Scroll to:
Molecular epidemiological and clinical aspects of enterovirus infection in the south of Russia
https://doi.org/10.21886/2219-8075-2023-14-1-83-92
Abstract
Objective: the study of molecular epidemiological and clinical aspects of EVI in the Rostov region for the period 2006-2020. to improve the disease surveillance system. Research methods: epidemiological, virological, molecular biological (PCR, sequencing, phylogenetic analysis), microbiological, statistical.
Materials and methods: the analysis of medical records of 139 patients with EVI was carried out. Samples of biomaterial (feces, throat swab) from 17293 people, samples from environmental objects (2710 samples), strains of enteroviruses (EV) in the amount of 142 specimens were studied.
Results: in the Rostov region until 2013. sporadic incidence of EVI prevailed with registration from 1 to 38 cases per year. June 2013 there was a sharp increase in the incidence of EVI with the formation of local foci in organized groups, associated with the circulation of a new genotype — EV 71 type of subgenotype C4 of “Chinese” origin (622 patients with EVI were registered, one death). The clinical features of the disease were determined: acute onset with manifestations of intoxication, foot and mouth disease-like syndromes, followed by the development of CNS pathology in 37.4% of patients. According to the results of sequencing of biomaterial samples from patients and virus carriers for the period 2006-2020. EVs of 22 types were detected.
Conclusions: EVs are subject to intense genetic variability, due to which new genovariants pathogenic for humans may appear. The change in EV genotypes, which dominated the circulation among the population of the Rostov region, determined the rise in the incidence of EVI in 2013. A significant diversity of non-polio EV genotypes was revealed, while the structure of EV genovariants changed in different years.
For citations:
Kovalev E.V., Tverdokhlebova T.I., Simovanyan E.N. Molecular epidemiological and clinical aspects of enterovirus infection in the south of Russia. Medical Herald of the South of Russia. 2023;14(1):83-92. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-1-83-92
Introduction
The beginning of the 21st century was marked by the emergence of the global threat of spreading definite infections caused by RNA-containing viruses. Among them are the so-called SARS, bird flu, enterovirus infection (EVI), and a new coronavirus infection. All of them are taken under the control of WHO. These infections can be conditionally referred to as “new” since their investigation was accompanied by the discovery of viral agents or the identification of unknown pathogen properties and extreme public attention.
EVI is characterized by the ubiquitous distribution, the abundance of pathogen serotypes (genotypes), its pantropism, the infection manifestation in the form of various clinical variants with injuries of one or more organs and systems, the large-scale nature of the disease, the variety of contamination mechanisms and transmission routes, the wide carriage, the high susceptibility to the infection (especially at an early age), the association between EVI and type 1 diabetes revealed for the recent years, numerous cases of EVI coinfection in patients with the coronavirus infection caused by SARS-CoV-2, as well as the high mortality [1–7].
In the last few years, there has been an evolution of EVIs. New enterovirus types of clinical significance have been identified. Currently, human enteroviruses are represented by more than 120 genetically heterogeneous serotypes [8]. The topicality of the continuous monitoring of the circulation of enteroviruses among the population by means of molecular genetics technologies [5] is stipulated by the presence of many types of non-polio enteroviruses (NPEVs), the constantly changing scales of their spreading and the spectrum of epidemically significant serotypes and genetic variants, which are dominating at a certain time in a particular geographical area.
The geography of EVI is wide and covers almost all countries of the world [9][10]. The countries of the Asia-Pacific region still remain endemic areas with a high incidence of EVI [9][11]. The largest outbreaks, described by researchers, were caused mainly by ECHO 6, 13, 30 viruses, as well as enterovirus 71, and happened in China, Taiwan, and Tunisia. In 2008, the largest outbreak of EVI was documented in China, involving 19 country administrative entities in the epidemic process; the number of cases was more than 30 thousand people with 39 deaths.
In the Russian Federation, official statistical reporting on EVI has been introduced since 2006. Outbreaks and cases of EVI have been recorded in the territories of the Northwestern Federal District, the Southern Federal District, the Volga Federal District, and the North Caucasian Federal District [12–15]. High incidence rates of this infection are typical for the territories of the Far Eastern Federal District [16][17]. Epidemiological investigations into the etiology of EVI outbreaks revealed in definite cases the facts of the entry of new enterovirus genotypes in the examined territories [18–20].
The investigation of enterovirus genovariants and the database bank creation are important for assessing the dynamics of already known and new isolates circulating in the Russian Federation, which have different abilities to spread, pathogenicity, and antigenicity, as well as for designing adequate preventive services, treatment regimens, methods, and diagnostic tools.
The purpose of the study is the investigation of the molecular epidemiological and clinical aspects of EVI in the Rostov Region for the period of 2006–2020 to improve the system of epidemiological surveillance for this disease.
Materials and methods
The material was collected within the territory of the Rostov Region. The objects of the study were patients with EVI (12,425 people), contact persons (974 people), patients with acute intestinal infections (200 people) and other diseases (3694 people), as well as samples of environmental objects including sewage water (2336 samples), surface watercourse (259 samples), drinking water (51 samples), food (64 samples), and strains of enteroviruses in the amount of 142 copies to determine their genotype.
The following investigation methods were used in the work: epidemiological, virological (neutralization reaction), molecular biological (PCR, sequencing, phylogenetic analysis), microbiological (traditional one, techniques based on the application of the Vitek-2 bacteriological analyzer and mass spectrometric MALDI-TOF), statistical with the software SPSS Statistics Base 22.0 and Microsoft Office Excel 2016.
