Scroll to:
Optimization of management approaches for patients with cervical intraepithelial neoplasia mildly (LSIL) associated with high oncogenic risk human papillomaviruses
https://doi.org/10.21886/2219-8075-2023-14-2-5-17
Abstract
Objective: To improve the management of mild cervical dysplasia by correcting serum folic acid as an epigenetic risk factor for disease progression.
Materials and methods: 90 patients with different outcomes of dynamic follow-up of LSIL associated with one type of HPV VCR were included in the study (16, 18, 33). Group I — 43 patients with regression of the disease; Group II — 30 patients with persistence of HPV infection and group III — 17 patients with LSIL progression. The effectiveness of the differentiated approach to the management of patients in the study groups was assessed based on the results of cytology of cervical smears and immunocytochemical reaction, the level of viral load, the results of colposcopic examination, and the dynamics of serum folic acid levels. Statistical processing of the results was performed using IBM SPSS Statistics 28.0.1.1, STATISTICA 13.5.0.17 and MedCalc 20.027.
Results: the prognostic significance of a number of anamnestic parameters and serum folate deficiency, the totality of which can be used as predictors of disease outcome progression, has been established; a computer program has been developed to calculate the individual risk of disease progression (IRD), which facilitates the use of the developed method for calculating progression in clinical practice.
Conclusions: The proposed method with a high degree of reliability and informativeness allows predicting the progression of LSIL without violating the algorithm of current clinical recommendations, timely forming among patients a high-risk group for the development of a more severe form of the disease (HSIL) and individually determining further treatment tactics.
For citations:
Anufrieva V.G., Lebedenko E.Yu., Voloshin V.V., Gaida O.V. Optimization of management approaches for patients with cervical intraepithelial neoplasia mildly (LSIL) associated with high oncogenic risk human papillomaviruses. Medical Herald of the South of Russia. 2023;14(2):5-17. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-5-17
Introduction
Human papillomavirus of high carcinogenic risk (HPV HCR) infection is by far the proven, most significant risk factor for cervical intraepithelial neoplasia (CIN) and an integral component of its progression to invasive squamous cell cancer. With a high lifetime incidence of HPV infection (80%), about 83% of infections are transient, the development of intraepithelial neoplasia will be reported in only 5–10% of infected women, and cervical cancer will develop in less than 1%.
Currently, the outcome of CIN (especially cervical low-grade squamous intraepithelial lesion (LSIL)) is difficult to predict; therefore, the often-unjustified use of active surgical tactics for cervical intraepithelial lesions that can regress on their own leads to a number of adverse reproductive health outcomes. Infertility, pregnancy failure, premature delivery, cervical insufficiency, and cesarean section in subsequent pregnancies determine the ongoing search for epigenetic factors that may serve as prognostic markers of the risk of CIN progression.
Methylation of deoxyribonucleic acid (DNA) at position C5 of the cytosine base within CpG-dinucleotides is a proven basic epigenetic mechanism, the disruption of which is registered already at the early stages of carcinogenesis and is an important step in the progression of neoplasia. Having epigenetic nature, these disorders trigger changes in gene expression, but without affecting the DNA nucleotide sequence and without leading to structural changes in the genome, they are potentially reversible [1][2].
It is known that HPV HCR persistence and progression of mild to severe CIN and the development of cervical cancer depend on DNA methylation of the virus itself. The full-fledged process of HPV HCR DNA methylation after introduction into cervical epithelial cells determines a number of important protective effects, such as inhibition of the synthesis of oncoproteins E6 and E7, which contributes to the suppression of cell proliferation processes, support of apoptosis of malignant cells, an increase in interferon synthesis, support of protective regulatory mechanisms of DNA repair, and stabilization of the genome in the end [3].
Hypomethylation of mobile DNA in the human genome triggers activation of transcription, which was proven by studying the epigenetics of a number of many malignant neoplasms. Disruption of DNA methylation in cells as a result of a decrease in the number of methyl groups correlates with the degree of tumor malignancy and is one of the earliest events in the multistep process of carcinogenesis in the breast, cervix, ovaries, and brain [3–6].
In modern obstetric professional and popular scientific literature, the high efficacy of folic acid and vitamin-mineral complexes containing vitamin B9 in the primary prevention of fetal neural tube defects when used in the periconceptional period has been widely reported [7–9].
Among the studies of recent years, the evidence of a relationship between serum folic acid levels and various cancers is of scientific and practical interest [10–14].
The association between folate deficiency and the risk of cancer development in various localizations was demonstrated by the results of a meta-analysis published in 2015 [15]. There is evidence that folate deficiency in combination with methylenetetrahydrofolate reductase (MTHFR) polymorphism is associated with a higher incidence of colorectal cancer [16] and breast cancer [17].
Despite the proven relationship between DNA methylation disorders and carcinogenesis, its causes and specific mechanisms are the subject of further research and discussion. In particular, new approaches to the treatment of malignant neoplasms by demethylation of tumor growth suppressor genes with their subsequent reactivation are widely discussed [18].
Whether the risk of progression with LSIL associated with HPV HCR can be reduced by correction of serum folic acid levels remains understudied.
The study included 90 patients with different outcomes of dynamic follow-up of LSIL associated with one of the HPV HCR types (16, 18, 33). Group I included 43 patients with regression of the disease (normalization of cervical smear results (NILM) associated with HPV HCR elimination or reduction of the viral load level to clinically insignificant (less than 103 genomic equivalents of HPV HCR), normalization of the visual state of the cervix according to the colposcopic examination (Table 1).
Group II was represented by 30 patients with persistent HPV infection, who after 6 months of follow-up still had changes in cervical epithelial cytology within LSIL, the same level of viral load (p=0.571, χ2=1.122), and no statistically significant changes in MRCI scores (Table 2).
Group III consisted of 17 patients with LSIL progression characterized by cytological changes in the cervical epithelium to high-grade squamous intraepithelial lesion (HSIL), positive p16ink4α/Ki-67 co-expression double staining test by immunocytochemical study, a statistically significant increase in viral load (p<0.001, χ2=39.192), and an increase in MRCI score (p<0.01, χ2=52.41) (Table 4).
Previous studies conducted at the Department of Obstetrics and Gynecology No. 3 of the Federal State Budgetary Educational Institution of Higher Professional Education of RostSMU of the Ministry of Health of Russia established the prognostic significance of a number of anamnestic parameters and deficiency of the serum folic acid level, the combination of which can be used as predictors of disease outcome progression [19]. The obtained results were used as a basis for the development of a method of LSIL progression prognosis (Russian Federation Patent No. 2766719 published on March 15, 2022) [20].
For the convenient application of the proposed method in clinical practice, a computer program was developed that allows the specialists to calculate automatically the numerical value of the progression risk index (PRI). If the RPI value is <1, the risk of LSIL progression to a more severe form of the disease is “low” and further follow-up is possible in accordance with clinical recommendations. If the PRI value >1, the risk of progression is considered “high”, and further dynamic follow-up, regulated by clinical recommendations [21], should be supplemented by verification of the etiology of folate deficiency and its correction.
