Scroll to:
Difficulties in diagnosing a myofibroblastic lung tumor in a child
https://doi.org/10.21886/2219-8075-2023-14-3-41-45
Abstract
Inflammatory myofibroblastic tumor (VMO) is a rare neoplasm, which is currently referred to as a mesenchymal neoplasm with intermediate biological potential. The tumor consists of differentiated myofibroblastic fusiform cells. The frequency of occurrence of VMO among primary lung neoplasms is up to 1%. This type of tumor is the most common type of primary lung tumors in children. VMO can occur both in childhood and in adulthood. VMO is found in the soft tissues of the abdominal cavity, in the pelvic organs, larynx, mammary gland, head and neck tissues. Extra-organ localization (retroperitoneal space, mediastinum) is also diagnosed. Lung VMO often has no clinical manifestations, and if present has nonspecific symptoms, such as cough, hemoptysis, shortness of breath, hyperthermia, chest pain. VMO was previously considered as a neoplasm with a benign course, but these tumors tend to local relapses and distant metastasis. The article presents data on the prevalence, clinical manifestations and treatment of VMO, as well as own clinical observation of the course of this disease in an 8-year-old child.
For citations:
Belykh N.A., Zakharova A.V., Piznyur I.V., Anikeeva N.A., Styazhkina E.V., Deeva Yu.V. Difficulties in diagnosing a myofibroblastic lung tumor in a child. Medical Herald of the South of Russia. 2023;14(3):41-45. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-3-41-45
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare type of neoplasm with distinctive histological heterogeneity. Besides, the development of IMT is based on molecular genetic changes [1].
This type of tumor can emerge at any age but it is more common in children and young adults [2]. The worldwide prevalence of this pathology ranges from 0.04% to 0.7%, regardless of race. The male to female ratio is 1:3 [3][4].
The IMT consists of differentiated myofibroblastic spindle cells and numerous plasma cells and/or lymphocyte infiltrates [2][5].
According to the classification of soft tissue tumors proposed by the World Health Organization (2020), this tumor belongs to the group of fibroblastic/myofibroblastic tumors [6].
The investigation history of the disease dates back to the 20th century, when Brunn et al. described the inflammatory pseudotumor of the chest in 1939 first. The term “inflammatory pseudotumors” was proposed by Umiker and Iverson in 1954 to describe a lung tumor, similar in some clinical and diagnostic characteristics to malignant neoplasms. Later, in 1955, Lane et al. coined the term “plasma granuloma” based on the histological picture of the tumor, which was similar to inflammatory pseudotumor but characterized by a predominance of plasma cells [2]. In 1990, Meis and Enzinger described a neoplasm similar in the histological picture to inflammatory pseudotumor but with a more aggressive clinical course and called it inflammatory fibrosarcoma. In 1994, IMT was defined as a neoplasm consisting of spindle cells specified as myofibroblasts and a large number of associated inflammatory cells, and then, in 1995, Coffin et al. introduced first the term “IMT”. In that study, they presented data from 84 patients with IMT of extrapulmonary localization, which had similar clinical and histological patterns to inflammatory fibrosarcoma. However, no case of distant metastasis detection was identified, and the rate of disease relapse was slightly lower than previously published data (25%) [1][7].
However, to date, the exact etiology and pathogenesis of IMT remain unclear. The main factors leading to the development of this neoplasia are inflammation, trauma, surgery, autoimmune process, and various bacterial and viral infections, in particular those caused by mycobacteria, corynobacteria, as well as viruses of hepatitis B, Epstein-Barr, and human papilloma. In addition, there are data on the relationship between IMT and chromosomal rearrangements in the 2p23 locus, which is located on the short arm of chromosome 2 next to the gene encoding anaplastic lymphoma kinase (ALK). ALK is a receptor-type tyrosine kinase, which is a driver of tumorigenesis, resulting from gene translocation in cases of anaplastic large-cell lymphoma, lung cancer, and IMT. To date, more than 21 partner genes are known to be involved in the pathogenesis of IMT, and the range of these genes is updated every year [1][3].
The clinical manifestations of IMT are closely related to the area of primary foci and may be accompanied by general symptoms such as pain and fever. Pulmonary IMT may manifest itself as chest pain and shortness of breath. Moreover, the manifestation of clinical symptoms does not depend on the size and weight of the tumor [5][8].