The material for microbiological and molecular biological investigations was feces and throat swabs. The polymerase chain reaction (PCR) method with hybridization-fluorescent detection in real time (RT-PCR) was done with the application of the AmpliSens® Enterovirus-FL and AmpliSens® Enterovirus 71-FL test systems in accordance with the manufacturer's instructions.
To determine the genotypes of circulating strains of enteroviruses, fragment sequencing of the RNA nucleotide sequences of virus strains isolated from PCR-positive samples was carried out according to Sanger [21]. Molecular typing of enteroviruses was based on RT-PCR and determination of the nucleotide sequence in the genome region at the 5'UTR (5'HTP) of the enterovirus RNA region with specific primers. The enterovirus genotype was determined by comparing the obtained sequence with the sequences of prototype human enteroviruses available in the GenBank Genetic Sequence Bank (NCBI) with the BLAST software.
A retrospective analysis of the epidemiological situation for EVI within the Rostov Region was carried out with maps of the epidemiological examination of foci and statistical reporting data, while the clinical picture of the disease was assessed by using inpatient and outpatient medical cards of those with the confirmed diagnosis.
Results
From 2006 (the beginning of the EVI official registration in the Russian Federation) to 2012, the incidence in the region was sporadic with registration from 1 to 38 cases per year (from 0.02 to 3.8 people per 100 thousand). The annual morbidity rates for this period were 3.8–6.2 times lower than the national ones. Monitoring of the enterovirus circulation in the environment (sewage water) attested that for the period from 2006 to 2012, the viral landscape was very diverse and changed almost annually (Table 1).
Таблица / Table 1
Пейзаж энтеровирусов, выделенных из объектов окружающей среды (сточные воды)
в Ростове-на-Дону в ходе вирусологического мониторинга в 2006–2012 гг.
Variety of enteroviruses isolated from environmental objects (wastewater)
in Rostov-on-Don during virological monitoring in 2006–2012
|
Показатель / Indicator |
Результаты вирусологического мониторинга в 2006-2012гг. / Results of virological monitoring in 2006-2012 |
||||||
|
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
|
|
Количество проб / Number of samples |
242 |
281 |
228 |
231 |
200 |
200 |
120 |
|
Пейзаж энтеровирусов / Variety of enteroviruses |
ECHO 6 ECHO 2 ECHO 25 Коксаки В/ Coxsackievirus В |
ECHO 4 ECHO 7 ECHO 25 Коксаки В 1-6/ Coxsackievirus В 1-6 |
ECHO 3 ECHO 5 ECHO 6 ECHO 7 ECHO 16 ECHO 20 |
Энтеровирусы из сточной воды не выделены / Enteroviruses have not been isolated from wastewater |
Коксаки В 1-6 / Coxsackievirus В 1-6 |
ECHO 17 |
|
The results presented in the table indicate that only enteroviruses related to ECHO and Coxsackie were registered in the environment in Rostov-on-Don in the period of 2006–2012. The same enteroviruses were also isolated from the material of patients: in 2008 — ECHO 30; in 2009 — ECHO 5 and ECHO 6; in 2010 — ECHO 6; in 2011 — ECHO 17 and Coxsackie A6; in 2012 — ECHO 6, ECHO 7, Coxsackie B3 and B4.
In 2013, an epidemic rise in the EVI incidence was registered in the Rostov Region with the formation of local foci in preschool educational institutions, due to the emergence of enterovirus 71 subgenotype C4, which had not previously been isolated in the region. The emergence of a new genetic variant of the EVI pathogen led to a sharp increase in the incidence, when the lack of immunity in the population contributed to the large and rapidly spreading infection. Over the period of epidemic trouble in 2013, 622 cases of EVI were registered (14.6 people per 100 thousand) with the involvement of different population age and social groups in the epidemic process and the appearance of local outbreaks and group diseases (Figure 1).