All patients with serum concentrations of folic acid outside the reference values (Table 1) were consulted by specialized professionals to clarify the genesis of insufficient vitamin B9 levels (nutritional deficiency, mutations in genes encoding enzymes of the folic acid cycle) and to choose the main direction of pharmacotherapy. The examination of the patients revealed no clinical and laboratory markers of folate deficiency anemia, and the main cause of folic acid deficiency was found to be an alimentary factor.
Table 1
Incidence of folic acid disorders in patients in the study groups, (%)
|
Serum folic acid levels (ng/ml) |
Clinical groups |
χ2 |
p |
||
|
group I (n = 43) |
group II (n = 30) |
group III (n = 17) |
|||
|
abs (%) [ 95% CI] |
abs (%) [ 95% CI] |
abs (%) [ 95% CI] |
|||
|
Normal (≥6) |
38 (88.4) [ 62.4-98.2] |
3 (10.0) [ 6.5-18.3] |
1 (5.9) [ 1.2-10.3] |
40.960 0.003 32.950 |
pI-II<0.001 pII-III=0.954 pI-III<0.001 |
|
Insufficiency (3.1-5.9) |
4 (9.3) [ 0.8-11.4] |
24 (80.0) [ 68.2-95.3] |
4 (23.5) [ 12.4-29.7] |
34.425 12.119 1.080 |
pI-II<0.001 pII-III<0.001 pI-III=0.299 |
|
Deficiency (<3.0) |
1 (2.3) [0] |
3 (10.0) [ 1.8-18.4] |
12 (70.6) [ 67.1-87.3] |
29.549 15.649 29.549 |
pI-II<0.001 pII-III<0.001 pI-III<0.001 |
Forty out of 43 patients (81.6%) with regression of the disease were recommended "routine" cervical screening in accordance with national clinical guidelines (HPV testing combined with a cytologic examination of cervical specimen (co-test) every three years). Three out of 43 (18.4%) patients of group I with vitamin B9 deficiency were recommended a prophylactic dose of folic acid (400 µg/day) by oral intake of a complex preparation containing a combination of folic acid (200 µg/g) and the active form of synthetic folic acid 5-MTHF (L-methylfolate) (200 µg/g) 1 tablet per day for three months. The choice of this preparation was determined by its ability to carry out folate supplementation without taking into account genetic polymorphisms of folate cycle enzymes. Due to the fact that absorption of metafolin can be carried out without the participation of various enzymatic systems of the intestinal tube, replenishment of the necessary level of folic acid and realization of the most important metabolic functions of this micronutrient (DNA replication and methylation) can also be realized in patients with a defect in the enzyme methylenetetrahydrofolate reductase (MTHFR). [22].
For group II patients (with HPV HCR persistence), the wait-and-see approach was supplemented with folate status correction depending on the presence and severity of serum vitamin B9 level abnormalities. Three out of 30 patients (10.0%) (with deficiency of this micronutrient) were prescribed folate therapy (oral intake of folic acid preparations 1000 µg/day) by specialized specialists. Twenty-four out of 30 patients (80.0%) in this group were recommended to take a complex preparation containing a combination of folic acid (200 µg) and the active form of synthetic folic acid 5-MTHF (L-methylfolate) (200 µg) 1 tablet per day for three months. In three out of 30 women in this group (10.0%), no disturbances in serum folic acid levels were detected.
Group III patients with disease progression underwent targeted cervical biopsy with subsequent morphologic verification of the biopsy specimen in accordance with current clinical guidelines. All 17 patients with verified CIN II in the biopsy specimen underwent loop electrosurgical excision of the cervix with a subsequent histologic examination of the excised cervical cone. According to the obtained results, the histologic conclusion (CINII) coincided with the results of the targeted biopsy in 15 out of 17 patients (88.2%). Two out of 17 (11.8%) patients had mismatched diagnoses: in one case, a higher degree of lesion was found, carcinoma in situ (CIS) was detected, and the patient was referred to a specialized institution for further follow-up; in the other case, a less pronounced lesion (CINI) was detected.
Active tactics in group III patients were accompanied by the medication correction of serum concentrations of folic acid. Fifteen out of 16 patients (93.7%) with vitamin B9 deficiency received folate therapy (oral intake of folic acid preparations 1000 µg per day) with control of its serum level in the dynamics of treatment. One of 16 patients (6.3%) with vitamin B9 deficiency took a complex preparation containing a combination of folic acid (200 µg) and active form of synthetic folic acid 5-MTHF (L-methylfolate) (200 µg) 1 tablet per day for three months. Postoperative drug treatment in patients of group III consisted in local intravaginal therapy with a drug having multitarget antitumor activity against cervical squamous cell intraepithelial lesions associated with HPV, containing 3,3’-diindolylmethane 1 vaginal suppository (100 mg) twice a day for three months.
The effectiveness of the differentiated approach to the management of patients in the study groups was evaluated by the results of cervical smear cytology and immunocytochemical reaction of p16ink4α/Ki-67 co-expression, viral load level, colposcopic examination results, and the dynamics of the serum folic acid level.
Determination of the serum level of folic acid concentration was performed by chemiluminescent immunoassay on microparticles, Architect i2000 (Abbott) using the ADVIA Centaur FOL reagent kit manufactured by Siemens Healthcare Diagnostics (Germany). The results of vitamin B9 concentration were interpreted according to the reference values – 3.1–20.5 ng/mL.
Statistical processing of the results was performed using methods of parametric and nonparametric analysis using the programs IBM SPSS Statistics 28.0.1.1 (developer – IBM Corporation), STATISTICA 13.5.0.17 (developer – StatSoft Inc.), and the MedCalc 20.027 package.
In group II, after 6 months, the overall proportion of patients with LSIL decreased significantly (from 100.0% to 20.0%; χ2=36.736, p<0.001). At the end of 12 months of follow-up, these cervical epithelial changes persisted in only two patients (6.7%; χ20-12=48.817, p<0.001). Progression of cervical intraepithelial lesions to HSIL was not detected in any patient. The frequency of normal cervical smears (NILM) increased significantly (χ2=24.955, p<0.001), and the proportion of patients with a clinically significant (>105 HPV HCR genomic equivalents) and marginally significant viral load (103-5 genomic equivalents) decreased (respectively χ20-12=5.822, p=0.001 and χ20-12=24.955, p<0.001).