The results of different IMT imaging methods vary. On X-ray radiography, the IMT may appear as several formations in the same anatomical area. The imaging may range from an infiltrate to a well-determined formation with varying proportions of inflammatory and fibrotic components. Computed tomography (CT) may reveal contrast deficiency in the fibrous component of the tumor. MRI imaging may also show low signal intensity due to fibrosis along with limited diffusion.
During laboratory diagnostics of IMT, neutrophilic leukocytosis, an increased level of C-reactive protein and erythrocyte sedimentation rate (ESR) may be observed [3].
As the standard treatment for IMT, surgical resection is applied. In cases if complete resection is not possible, radiation and hormonal or molecular targeted drug therapy are used.
The discovery of ALK fusions in patients with non-small cell lung carcinoma has facilitated the clinical elaboration of ALK inhibitors, including the molecularly targeted drug crizotinib, which has emerged in recent years and demonstrated high efficacy [3][9].
IMT has a low metastatic potential and has a tendency to relapse, the frequency of which depends on the localization of the process and varies from < 2% for pulmonary localization to 37% for extrapulmonary localization.
The most common areas of metastasis include lymph nodes, lungs, and bones. Therefore, long-term observation of the patient is necessary to prevent relapse [10].
Description of a clinical case
Female patient N., born in 2014, belongs to the Slavic nationality. A girl is from the 4th pregnancy, which occurred against the background of the threat of miscarriage in the 1st and 2nd trimesters; the birth was the 3rd, urgent and spontaneous. Body weight at birth was 3620 g, body length was 55 cm, head circumference was 36 cm, chest circumference was 35 cm, and Apgar score was 8/9 points. The child was discharged from the maternity hospital on the 5th day in satisfactory condition. The neonatal period was uneventful.
The girl grew and developed according to her age, she was breastfed for up to 6 months, and was vaccinated according to the calendar. Past diseases included chicken pox at the age of 3 years, and ARVI 2–3 times a year. Allergological and genealogical anamneses were not burdened.
The girl contacted the infectious diseases department of the State budgetary institution of the Ryazan region “City Clinical Hospital No. 11” in Ryazan on November 5, 2020 with complaints of subfebrile temperature and shortness of breath. Upon admission, the general condition was assessed as moderate; body temperature was 37.5°C. An unproductive cough and hyperemia of the oropharynx were also revealed. In the lungs, harsh breathing, dry wheezing, and prolonged exhalation were found by auscultation, the respiratory rate was 46 per minute, and SpO2 was 95%. The preliminary diagnosis was “Community-acquired pneumonia.”
Blood tests showed no signs of anemia, leukocytosis was up to 15.3 × 10⁹/l, neutrophilia was up to 93%, and acceleration of ESR was up to 25 mm/h.
Urinalysis showed no pathology.
ELISA to antibodies against M. pneumonia revealed that IgM and IgG gave positive results, while against Chl. pneumoniae revealed that IgM and IgG were negative. On the chest x-ray pattern dated November 5, 2020, the infiltration of the lung tissue was determined on the left in the projection of the 1st segment and the inferior medial lung segment. The conclusion was as follows: left-sided segmental pneumonia (Figure 1).
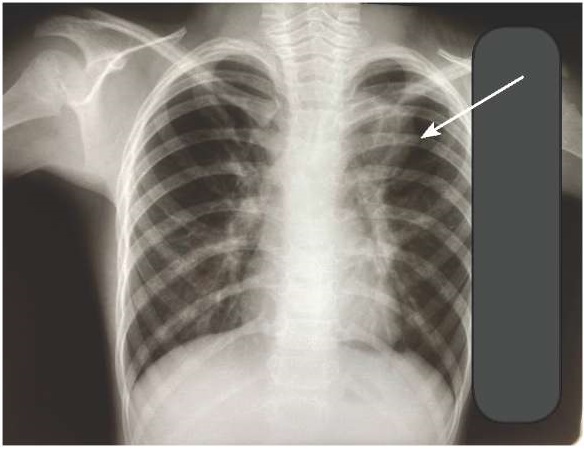
Figure 1. Chest X-ray during hospitalization
Against the backdrop of antibacterial and symptomatic therapy, the symptoms of intoxication and bronchial obstruction were relieved. On the control X-ray pattern of the chest organs (November 13, 2020), infiltration was not found but a darkening with clear contours was seen in the upper lobe on the left. Then in the left upper lobe during a CT scanning of the chest organs (November 23, 2020), specialists revealed a formation with clear even contours of isodense density with entry and breakage of the upper lobe bronchus; wherein the trachea was displaced to the right, and there was no infiltration. The conclusion was the following: CT data of a bronchogenic cyst.