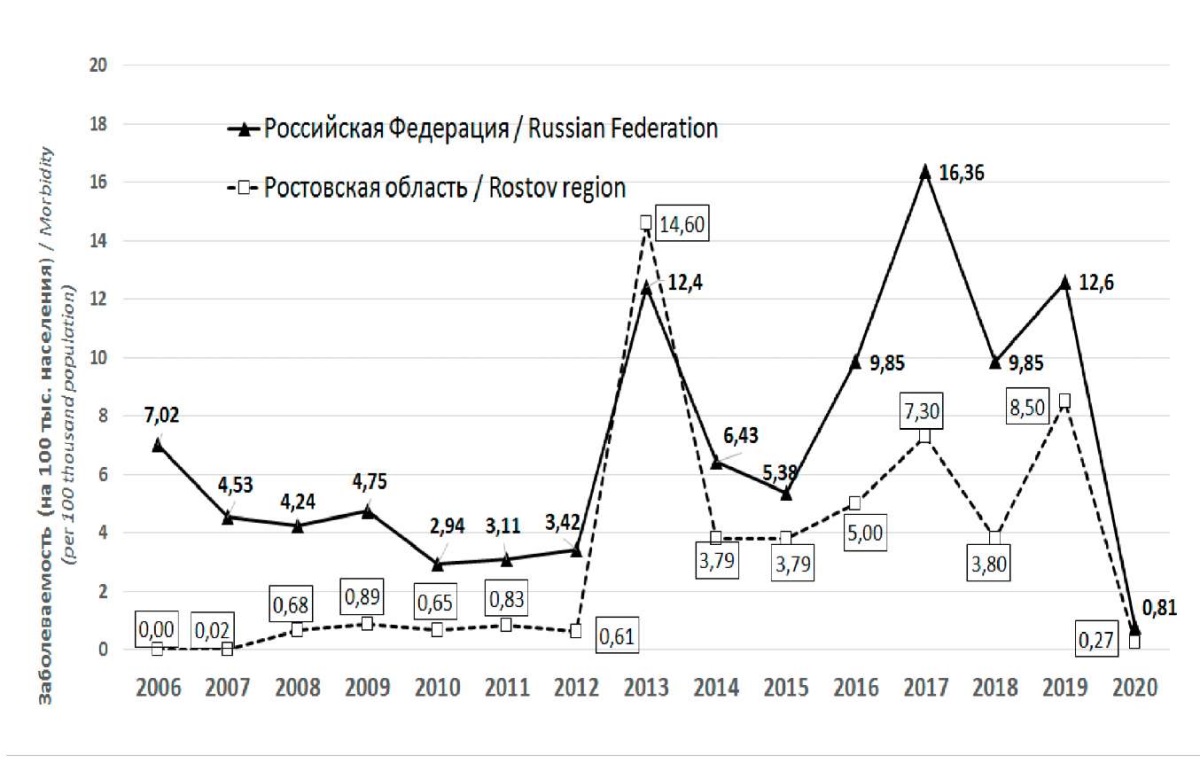
Рисунок. 1. Заболеваемость ЭВИ в Ростовской области
и Российской Федерации за период 2006–2020 гг.
Figure 1. The incidence of EVI in the Rostov Region
and the Russian Federation for the period of 2006–2020
At the end of May 2013, in one organized group of the Pervomaisky district of Rostov-on-Don, in the municipal budgetary preschool educational institution (MBPEI) No. 83 (kindergarten “Teremok”), the outbreak of acute respiratory viral and acute intestinal infections (ARVI and AII) was registered.
The first case of the disease was noted on May 28, 2013 in the younger group. In total, 78 children fell ill in this preschool institution, among which 53 people had EVI, and 25 people had enteroviral meningitis (EVM). One child aged 3 years with symptoms of meningoencephalitis died on June 2, 2013. According to the results of a post-mortem virological and bacteriological investigation of biological material, his death was revealed to be stipulated by a mixed infection of enteroviral and pneumococcal etiology against the background of persistent cytomegalovirus. The child was on dispensary registration in the group of frequently ill children.
The second child with meningeal syndrome from the same group of the kindergarten was admitted to the intensive care unit on June 3, 2013. The next day, June 4, 2013, a team of doctors organized an examination of all 270 children attending the MBPEI No. 83. Among them, 9 children with high temperature and catarrhal symptoms were promptly identified and hospitalized in the infectious department.
The age composition of ill children varied from 1 to 7 years with a predominant structure of 1–2 years (14 people) and 3–6 years (63 people). The age distribution of children who fell ill in 2013 at MBPEI No. 83 coincides with generally accepted ideas about the age groups most vulnerable to EVI.
Clinical characteristics of EVI in children caused by enterovirus 71 subgenotype C4 are shown in Figure 2.
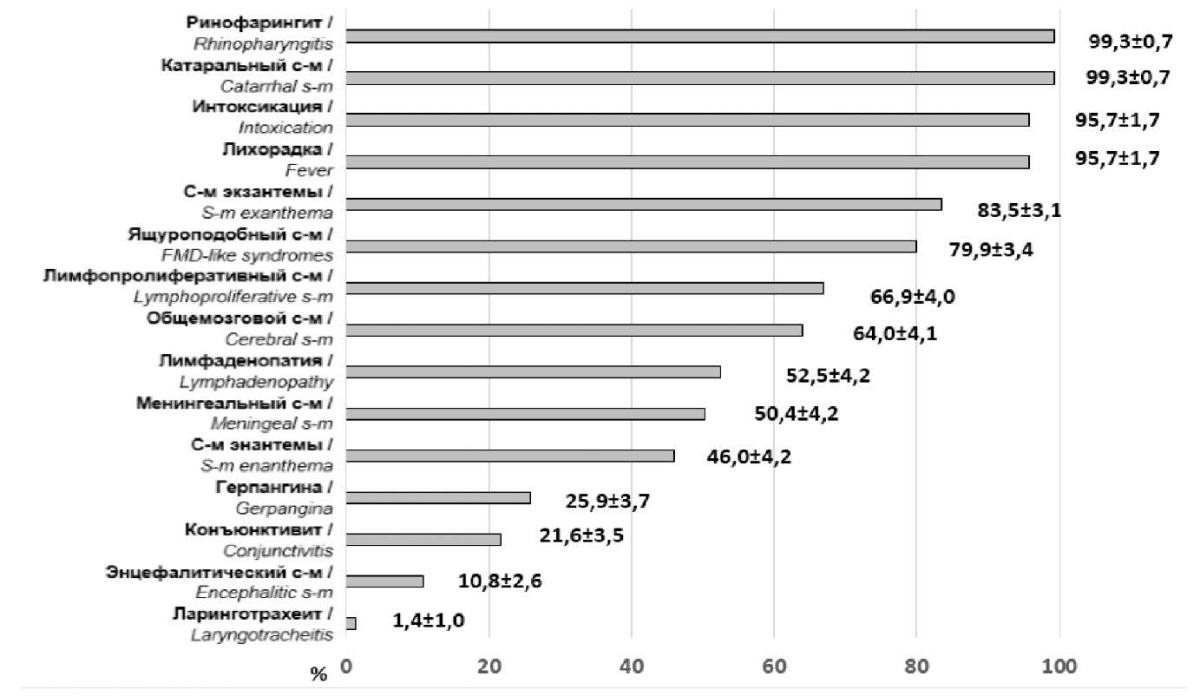
Рисунок 2. Клиническая характеристика энтеровирусной инфекции у детей,
обусловленной энтеровирусом 71 типа субгенотипа С4 (%, M±m)
Figure 2. Clinical characteristics of enterovirus infection in children
caused by enterovirus type 71 subgenotype C4 (%, M±m)
Dynamics analysis of the disease symptoms showed that the infectious process was represented by a two-phase course. In the first phase, general infectious, catarrhal, "hand-foot and mouth" disease-like and lymphoproliferative syndromes prevailed. The second phase peculiarity was marked by the overlay of central nervous system (CNS) injury symptoms.