Table 2
Dynamics of cytological, immunobiological, immunocytochemical, colposcopic, and laboratory parameters during the observation of patients with disease persistence (group II)
|
Parameters |
Group II (n=30) |
χ2; p |
|
|||||
|
abs. |
% |
abs. |
% |
abs. |
% |
|
||
|
Before treatment |
6 months |
12 months |
|
|||||
|
Cervical cytology |
||||||||
|
NILM |
0 |
0 |
19 |
63.3 |
28 |
93.3 |
χ2 0-6=24.955; p<0.001 χ2 6-12=6.285; p=0.013 χ2 0-12=0.517; p=0.473
|
|
|
ASC-US |
0 |
0 |
5 |
16.7 |
0 |
0 |
χ2 0-6=3.491; p=0.062 χ2 6-12=3.491; p=0.062
|
|
|
LSIL |
30 |
100.0 |
6 |
20.0 |
2 |
6.7 |
χ2 0-6=36.736; p< 0.001 χ26-12=1.298; p=0.255 χ20-12=48.817; p< 0.001 |
|
|
Viral load of oncogenic HPV |
||||||||
|
HPV negative |
0 |
0 |
0 |
0 |
28 |
93.3 |
χ20-12=0.185; p=0.667 |
|
|
<103 HPV |
4 |
13.3 |
26 |
80.0 |
0 |
0 |
χ20-6=48.817; p<0.001 χ26-12=35.424; p<0.001 χ20-12=0.185; p=0.667 |
|
|
103-5 HPV |
19 |
63.3 |
3 |
10.0 |
2 |
6.7 |
χ20-6=31.822; p< 0.001 χ26-12=0.268; p=0.605 χ20-12=13.983; p<0.001 |
|
|
>105 HPV |
7 |
23.3 |
1 |
10.0 |
0 |
0 |
χ20-6=1.080; p=0.299 χ26-12=0.0001; p=1.000 χ20-12=5.822; p=0.016
|
|
|
Immunocytochemical study of expression p16ink 4α/Ki-67 |
||||||||
|
p16ink4α/Ki-67 (+) |
0 |
0 |
1 |
3.3 |
0 |
0 |
χ20-6=0.0001; p=1.000 χ26-12=0.0001; p=1.000 |
|
|
p16ink4α/Ki-67 (-) |
30 |
100.0 |
29 |
96.7 |
0 |
0 |
χ20-6=0.0001; p=1.000 χ26-12=0.0001; p=1.000 |
|
|
Colposcopic index R.Reid |
||||||||
|
0-2 scores |
28 |
93.3 |
29 |
93.3 |
28 |
93.3 |
χ26-12=0.0001; p=1.000 |
|
|
3-5 scores |
2 |
6.7 |
1 |
3.3 |
2 |
6.7 |
χ26-12=0.0001; p=1.000 |
|
|
Serum folic acid levels |
||||||||
|
Normal B9 (≥6.0 mg/ml) |
3 |
10.0 |
26 |
87.7 |
28 |
93.3 |
χ20-6=32.303; p< 0.001 χ26-12=1.185; p=0.667 χ20-12=38.443; p<0.001 |
|
|
Insufficiency (3.1-5.9 ng/ml) |
24 |
80.0 |
4 |
13.3 |
2 |
6.7 |
χ2 0-6= 24.174; p< 0.001 χ26-12=0.185; p=0.667 χ20-12=29.932; p<0.001 |
|
|
Deficiency B9 (<3.1 ng/ml) |
3 |
10.0 |
0 |
0 |
0 |
0 |
χ20-6=1.104; p=0.237 χ20-12=1.404; p=0.237 |
|
Evaluation of the colposcopic examination results in the dynamics of management revealed no statistically significant increase in the scores corresponding to the average degree of lesions (χ20-12=0.0001, p=1.000). Such scores were maintained only in two (6.7%) patients. In 28 (93.3%) women, a colposcopic normal or minimal degree of epithelial damage (0–2 points) was registered after 12 months (Table 3).
Table 3
Comparative analysis of the modified R. Reid colposcopic index (ICID) in patients in the study groups over time (scores)
|
|
Clinical groups |
||||||
|
Scores |
Group I (n= 43) |
Group II (n=30) |
Group III (n=17) |
χ2 p
|
|||
|
Initially |
6 months |
Initially |
6 months |
Initially |
6 months |
||
|
|
abs. (%) |
abs. (%) |
abs. (%) |
||||
|
0-21 |
40 (93.0) |
43 (100.0) |
28 (93.3) |
26 (86.7) |
15 (88.2) |
0 |
χ2 I (f-6 months)=3.108 p=0.078 χ2 II (f-month)=0.185 p=0.667 χ2III (f-6 month)=23.382 p<0.001 |
|
3-52
|
3 (7.0) |
0 |
2 (4.7) |
4 (13.3) |
2 (11.8) |
1 (5.9) |
χ2 I (f-6 month)=1.382 p=0.240 χ2 II (f-month)=0.185 p=0.667 χ2 III (f-6 months)=0.001 p=1.000 |
|
6-83
|
0 |
0 |
0 |
0 |
0 |
16 (94.1) |
χ2III (f-month)=26.563 p<0.001 |
Note. Gradation according to the modified colposcopic index R. Reid: 1minimal epithelial damage; 2mean degree of damage; 3high degree of damage. Gradation of the degree of damage according to the clinical-colposcopic index: 4significant lesions; 5CIN I–II; 6high degree lesion.
LSIL regression in group II patients (according to cytologic, molecular-biologic, and colposcopic studies) was also accompanied by statistically significant positive dynamics of serum concentrations of folic acid (Table 2).
Thus, the proportion of women with physiologic serum levels of vitamin B9 increased from 10.0% to 93.3% (χ20-12=38.443, p<0.001), with “insufficiency” – decreased from 80.0% to 6.7% (χ20-12=29.932, p<0.001). The “insufficiency” of the serum level of vitamin B9, which was detected in 2/30 patients of this group (6.7%), was associated with the disease persistence: cytologic changes of the cervical smear within the limits of LSIL persisted, a clinically insignificant level of viral load (103-5 genomic equivalents of HPV HCR), the “average” degree of cervical epithelium lesion, according to colposcopic examination (MRCI), was 4 points; positive p16ink4α/Ki-67 co-expression reaction was noted (in one out of 30 patients (3.3%). This patient underwent a targeted cervical biopsy with subsequent morphologic verification of the biopsy specimen, which revealed CINI. Dynamic follow-up continued within the framework of clinical recommendations.
In group III patients, 3 months after active surgical tactics, the proportion of NILM cytologic findings increased statistically significantly (χ2=28.235, p<0.001). In 1/16 women (6.25%), cytologic changes in the cervical epithelium corresponded to ASC-US (Table 4). At the end of 12 months of the follow-up, the absence of cervical epithelial changes was noted in all 16/16 patients (100.0%).
