyan
Россия
Edward A. Maylyan - Dr. Sci. (Med.), Professor, head of the Department of microbiology, virology, immunology and allergology, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
D. A. Lesnichenko
Россия
Denis A. Lesnichenko - Cand. Sci. (Med.), associate Professor, assoc. prof of the Department of microbiology, virology, immunology and allergology, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
I. G. Nemsadze
Россия
Ilona G. Nemsadze - Cand. Sci. (Med.), assoc. prof. of the Department of obstetrics and gynecology, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
А. S. Prilutskii
Россия
Аleksandr S. Prilutskii - Dr. Sci. (Med.), Professor, professor of the Department of microbiology, virology, immunology and allergology, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
A. E. Bagriy
Россия
Andrey E. Bagriy - Dr. Sci. (Med.), Professor, head of the Department of internal medicine No 2, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
O. A. Trunova
Россия
Olga A. Trunova - Dr. Sci. (Med.), Professor, professor of the Department of higher education organization, health management and epidemiology, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
E. V. Prochorov
Россия
Evgeniy V. Prochorov - Dr. Sci. (Med.), Professor, head of the Department of pediatrics No 1, Donetsk State Medical University.
Donetsk
Competing Interests:
The author declare that there is no conflict of interests
Review
For citations:
Reznichenko N.A., Zoloto E.V., Maylyan E.A., Lesnichenko D.A., Nemsadze I.G., Prilutskii А.S., Bagriy A.E., Trunova O.A., Prochorov E.V. Correlations of cytokines, regulating the synthesis of structural macromolecules of connective tissue, in undifferentiated connective tissue dysplasia in adolescent girls. Medical Herald of the South of Russia. 2023;14(3):92-100. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-3-92-100
JATS XML







