Despite the fact that EVI proceeded in a moderate form for most patients, one-third of them revealed pathologies of the CNS (meningitis, meningoencephalitis), rhomboid fossa (rhombencephalitis), and/or cerebellum. All the children had a burdened premorbid background and belonged to risk groups in different age periods including antenatal, newborn, and aged over one month.
When conducting laboratory investigations of biological material, the following results were obtained: among 156 ill kindergarten children, the diagnosis of EVI was revealed in 78 people and laboratory-confirmed in 59 cases. Enterovirus RNA was found in the feces of 53 patients, and in throat swabs in 23 patients. EV 71 was identified in 24 children.
During the epidemiological investigation, kindergarten No. 83 was revealed to be located in a residential microdistrict of the city of Rostov-on-Don, next to the “Temernik” clothing market, where migrants often traveling outside of Russia work, whose children attended this children's institution.
At the same time, an investigation on isolating pathogenic bacteria and viruses from environmental objects (50 samples of drinking water and sewage water) was carried out. Enterovirus RNA was found in 2 sewage samples, namely, from the sewer collector, whereinto wastewater is discharged from the kindergarten and residential buildings with ill residents, as well as from the sewer collector whereinto wastewater is discharged from the children's infectious disease department No. 6 of Municipal budgetary healthcare institution (MBHCI) “the City Hospital No. 1 named after N.A. Semashko”, where children with EVI were hospitalized.
The presence of enterovirus in feces, material from the throat of carriers and patients with a typical clinical picture (lesion of the oropharynx and upper respiratory tract, hands) indicates the implementation of the fecal-oral and aspiration mechanisms of pathogen transmission.
In addition to the MBPEI No. 83, cases with a preliminary diagnosis of “Enterovirus infection” were registered simultaneously within the period from May 28 to June 10, 2013 in 11 more preschool institutions located in the Pervomaisky (5), Voroshilovsky (2), Proletarsky (2), Sovetsky (1) districts of Rostov-on-Don, as well as in the towns of the Rostov Region (Taganrog, Bataysk, and Azov). In all the institutions, the epidemiological features of EVI were the earlier onset of the epidemic season, the large-scale involvement, the rapidity of the epidemic process development with the defeat of mainly children's organized contingent (2–6 years old), and the clinical picture inherent to respiratory viral or bacterial infections accompanied by meningitis.
To determine the genotype of the EVI pathogen and reveal epidemiological links between the formed foci in the Rostov Region, the fragment sequencing of biological material samples from patients and carriers with positive results tested by RT-PCR was carried out at the Federal Budgetary Institution “Rostov Research Institute of Microbiology and Parasitology” of Rospotrebnadzor [22].
The results of sequencing showed that among the examined 74 strains of human enteroviruses, the largest number (48 strains or 64.9%) were identified as human enteroviruses A71, 9 strains (12.2%) were identified as human enteroviruses A Coxsackie A16. As rare genotypes of human enteroviruses, there were registered 5 strains (6.8%) of human enterovirus A Coxsackie A6, 4 strains (5.4%) of human enterovirus B ECHO 30, 2 strains (2.7%) of human enterovirus B ECHO 11, 2 strains (2.7%) of human enterovirus B Coxsackie B5, and one strain each (1.4%) of human enterovirus B ECHO 9, human enterovirus A Coxsackie A4, human enterovirus B Coxsackie B3, as well as human enterovirus C Coxsackie A24 (Figure 3).