Table 4
Dynamics of cytological, immunobiological, immunocytochemical, colposcopic, and laboratory parameters after HSIL surgery in patients with disease progression (group III)
|
Parameters |
Group III (n =16)* |
χ2, p |
|||||
|
abs. |
% |
abs. |
% |
abs. |
% |
||
|
Prior to surgery |
3 months |
12 months |
|||||
|
Cervical cytology |
|||||||
|
NILM |
0 |
0 |
15 |
93.7 |
16 |
100.0 |
χ2 0-3=28.235; p<0.001 χ23-12=0.0001; p=1.000 |
|
ASC-US |
0 |
0 |
1 |
6.3 |
0 |
0 |
χ20-3=0.0001; p=1.000 χ23-12=0.0001; p=1.000 |
|
LSIL |
1 |
6.3 |
0 |
0 |
0 |
0 |
χ20-3=0.0001; p=1.000 χ23-12=0.0001; p=1.000 |
|
HSIL |
15 |
93.7 |
0 |
0 |
0 |
0 |
χ2 0-3=28.235; p<0.001 |
|
Viral load of oncogenic HPV |
|||||||
|
<103 HPV |
0 |
0 |
2 |
12.5 |
0 |
0 |
χ20-3=0.533; p=0.466 χ23-12=35.424; p<0.001 χ20-12=0.185; p=0.667 |
|
103-5 HPV |
1 |
6.3 |
0 |
0 |
0 |
0 |
χ20-3=0.0001 p=1.000 |
|
>105 HPV |
15 |
93.7 |
0 |
0 |
0 |
0 |
χ2 0-3=28.235 p<0.001 |
|
Immunocy to the chemical study of expression p16ink 4α/Ki-67 |
|||||||
|
p16ink4α/Ki-67 (+) |
14 |
87.5 |
0 |
0 |
0 |
0 |
χ20-3=21.460; p< 0.001 |
|
p16ink4α/Ki-67 (-) |
2 |
12.5 |
0 |
0 |
0 |
0 |
χ20-3=0.533; p=0.466 χ20-12=0.0001; p=1.000 |
|
Colposcopic index R.Reid |
|||||||
|
0-2 scores |
0 |
0 |
15 |
93.7 |
16 |
100.0 |
χ2 0-3=28.235; p<0.001 χ23-12=0.0001; p=1.000 |
|
3-5 scores |
1 |
6.3 |
1 |
6.3 |
0 |
0 |
χ20-3=0.0001; p=1.000 |
|
6-8 scores |
15 |
93.7 |
0 |
0 |
0 |
0 |
χ2 0-3=28.235; p<0.001 χ20-12=0.0001; p=1.000 |
|
Serum folic acid levels |
|||||||
|
normal B9 (≥6.0 ng/ml) |
0 |
0 |
7 |
43.8 |
13 |
81.3 |
χ20-3=6.583; p=0.011 χ23-12=3.333; p=0.068 χ20-12=18.656; p<0.001 |
|
Insufficiency (3.1–5.9 ng/ml) |
1 |
6.3 |
6 |
37.5 |
3 |
18.7 |
χ2 0-3= 2.926; p=0.088 χ23-12=0.618; p=0.432 χ20-12=0.286; p=0.593 |
|
Deficiency B9 (<3.1 ng/ml) |
15 |
93.7 |
3 |
18.7 |
0 |
0 |
χ20-3=15.365; p<0.001 χ23-12=1.471; p=0.226 χ20-12=24.596; p<0.001 |
Note: * — patients in group III with histologically verified CIN II.
During the follow-up from 3 to 12 months, the clinically significant HPV HCR viral load decreased statistically significantly (χ20-12=28.235, p<0.001). An immunocytochemical study of p16ink4α/Ki-67 co-expression did not reveal a positive reaction in any patient, which had statistically significant differences compared to the results for this parameter before surgical treatment (χ20-12=21.460, p<0.001) (Table 4).
The results of the colposcopic examination after 12 months registered reliably significant changes: a minimal degree of epithelial damage (0–2 points) was detected in all (100.0%) patients of this group (χ20-12=28.235, p<0.001).
After active surgical tactics for HSIL and correction of folate status, positive dynamics of cytological, immunobiological, immunocytochemical, and colposcopic parameters were accompanied by the normalization of serum concentrations of vitamin B9, which reached the level of normal values by the end of the third month in 43.8% of patients (χ2=6.583, p=0.011), and at the end of 12 months – in 81.3% (χ2=18.656, p<0.001) (Table 4).
Further follow-up of group III patients was carried out according to clinical recommendations: a cytologic examination of cervical microdissection and a molecular biological examination of cervical canal secretions for HPV HCR for the early detection of recurrence [21].
Statistical hypothesis testing and validation of the developed method of LSIL progression prediction were performed in 43 patients of group I (with disease regression) and 17 patients of group III (with LSIL progression). Forty-three women of group I, who had disease regression by the results of the study, had LSIL RPI values < 1 (mean value – 0.81±0.02), characterized by “low” risk of progression in 38 patients (88.4%) (“true-negative” result). In 5 patients of this group (11.6%), LSIL RPI values were >1, which corresponded to a “high” risk of CIN progression (“false-positive” result).
In group III patients (with LSIL progression to a more severe form of cervical dysplasia), the mean numerical LSIL RPI values corresponding to “high” risk (>1) were 1.53±0.29 in 16 of 17 patients (88.2%), (“true-positive” results). Two out of 17 patients of group III (11.8%) with confirmed LSIL progression had “false-negative” results, as LSIL RPI values were <1 (0.941 and 0.821).
The ability of the developed method to predict LSIL progression is demonstrated by the clinical observations presented below.
Clinical Case 1
In patient D-eva, born in 1990, a cytologic examination of the cervical smear revealed LSIL (Fig. 1), HPV testing with genotyping revealed HPV type 16 infection at a clinically significant concentration (5.67 lg).

Figure 1. Patient D-eva, born in 1990. Liquid cytology, color according to Pap, ×400. LSIL, HPV features. Cells of the intermediate and parabasal layers, metaplastic with mild dyskaryosis. Koilocytosis in the gap layer cell. Paraceratosis in surface layer cells
Dilated colposcopy revealed the presence of grade I abnormal patterns (Fig. 2).
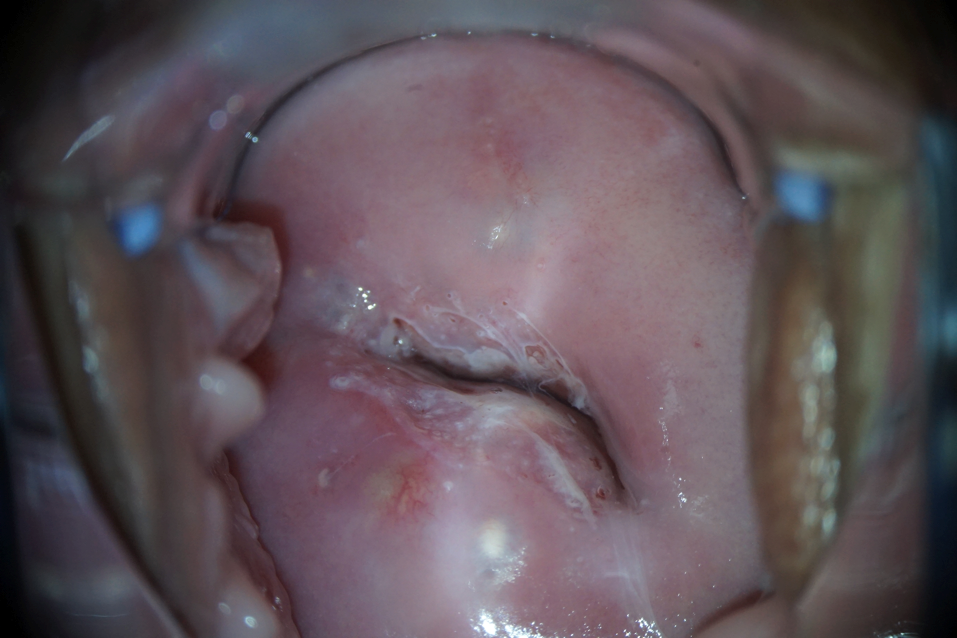 |
 |
|
Note: a) pale acetowhite area edging the junction of the transition of the flat epithelium into a cylindrical one. Open ducts of cervical glands with corneal rims (photo after exposure to 3% acetic acid). |
b) Schiller’s test is positive: application of 1% water solution of Lugol did not color the lesion, as well as the rims of the open ducts of the cervical glands. |
Figure 2. Patient D-eva, born in 1990. Abnormal colposcopic picture of the 1st degree.