For further examination, the girl was transferred to the pulmonology department of the Russian Children’s Clinical Hospital named after N.V. Dmitrieva, Ryazan, with a diagnosis of “Community-acquired pneumonia, segmental, left-sided, upper lobe, acute, respiratory failure stage 1 on the background of mycoplasma infection. Bronchogenic cyst of the upper lobe of the left lung?”
Upon further examination, on the left upper lobe a chest X-ray pattern (dated November 25, 2020), an additional formation of a homogeneous structure with clear contours adjacent to the upper mediastinum and the left root was revealed. The left root was compacted.
The results of CT with intravenous contrast (November 27, 2020) were as follows: a CT picture of an additional cystic-solid formation in the projection of the upper lobe of the left lung. The size of the mediastinal lymph nodes was within normal limits. Spirometry showed a moderate decrease in the patency of the small bronchi.
Ultrasound of the abdominal and pelvic organs (December 8, 2020) and the thyroid gland (November 25, 2020) revealed no pathology. In addition, the results of bronchoscopy (November 25, 2020) rejected endobronchial pathology. Diaskintest (December 4, 2020) was negative.
Contrast-enhanced MRI of the chest (December 10, 2020) revealed signs of a large solid formation in the left hemithorax.
The results of the investigation of the level of tumor markers were as follows: alpha-fetoprotein was less than 1.66 IU/ml (normalcy from 0 to 5.5 IU/ml), NSE (neuron-specific enolase) was 27.6 ng/ml (normalcy less than 17 ng/ml), b — total human chorionic gonadotropin was <1.20 (normalcy 0–5 mU/ml).
For further examination, the girl was sent to the Pirogov Children’s Clinical Hospital in Moscow.
Upon admission (January 26, 2021), general blood and urine tests were within formal normative limits.
The X-ray pattern of the chest organs dated January 27, 2021 revealed the following: in the SⅠ-Ⅱ projection of the upper lobe of the left lung, there was determined an elongated formation (45×25×43 mm), with intense inclusions, with a clear contour, adjacent and obviously closely connected with the root of the left lung, with costal pleura reaction and interstitial deformation.
Multislice CT, dated January 28, 2021, revealed a solid neoplasm SⅠⅡ-Ⅰ of the upper lobe in the left lung, which reached or emanated from the root, with the same dimensions (33.5×24×45.7 mm) of an irregular elongated shape (+ 36-39-42 HU), closely fused with the segmental bronchi SⅠ-Ⅱ-ⅠⅡ.
Ultrasound of the abdominal and pelvic organs revealed no pathology.
On February 4, 2021, the child underwent left upper lobe tumor lobectomy. The histological examination revealed the following: on the section, a dense whitish lesion focus with clear boundaries measuring 3×2.5×1.2 cm was adjacent to the root of the lung.
Immunohistochemical investigation results were as follows: tumor cells were positive for SMA antibodies, focally Desmin, S100, negative reactions with anti-RSK, PR, ALK (p80), EMA, CD34, TTFI, TRK, and ROS1. The conclusion was the following: the described pathological changes correspond to an IMT.
The clinical diagnosis was “Inflammatory myofibroblastic tumor of the upper lobe of the left lung; condition after removal of the upper lobe of the left lung with a tumor.”
In August 2021, the girl underwent a routine examination at the pulmonology department of the Russian Children’s Clinical Hospital named after N.V. Dmitrieva, Ryazan. According to the results of the laboratory examination, no pathology was detected. CT scanning of the chest organs (dated August 10, 2021) showed no evidence of the presence of fresh focal and infiltrative changes. An ultrasound of the abdominal and pelvic organs (dated August 10, 2021) revealed no pathology
Currently, the girl has no complaints. She is under the dynamic supervision of a pediatrician and pulmonologist.
Conclusion
IMT is a rare disease that can camouflage under various clinical patterns, which makes it hard to diagnose this pathology.