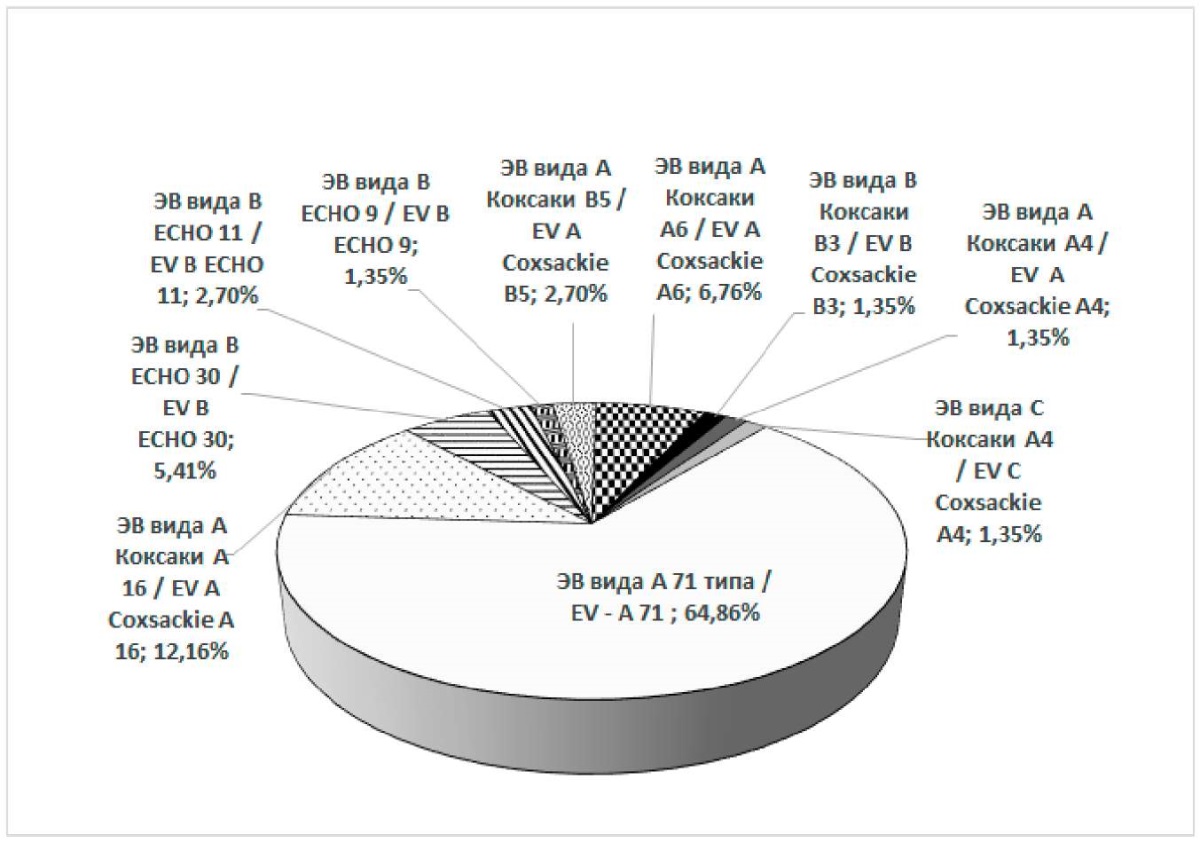
Рисунок 3. Структура генотипов энтеровирусов человека,
выделенных из клинического материала в Ростовской области в 2013 г.,
по данным секвенирования последовательностей РНК
и результатам их программного анализа
(за период 26.06.2013–10.10.2013 гг.)
Figure 3. The structure of human enterovirus genotypes
isolated from clinical material in the Rostov Region in 2013,
according to RNA sequence sequencing data and the results of their program analysis
(for the period of June 26, 2013 – October 10, 2013)
The phylogenetic analysis resulted in the identification of 4 isolated groups of viruses. Three of them (46 strains) were represented by RNA nucleotide sequences of enteroviruses 71 isolated from individuals living in Rostov-on-Don city and the Aksai town, which differed essentially from each other in genetic characteristics. The fourth group (7 strains) was represented by RNA nucleotide sequences of enteroviruses, which were isolated from individuals living in the Taganrog town. This group of strains had a high concordance level of genetic properties with enterovirus strains, characterized in the international database as human enterovirus A Coxsackie A16, isolated in 2013 in China [23].
Identification of three groups of human enteroviruses A 71 with a high degree of genetic similarity of RNA nucleotide sequences within each of them but essential differences in genetic characteristics between groups, as well as a detection of a group of enteroviruses A Coxsackie A16 with similar genetics features, suggests the formation of conditions for the spreading infection in at least four EVI foci, which are not interconnected.
Phylogenetic analysis of RNA nucleotide sequences isolated out of enterovirus strains from patients and virus carriers in the Rostov Region during the epidemic rise in EVI in 2013 showed that enterovirus 71 strains circulating in the Rostov Region had a high genetic relatedness with strains of the “Chinese” origin of 2008–2011, which had predominantly the 4C subgenotype. Apparently, the strain was brought to Russia from China 2–3 years before the epidemic rise of EVI in the Rostov Region and other Southern Russia territories and circulated without causing clinically registered morbidity [23][24].
The landscape of enteroviruses isolated from environmental objects in the Rostov Region in 2013 is represented by EV 71; Coxsackie A24; ECHO 11, 23, 30; that is, by 5 genotypes.
In subsequent years after the outbreak of EVI in 2013, singular events of sporadic EVI were registered. In 2014, 2015, and 2018, the incidence rate of EVI in the Rostov Region was at the same level, amounting to 3.8 people per 100 thousand, which is 3.8 times less than in 2013 (Figure 1). Since 2016, there has been a trend toward an increase in the incidence from 5.0 to 7.3 people per 100,000. In 2019, the incidence rate of EVI reached 8.5, and by 2020, it decreased to 0.27 people per 100,000 that, apparently, was associated with the introduced anti-COVID restrictions. The maximum number of EVI cases was registered in 2013, 2017, and 2019, which was stipulated by the circulation of new for the region enterovirus variants.