The patient had a serum folic acid concentration of 3.0 ng/mL. The patient had a history of chlamydia infection. Sexual debut at the age of 18 years. The patient was married and had a regular sexual partner. These anamnestic factors were entered into the computer program. The RPI values were 1.074, which corresponded to a “high” risk of LSIL progression (Fig. 3).
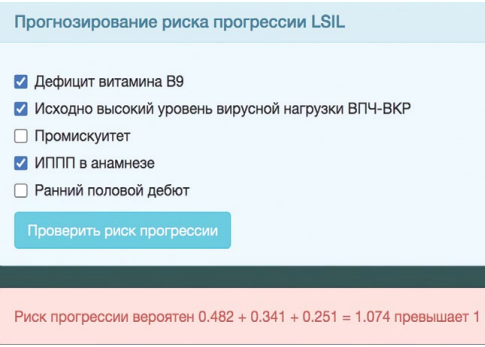
Figure 3. Computer program window for automatic calculation of risk index of LSIL progression in patient D-eva. IRD value > 1, risk “high”.
The patient was consulted by specialized professionals, recommendations on the correction of folate status were given. The patient did not undergo treatment, and 6 months later, she did not show up for an appointment. The repeat examination in 12 months revealed disease progression: the cytologic examination revealed a high-grade lesion (HSIL) (Fig. 4).
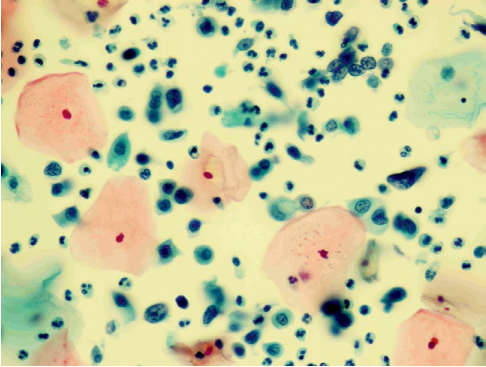
Figure 4. Patient D-eva, born in 1990 (after 12 months). Liquid cytology, Pap coloration, ×400. HSIL. Pronounced dyskaryosis of flat epithelium cells of the intermediate parabasal and basal layers.
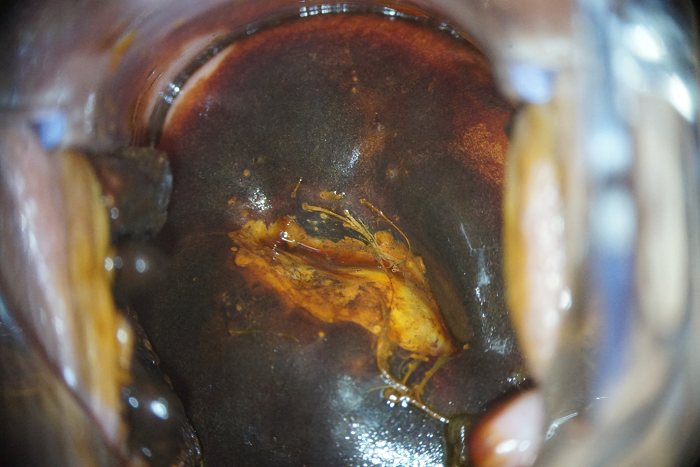
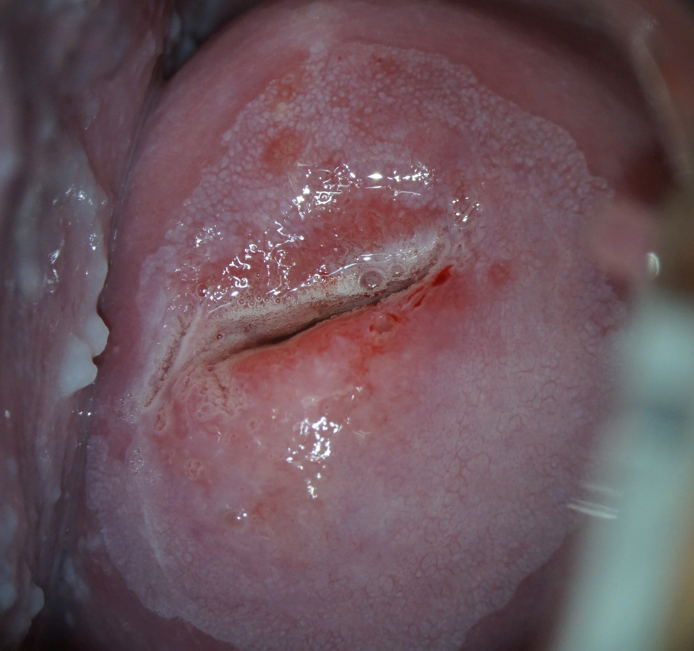
HPV testing with genotyping revealed a further increase in the HPV type 16 viral load at a clinically significant concentration (6.41 lg). Extended colposcopy revealed the presence of grade II abnormal patterns (Fig. 5). The serum level of vitamin B9 remained without a significant positive trend (3.7 ng/mL). The immunocytochemical study revealed a positive reaction to p16ink 4α/Ki-67 co-expression in the nuclei of epithelial cells with dyskaryosis (Fig. 6).
 |
 |
|
Note: a) large acetowhite area with fibrous-toothed edges, combination of delicate and coarse mosaic after the application of 3.0% acetic acid solution. |
b) the Schiller test is positive: the application of 1% Lugol aqueous solution did not paint the lesion. |
Figure 5. Patient D-eva, born in 1990 (after 12 months of observation). Grade II abnormal colposcopic pattern.