References
1. Suleymanova A.M., Kachanov D.Yu., Imyanitov E.N., Roshchin V.Yu., Shamanskaya T.V., Varfolomeeva S.R. Inflammatory myofibroblastic tumors in children: literature review. Russian Journal of Pediatric Hematology and Oncology. 2020;7(2):64-77. (In Russ.) https://doi.org/10.21682/2311-1267-2020-7-2-64-77
2. Korolczuk A, Jarosz P, Jasielski P, Mitura P, Bar K. Inflammatory myofibroblastic tumor of the kidney in patient with nephron-sparing surgery. Case report and review of the literature. Indian J Pathol Microbiol. 2022;65(1):176-180. https://doi.org/10.4103/ijpm.ijpm_11_21
3. Gros L, Dei Tos AP, Jones RL, Digklia A. Inflammatory Myofibroblastic Tumour: State of the Art. Cancers (Basel). 2022;14(15):3662. https://doi.org/10.3390/cancers14153662
4. Lopukhova VA, Tarasenko IV, Shestavina NV, Ilyin MYu. Tendencies of primary morbidity and mortality of population of the Kursk region from malignant neoplasms. Science of the young (Eruditio Juvenium). 2020;8(2):202-7. https://doi.org/10.23888/HMJ202082202-207
5. Shi P, Zhang L, Shi H, Wu Y. Inflammatory Myofibroblastic Tumor in the Thyroid Gland: A Retrospective Case Series Study and Literature Review. Oncol Res Treat. 2022;45(6):353-365. https://doi.org/10.1159/000524489
6. Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021;113(2):70-84. https://doi.org/10.32074/1591-951X-213
7. Chen C, Huang M, He H, Wu S, Liu M, et al. Inflammatory Myofibroblastic Tumor of the Urinary Bladder: An 11-Year Retrospective Study From a Single Center. Front Med (Lausanne). 2022;9:831952. https://doi.org/10.3389/fmed.2022.831952
8. Park JW, Han SH, Kim DK. [Inflammatory Myofibroblastic Tumor Misdiagnosed as Intrahepatic Cholangiocarcinoma]. Korean J Gastroenterol. 2022;79(1):41-44. (In Korean). https://doi.org/10.4166/kjg.2021.146
9. Wu S, Jian F. A Case Report of Inflammatory Myofibroblastic Tumor of the Sphenoidal Sinus. Ear Nose Throat J. 2021:1455613211065990. Epub ahead of print. PMID: 34933588. https://doi.org/10.1177/01455613211065990
10. Shustova S.A., Miroshkina T.A. Protective mechanisms of lungs. I.P. Pavlov Russian Medical Biological Herald. 2020;28(4):567-577. https://doi.org/10.23888/PAVLOVJ2020284567-577
About the Authors
N. A. BelykhРоссия
Natalya A. Belykh - Dr. Sci. (Med.), Docent, Head of the Department of Polyclinic Pediatrics with the Course of Pediatrics of the Faculty of Additional Professional Education, Ryazan State Medical University.
Ryazan
Competing Interests:
None
A. V. Zakharova
Россия
Anastasia V. Zakharova - Student, Ryazan State Medical University.
Ryazan
Competing Interests:
None
I. V. Piznyur
Россия
Inna V. Piznyur - assistant of the Department of Polyclinic Pediatrics with the Course of Pediatrics of the Faculty of Additional Professional Education, Ryazan State Medical University.
Ryazan
Competing Interests:
None
N. A. Anikeeva
Россия
Nataliya A. Anikeeva - MD, PhD, Assistant Professor of the Department of Polyclinic Pediatrics with the Course of Pediatrics of the Faculty of Additional Professional Education, Ryazan State Medical University.
Ryazan
Competing Interests:
None
E. V. Styazhkina
Россия
Elena V. Stezhkina - MD, PhD, Assistant Professor of the Department of Polyclinic Pediatrics with the Course of Pediatrics of the Faculty of Additional Professional Education, Ryazan State Medical University.
Ryazan
Competing Interests:
None
Yu. V. Deeva
Россия
Yulia V. Deeva - assistant of the Department of Polyclinic Pediatrics with the Course of Pediatrics of the Faculty of Additional Professional Education, Ryazan State Medical University.
Ryazan
Competing Interests:
None
Review
For citations:
Belykh N.A., Zakharova A.V., Piznyur I.V., Anikeeva N.A., Styazhkina E.V., Deeva Yu.V. Difficulties in diagnosing a myofibroblastic lung tumor in a child. Medical Herald of the South of Russia. 2023;14(3):41-45. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-3-41-45
JATS XML







