Among the diseased, the proportion of children ranged from 21% in 2009 to 94% in 2013.
Enteroviral meningitis ranged from 3.7% of the total number of EVI cases in 2014 to 35.7% in 2010. In the clinical aspect, for all the years of observation, except for 2013, “small” EVI forms with signs of herpangina, “hand-foot and mouth” disease syndrome, and ARVI prevailed.
The EVI incidence in the Rostov Region was registered over the analyzed period in 33 territories, namely, 12 towns and 21 districts.
Analyzing the intra-annual EVI dynamics over a years-long period revealed a clearly defined summer-autumn seasonality with the beginning of the rise in June-July, the peak in August, and the decline in September-October, which is associated with the natural and climatic conditions in the South of Russia and the period of public bathing in water sources.
Within the 2014–2020 period, in the landscape of enteroviruses isolated from patients and carriers of EVI in the Rostov Region, the prevailing forms were enterovirus Coxsackie A (1, 2, 4, 5, 6, 9, 10, 16) and Coxsackie B (2.5) accounting for more than 48%, as well as enteroviruses ECHO (6, 9, 30), which were within 46%. The role of EV 71 in EVI pathology during the specified period was insignificant (about 6%).
Discussion
The presented features of the EVI clinical course in children during the disease outbreak in the Rostov Region had a clear similarity with the clinical course of EVI caused by enterovirus 71, namely, a peculiar cyclicity, characterized by the initial appearance of “hand-foot and mouth” disease-like syndrome with the subsequent development of CNS pathology including meningitis, encephalitis, and rhombencephalitis in some of the patients [25][26]. According to the classification of V.F. Uchaikin (2002), in all the children who fell ill in the period of the EVI epidemic rise, the combined form of EVI-71 was diagnosed, including that without CNS injury in 87 children (62.6%), and with CNS injury in 52 children (37.4%). CNS pathology was stipulated by meningitis in 42 (32.0%) patients and meningoencephalitis in 10 patients (7.2%) [4][22].
The data of phylogenetic analysis attests to a high level of concordance of genetic properties of human EV A71 isolated in the Rostov Region in 2013 with enterovirus strains isolated in 2008–2011 on the territory of China. This could be facilitated by the activation of migration flows from the countries of the Asia-Pacific region to Russia and vice versa [9]. In particular, according to Rosstat data in 2012, compared to 2011, the tourist flow from China to Russia increased by 47%, and totally for 5 years from 2011 to 2015, the tourist flow from China to the country increased from 233 thousand to 1.3 million people, that is, by 5.6 times.
The complication of the epidemiological situation with enterovirus in the southern regions of the Russian Federation in 2013 was associated with the circulation of highly pathogenic type EV 71, which had not been encountered in this area until 2013, which determined its high epidemiological significance and facilitated its wide spreading. This indicates that in the development of the EVI epidemiological process, the infectious-immunological relationship between the pathogen and the immunological insusceptibility state in the population, especially in children age, is important. In the authors’ opinion, this fact is consistent with the theory of self-regulation of parasitic systems developed by V.D. Belyakov [27–29]. This theory is based on the concept of the heterogeneity of the pathogen population in terms of virulence and antigenicity, as well as the heterogeneity of the human population in terms of immunity. During the epidemic process, the rate and nature of the heterogeneity in the pathogen population are altering, and that is coming along with changes in the population’s collective immunity.
Conclusions
- In the period of 2006–2020, the course of the epidemic process of EVI in the Rostov Region was characterized by sporadic incidence with some fluctuations in incidence rates for the years from 0.02 to 14.6 people per 100 thousand. When analyzing the intra-annual distribution of EVI cases over a years-long period, a clearly defined summer-autumn seasonality was revealed, which is associated with natural and climatic conditions in southern Russia and the period of public bathing in water sources.
- The registered rise in the incidence of EVI in 2013 accompanied by the forming local foci in organized children's groups with the number of ill people 622 and one death was stipulated by the emergence and spreading of a new type human enterovirus 71, having one of the most pathogenic genotypes of enteroviruses, which has not been previously encountered in the Rostov Region.
- According to molecular genetics investigations and phylogenetic analysis, EV 71 subgenotype C4, which caused an outbreak of EVI in the Rostov Region, was closely related to the strain examined in China for 2008–2011 and brought to the Russian Federation due to migration processes. In the period from 2006 to 2020, according to the results of the sequencing of samples of biological material from patients and virus carriers in the Rostov Region, enteroviruses of 22 types were detected, namely, Coxsackie A1, 2, 4, 5, 6, 9, 10, 16; Coxsackie B1, 2, 3, 4, 5; ECHO 5, 6, 7, 9, 11, 12, 17, 30; enterovirus A 71 subgenotype C4.
- The clinical course of EVI during the outbreak in 2013 in the vast majority of children (94.9%) was characterized by an acute onset, the development of an intoxication syndrome, a peculiar cyclicity with the initial appearance of a “hand-foot and mouth” disease-like syndrome and subsequent development of the CNS pathology (meningitis 32.0%, meningoencephalitis 7.2%) in some patients (37.4%). All the children to be examined had an aggravated premorbid background and belonged to risk groups.
- Optimization of the epidemiological surveillance system for EVI in the Rostov Region has led to a decrease in the incidence of EVI from 14.5 to 0.27 people per 100,000 when comparing 2013 and 2020.