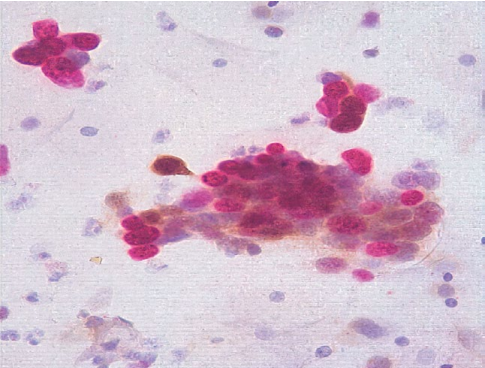
Figure 6. Patient D-eva, born in 1990. Immunocytochemical study, ×400. HSIL. Co-expression of p16ink 4α/Ki-67 in the nuclei of epithelial cells with dyskaryosis. Brown color — p16ink 4α, red — Ki-67.
The results of the histologic examination of the cervical biopsy specimen revealed CIN II, and the flap margins were negative (Fig. 7).

Figure 7. Patient D-eva, born in 1990. Cervical biopsy, hematoxylin-eosin staining, ×200. CIN II (HSIL), chronic cervicitis, HPV infection. Proliferating basaloid cells with dyskaryosis occupy 2/3 of the epithelial stratum. Dyskeratosis and koilocytosis in surface and intermediate layer cells. Subepithelial angiomatosis and mild lymphocytic infiltration.
This patient underwent active therapeutic tactics (complex therapy): cervical conization (confirmed CINII) + local intravaginal therapy with a drug with multitarget antitumor activity against cervical squamous cell intraepithelial lesions associated with HPV, containing 3,3’-diindolylmethane 1 vaginal suppository (100 mg) twice a day for three months. Correction of folate status. Follow-up was scheduled in accordance with clinical recommendations for patients with CINII.
Clinical Case 2
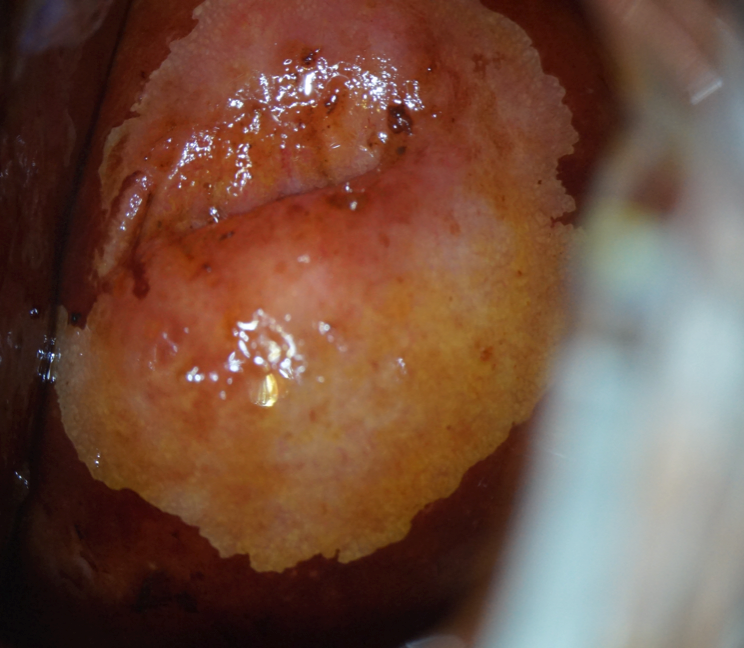
Patient B-eva, born in 1994, was diagnosed with LSIL associated with HPV type 16 infection at a concentration of 4.31 lg (Fig. 8).
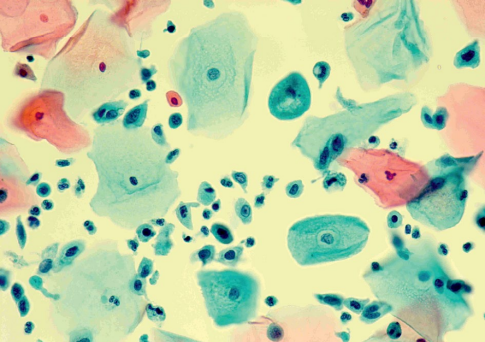
Figure 8. Patient B-eva, born in 1994. Liquid cytology, color according to Pap, ×400. LSIL, HPV features. Cells of the intermediate and parabasal layers, metaplastic with mild dyskaryosis. Koilocytosis in the gap layer cell. Paraceratosis in surface layer cells.
Extended colposcopy revealed abnormal colposcopic changes of the first degree (Fig. 9). From the anamnesis: sexual debut at the age of 14, no STIs, not married, no permanent sexual partner.
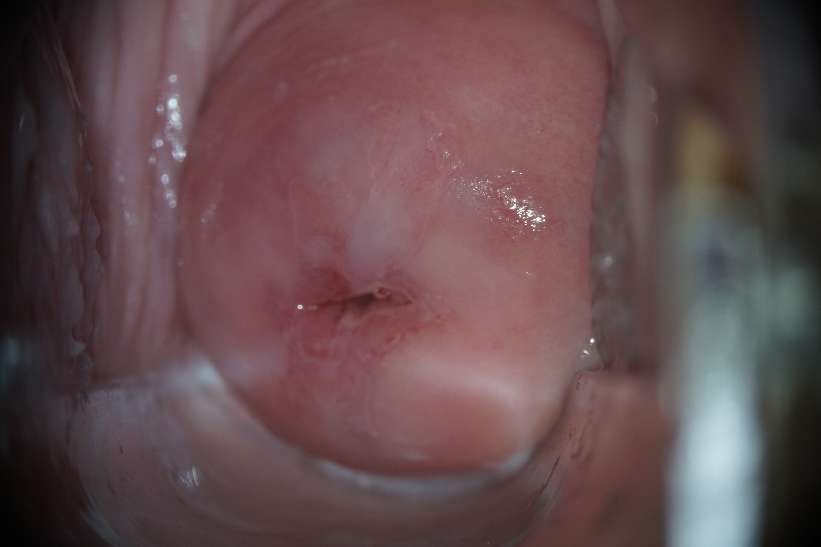 |
 |
|
Note: a) delicate acetowhite epithelium in the type I transformation zone after the application of 3.0% acetic acid solution. |
b) Schiller test positive: the application of 1% Lugol aqueous solution did not paint the lesion. |
Figure 9. Patient B-eva, born in 1994. Abnormal colposcopic picture of the 1st degree.
The serum level of folic acid in this patient was 2.6 ng/mL, and consultation by specialized professionals was recommended to verify the cause of folate status deficiency and its correction.
The identified factors were entered into the window of the computer program. The IRP values were 1.533, which corresponded to a “high” risk of LSIL progression (Fig. 10).
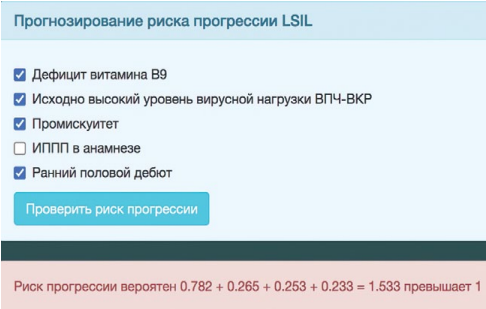
Figure 10. Computer program window for the automatic calculation of the LSIL progression risk index in patient B-eva. IRD value > 1, risk “high”.