References
1. Nosov S.D., ed. Rukovodstvo po infekcionnym boleznyam u detej. Moscow: Medicina; 1980. (In Russ.)
2. Lukashev A.N. The role of recombination in the evolution of enteroviruses. Problems of Virology. 2005;50(3),46-51. (In Russ.) eLIBRARY ID: 9150593
3. Lukashev A.N., Koroleva G.A., Lashkevich V.A., Mikhailov M.I. Enterovirus 71: epidemiology and diagnostics. Journal of Microbiology, Epidemiology and Immunobiology (JMEI). 2009;(3):110-116. (In Russ.) eLIBRARY ID: 23087718
4. Onishchenko G.G.,, Popova A.YU., Ezhlova E.B., Smolenskij V.YU., Demina YU.V. et al.; Onishchenko G.G., ed. Enterovirusnaya infekciya v yuzhnyh sub»ektah Rossijskoj Federacii (epidemiologiya, diagnostika, klinika, profilaktika). Rostov-on-Don; 2016. (In Russ.)
5. Alimov A.V., Feldblyum I.V., Akimkin V.G., Zakharova Yu.A., Sergeev A.G., Pitersky M.V. Epidemiological surveillance and control of enterovirus (non-polio) infection: current problems and solutions. Yekaterinburg: Unika; 2021. (In Russ.)
6. Brotons P, Jordan I, Bassat Q, Henares D, Fernandez de Sevilla M, et al. The Positive Rhinovirus/Enterovirus Detection and SARS-CoV-2 Persistence beyond the Acute Infection Phase: An Intra-Household Surveillance Study. Viruses. 2021;13(8):1598. doi: 10.3390/v13081598
7. Wang K, Ye F, Chen Y, Xu J, Zhao Y, et al. Association Between Enterovirus Infection and Type 1 Diabetes Risk: A Meta-Analysis of 38 Case-Control Studies. Front Endocrinol (Lausanne). 2021;12:706964. DOI: 10.3389/fendo.2021.706964
8. Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Dempsey DM, et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch Virol. 2019;164(9):2417-2429. doi: 10.1007/s00705-019-04306-w
9. Butakova L.V., Trotsenko O.E., Sapega E.Yu. Enterovirus infection: overview of the current global situation in the context of intensified migration flows. Public Health and Life Environment – PH&LE. 2018;(4):55-60. (In Russ.)
10. Rankin DA, Speaker A, Perez A, Haddadin Z, Probst V, et al. Circulation rhinovirus/ Enterovirus respiratory infections in children during 2020-2021 in the United Statutes. Open forum infectious diseases. Oxford University Press, 2021;8(Suppl 1):S93. DOI: 10.1093/ofid/ofab466.154
11. Golitsyna L.N., Nguyen T.T.T., Romanenkova N.I., Luong M.T., Vo L.T., Kanaeva O.I. et al. Enterovirus infection in the Socialist Republic of Vietnam. Russian Journal of Infection and Immunity. 2019;9(3–4):467–475. (In Russ.) DOI: 10.15789/2220- 7619-2019-3-4-467-475
12. Zhukova L.I., Rafeenko G.K., Larin F.I., Scherbina L.I., Shut I.N. et al. Enterovirus non-poliomyelitis infections in Krasnodar region. Journal of Microbiology, Epidemiology and Immunobiology (JMEI) 2014;(4):13-17. (In Russ.) eLIBRARY ID: 23929480
13. Lukashev A.N., Golitsina L.N., Vakulenko Y.A., Akhmadishina L.V., Romanenkova N.I. et al. Current possibilities and potential development of molecular enterovirus surveillance. Experience of Russian Federation. Russian Journal of Infection and Immunity. 2018;8(4):452-464. (In Russ.) doi: 10.15789/2220-7619-2018-4-452-464
14. Romanenkova N.I., Bichurina M.A., Golitsyna L.N., Rozaeva N.R., Kanaeva O.I., et al. Nonpolio enteroviruses which caused the rise of enterovirus infection on some territories of Russia in 2016. Journal Infectology. 2017;9(3):98-108. (In Russ.) https://doi.org/10.22625/2072-6732-2017-9-3-98-108
15. Sapega E.Yu., Butakova L.V., Trotsenko O.E., Goryaev D.V., Dmitrieva G.M. Molecular-epidemiologic analysis of enteroviruses circulating on the territory of the Far Eastern and Siberian federal districts of the Russian Federation including those that caused outbreaks of the disease. The Far Eastern Journal of Infectious Pathology. 2018;(35):5-14. (In Russ.) eLIBRARY ID: 36610120
16. Sapega E.Yu., Yanovich V.A., Trotsenko O.E., Onishchenko G.G., Korita T.V., et al. Epidemiological Features of Enterovirus Infection during Flood on the Territory of Jewish Autonomous Region. Problems of Particularly Dangerous Infections. 2014;(1):71-74. (In Russ.) https://doi.org/10.21055/0370-1069-2014-1-71-74
17. Trotsenko O.E., Kurganova O.P., Zaitseva T.A., Ezhalova E.B., Yanovich V.A., et al. Utilization of scientific potential for international cooperation between Russia and China for prevention of epidemic spread of enterovirus infections. The Far Eastern Journal of Infectious Pathology. 2015;28(28):6-12. (In Russ.) eLIBRARY ID: 24312352
18. Golicyna L.N., Zverev V.V., Epifanova N.V., Sanina T.A., Kashnikov A.YU. i dr. Nepoliomielitnye enterovirusy v Rossijskoj Federacii v 2016 godu. Infekcionnye bolezni. Zabolevaemost’, etiologiya, struktura i voprosy profilaktiki enterovirusnoj (nepolio) infekcii. 2017;(4):25-30. (In Russ.)
19. Sapega E.Yu., Butakova L.V., Kotova V.O., Amyaga E.N., Trotsenko O.E. Molecular Genetic peculiarities of enterovirus circulation in the Far Eastern federal district of the Russian Federation in 2014-2015. The Far Eastern Journal of Infectious Pathology. 2016;(30):38-44. (In Russ.) eLIBRARY ID: 26468242
20. Romanenkova, N. I., Rozaeva, N. R., Bichurina, M. A., Kanaeva, O. I., Galimov, R. R. I dr. Osobennosti epidemicheskogo processa i etiologii enterovirusnoj infekcii na otdel’nyh territoriyah Rossii. Vserossijskaya nauchnoprakticheskaya konferenciya s mezhdunarodnym uchastiem «Epidemiologicheskij nadzor za aktual’nymi infekciyami: novye ugrozy i vyzovy»; 2021; Nizhnij Novgorod:188-192. (In Russ.) DOI: 10.21145/978-5-6046124-2-2_2021
21. Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94(3):441-8. doi: 10.1016/0022-2836(75)90213-2
22. Kovalev E.V. , Yagovkin E.A. , Onischenko G.G., Simovanyan E.N., Nenadskaya S.A. et al. Epidemiological and clinical features of enterovirus (nepolio) infection of 71st type in children in the city of Rostov-on-Don Infectious diseases: News, Opinions, Training. 2018;4(27): 44-51. (In Russ.) DOI: 10.24411/2305-3496-2018-14007
23. Kolpakov D.S., Kucherenko N.B., Yagovkin E.A., Shemshura A.B., Sauhat S.R. i dr. Molekulyarno-geneticheskaya harakteristika shtammov enterovirusov cheloveka, vydelennyh ot lyudej v period pod»ema zabolevaemosti naseleniya Rostovskoj oblasti enterovirusnoj (nepolio) infekciej v 2013g. VIII Vserossijskaya nauchno-prakticheskaya konferenciya s mezhdunarodnym uchastiem «Molekulyarnaya diagnostika 2014»; 18-20 marta 2014; Moskva. (1):425-426. (In Russ.)
24. Lukashev A.N., Ahmadishina L.V., Bajkova O.YU., Govoruhina M.V., Nenadskaya S.A. i dr. Molekulyarnaya epidemiologiya enterovirusov 71-go tipa — vozbuditelya vspyshki v Rostove-na-Donu v 2013g. VIII Vserossijskaya nauchno-prakticheskaya konferenciya s mezhdunarodnym uchastiem «Molekulyarnaya diagnostika 2014»; 18-20 marta 2014; Moskva. (1):390-391. (In Russ.)
25. Skripchenko N.V., Ivanova V.V. Enterovirusnaya infekciya u detej (epidemiologiya, etiologiya, diagnostika, klinika, terapiya, profilaktika). St. Petersburg: NIIDI; 2009. (In Russ.)
26. Koroleva G.A., Lukashev A.N., Khudyakova L.V., Mustafina A.N., Lashkevich V.A. Encephalomyelitis caused by enterovirus type 71 in children. Problems of Virology. 2010;55(6), 4-10. (In Russ.) eLIBRARY ID: 15528383
27. Belov A.B. The Academician V. D. Belyakov - the Founder of the Domestic Theory of Epidemiological Science of the XXI Century. Epidemiology and Vaccinal Prevention. 2016;15(6):9-15. (In Russ.)
28. Belyakov V. D. Obshchie zakonomernosti funkcionirovaniya parazitarnyh sistem (mekhanizmy samoregulyacii) Parazitologiya. 1986;20(4):249-255. (In Russ.)
29. Belyakov V.D., Golubev D.B., Kaminskij G.D., Tec V.V. Samoregulyaciya parazitarnyh sistem: molekulyarnogeneticheskie mekhanizmy. Medicina: Leningradskoe otdelenie; 1987. (In Russ.)
About the Authors
E. V. KovalevRussian Federation
Evgeny V. Kovalev, Head of Rospotrebnadzor Department for the Rostov Region
Rostov-on-Don
Competing Interests:
The authors declare no conflict of interest.
T. I. Tverdokhlebova
Russian Federation
Tatyana I. Tverdokhlebova, Dr. Sci. (Med.), Director of the Rostov research Institute of Microbiology and Parasitology
Rostov-on-Don
Competing Interests:
The authors declare no conflict of interest.
E. N. Simovanyan
Russian Federation
Emma N. Simovanyan, Dr. Sci. (Med.), professor, Head of the Department of Pediatric Infectious Diseases
Rostov-on-Don
Competing Interests:
The authors declare no conflict of interest.
Review
For citations:
Kovalev E.V., Tverdokhlebova T.I., Simovanyan E.N. Molecular epidemiological and clinical aspects of enterovirus infection in the south of Russia. Medical Herald of the South of Russia. 2023;14(1):83-92. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-1-83-92







