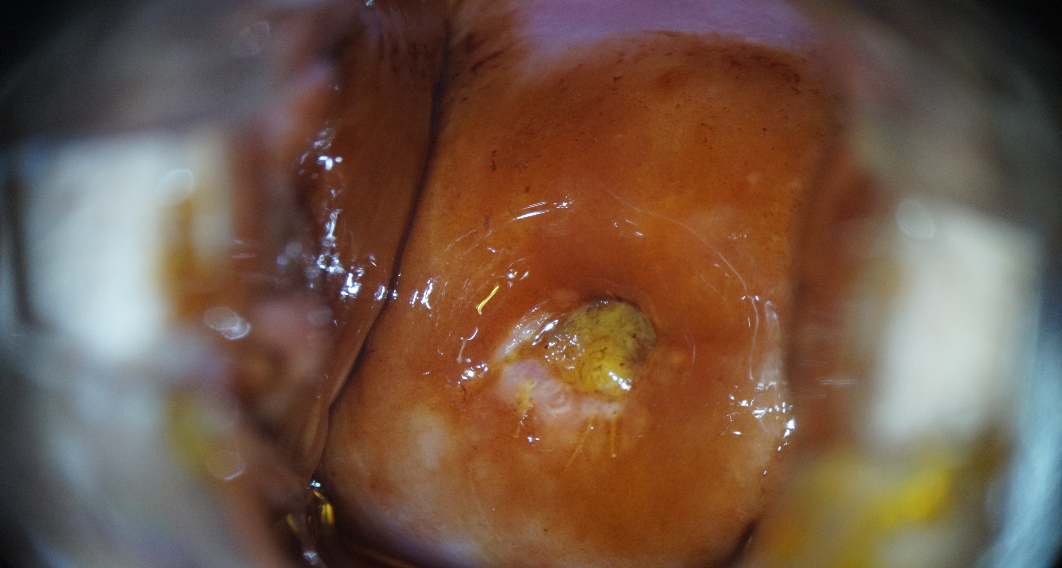
After 6 months, according to clinical recommendations, a list of regulated examinations was performed. The dynamics of laboratory and instrumental methods of cervical diagnostics (cytologic examination of ectocervical smear, molecular biological examination of cervical canal secretions for HPV with genotyping and determination of viral load, extended colposcopy, examination of the folic acid level in the blood serum) were evaluated during the repeated consultation. After an increase in vitamin B9 concentrations (up to 16.1 ng/ml), normalization of the results of the cervical smear (Fig. 11) and colposcopic picture (Fig. 12) was noted. The results of HPV HCR genotyping showed a decrease in the level of HPV type 16 viral load to clinically insignificant (102 genomic equivalents of HPV HCR).
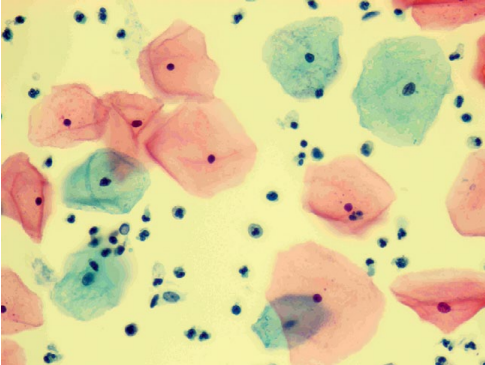
Figure 11. Patient B-eva, born in 1994 (after 6 months of follow-up). Liquid cytology, Pap coloration, ×400. NILM. Surface and intermediate cells of a multilayer flat non-threshold epithelium, cylindrical endocervical cells located singly and, in a group, white blood cells.
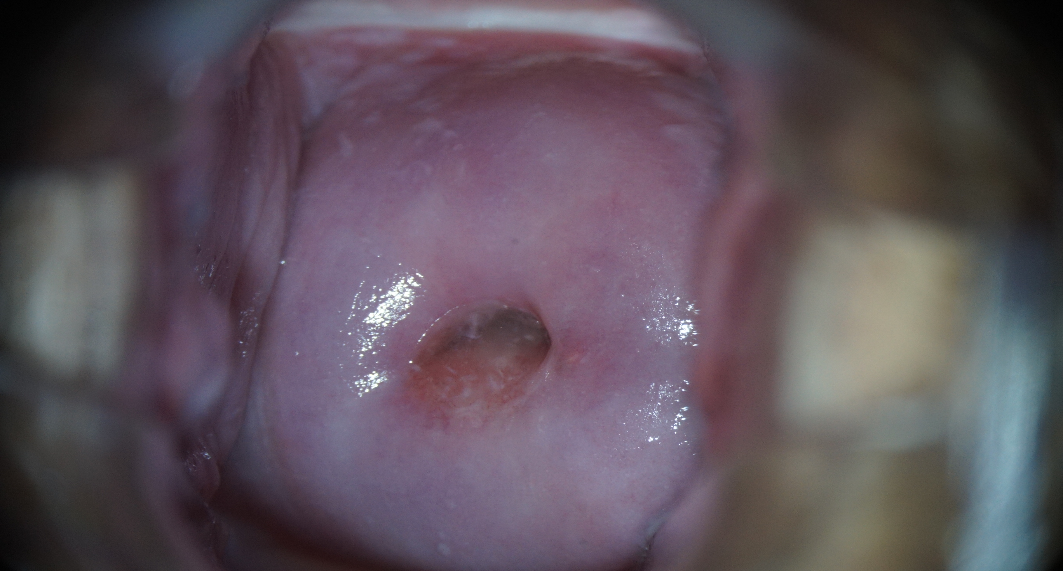 |
 |
|
Note: a) original flat epithelium pale pink, smooth-shiny surface. |
b) Schiller's test is negative. Uniform ectocervix staining with 1% Lugol aqueous solution. |
Figure 12. Normal colposcopic pattern in patient B-eva, born in 1994, after 6 months of follow-up.
The performed immunocytochemical study did not reveal a positive p16ink4α/Ki-67 co-expression reaction (Fig. 13). Routine cervical screening was recommended according to current clinical guidelines.

Figure 13. Patient B-eva, born in 1994. Immunocytochemical study, ×400. NILM. No co-expression of p16ink 4α/Ki -67 in epithelial cell nuclei.
Conclusion
The obtained results allowed the authors to optimize approaches to the management of patients with LSIL associated with HPV HCR. The proposed method with a high degree of reliability and informativeness allows the specialists to predict the progression of cervical LSIL associated with HPV and, without violating the algorithm of current clinical recommendations, to timely form among these patients a group of “high” risk of developing a more severe form of the disease, to individually determine further therapeutic tactics, and to reasonably undertake invasive therapeutic and diagnostic interventions on the cervix.
References
1. Matsuyama M., Søraas A., Yu S., Kim K., Stavrou E. X., et al. Analysis of epigenetic aging in vivo and in vitro: Factors controlling the speed and direction. Exp Biol Med (Maywood). 2020; 245 (17): 1543-1551. doi: 10.1177/1535370220947015
2. Zhang L, Tan W., Yang H., Zhang S., Dai Y. Detection of Host Cell Gene / HPV DNA Methylation Markers: A Promising Triage Approach for Cervical Cancer. Front Oncol. 2022; 12: 831949. doi: 10.3389/fonc.2022.831949
3. Abramov P. M., Katargin A. N., Fedorova M. D., Kisseljova N. P., Pavlova L. S., Vinokurova S. V. The role of HPV16 regulatory region methylation in viral oncogenes E6 and E7 eхpression in primary cervical cancer lesions. Advances in Molecular Oncology. 2018; 5 (4): 110-116. (In Russ.) doi: 10.17650/2313-805x-2018-5-4-110-116
4. Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018; 19 (2): 81-92. doi: 10.1038/nrg.2017.80
5. Mandaviya P. R., Joehanes R., Brody J., Castillo-Fernandez J. E., Dekkers K. F., et al. Association of dietary folate and vitamin B-12 intake with genome-wide DNA methylation in blood: a large-scale epigenome-wide association analysis in 5841 individuals. Am J Clin Nutr. 2019; 110 (2): 437-450. doi: 10.1093/ajcn/nqz031
6. Di Domenico M., Giovane G., Kouidhi S., Iorio R., Romano M., et al. HPV epigenetic mechanisms related to Oropharyngeal and Cervix cancers. Cancer Biol Ther. 2018; 19 (10): 850-857. doi: 10.1080/15384047.2017.1310349
7. Gromova O. A., Torshin I. Yu., Tetruashvili N. K., Limanova O. A. New trends in nutritional support for pregnancy. Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2018;(1): 21-28. (In Russ.). doi: 10.18565/aig.2018.1.21-28
8. Kareva E. N., Zorina L. A., Sudnitsyna M. V. Tetrahydrofolate: role in periconceptional supplementation and prenatal care. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie [Obstetrics and Gynecology: News, Opinions, Training]. 2019; 7 (2): 59-63. (In Russ.) doi: 10.24411/2303-9698-2019-12007
9. Kuznetsova I. V. Role ofpreconception endothelial dysfunction in development of obstetric complications. Medical alphabet. 2019; 1 (1): 53-58. (In Russ.) doi: 10.33667/2078-5631-2019-1-1(376)-53-58
10. Pieroth R., Paver S., Day S., Lammersfeld C. Folate and Its Impact on Cancer Risk. Curr Nutr Rep. 2018; 7 (3): 70-84. doi: 10.1007/s13668-018-0237-y
11. Sanderson S. M., Gao X., Dai Z., Locasale Jw. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer. 2019; 19 (11): 625-637. doi: 10.1038/s41568-019-0187-8
12. FolicAcid. Available online: https://www.breastcancer.org/managing-life/diet-nutrition/dietary-supplements/known/folic-acid (accessed on 7 September 2022)
13. Franco C. N., Seabrook L. J., Nguyen S. T., Leonard J. T., Albrecht L. V. Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites. 2022; 12 (10): 961. doi: 10.3390/metabo12100961
14. Shulpekova Y., Nechaev V., Kardasheva S., Sedova A., Kurbatova A., et al. The Concept of Folic Acid in Health and Disease. Molecules. 2021; 26 (12): 3731. doi: 10.3390/molecules26123731
15. Zhang D., Wen X., Wu W., Guo Y., Cui W. Elevated homo cysteine level and folate deficiency associated with increased overall risk of carcinogenesis: meta-analysis of 83 case-control studies involving 35,758 individuals. PLoS One. 2015; 10 (5): e0123423. doi: 10.1371/journal.pone.0123423
16. Panprathip P., Petmitr S., Tungtrongchitr R., Kaewkungwal J., Kwanbunjan K. Low folate status, and MTHFR 677C > T and MTR 2756A > G polymorphisms associated with colorectal cancer risk in Thais: a case-control study. Nutr Res. 2019; 72: 80-91. doi: 10.1016/j.nutres.2019.10.008
17. Sun Y., Fowke J. H., Liang X., Mozhui K., Sen S., et al. Changes in Dietary Intake of Methionine, Folate / Folic Acid and Vitamin B12 and Survival in Postmenopausal women with Breast Cancer: A Prospective Cohort Study. Nutrients. 2022; 14 (22): 4747. doi: 10.3390/nu14224747
18. Liu M. C., Oxnard G. R., Klein E. A., Swanton C., Seiden M. V.; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020; 31 (6): 745-759. doi: 10.1016/j.annonc.2020.02.011
19. Anufrieva V. G., Lebedenko E. Yu., Gaida O. V., Mikhelson A. A., Karnushin T. E., Evseev P. A. Manageable risk factors for progression of HPV-associated cervical intraepithelial neoplasia. Medical Herald of the South of Russia. 2022; 13 (2): 34-43. doi: 10.21886/2219-8075-2022-13-2-34-43
20. Patent No. 2766719, Russian Federation, IPC A61V 10/00 (2006.01). Method for prediction of progression of cervical squamous intraepithelial lesion of low degree against background of papillomavirus infection. V. G. Anufrieva, E. Yu. Lebedenko, O. V. Gaida. Application No. 2021127672, 20. 09. 2021; Opubl.15. 03. 2022., Bulletin No. 8.
21. Clinical Guidelines "Cervical Intraepithelial Neoplasia, Erosion and Ectropion of the Cervix," 2020. (In Russ.).
22. Kamilova I. K., Miklin O. P., Gudz O. V., Zinchenko A. A. Correction of folate status - problems and prospects in the Russian Federation. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie [Obstetrics and Gynecology: News, Opinions, Training]. 2019; 7 (3): 120-129. (In Russ.) doi: 10.24411/2303-9698-2019-13018.
About the Authors
V. G. AnufrievaRussian Federation
Vitaliyа G. Anufrieva, obstetrician-gynecologist
Rostov-on-Don
E. Yu. Lebedenko
Russian Federation
Elizaveta Yu. Lebedenko, Dr. Sci. (Med.), associate professor, head of the Department
Department of Pathological Anatomy
Rostov-on-Don
V. V. Voloshin
Russian Federation
Vladimir V. Voloshin, Cand. Sci. (Med.), associate professor
Department of Obstetrics and Gynecology
Rostov-on-Don
O. V. Gaida
Russian Federation
Oksana V. Gaida, Cand. Sci. (Med.), associate professor
Department of Obstetrics and Gynecology
Rostov-on-Don
Review
For citations:
Anufrieva V.G., Lebedenko E.Yu., Voloshin V.V., Gaida O.V. Optimization of management approaches for patients with cervical intraepithelial neoplasia mildly (LSIL) associated with high oncogenic risk human papillomaviruses. Medical Herald of the South of Russia. 2023;14(2):5-17. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-5-17







































