Scroll to:
Hypoxic-ischemic brain damage of the fetus and newborn with hemodynamic disorders in the “mother-placenta-fetus” system
https://doi.org/10.21886/2219-8075-2022-13-4-88-99
Abstract
Objective: to identify the dependence of the severity of cerebral disorders in newborns and children of the first year of life on the indicators of blood flow in the utero-placental complex. Materials and methods: a total of 184 full-term newborns were examined in the period from birth to one year of life. The main group included children with cerebral ischemia of II and III severity and its consequences (group II, n=78; group III, n=42). Group I included newborns without signs of central nervous system damage, 14 of them had neurological symptoms by the end of the neonatal period (after a month, group I n =50, group II n =92). All children underwent general clinical examination, assessment of neurological status, ultrasound examination of the brain, transcranial dopplerography of cerebral vessels, electroencephalography. Maternal medical records were analyzed to identify hemodynamic parameters in the "mother-placenta-fetus" system at 12-13, 20-21, 28-32, and 36-40 weeks of gestation. Results: The features of uterine and fetal blood flow in the dynamics of gestation in mothers of the examined groups of children were determined. The associations between the values of Pi AUD, AUS, and AUM in the second and third trimesters of pregnancy in different groups were shown. The relationship between disorders of uteroplacental hemodynamics and the severity of cerebral pathology was revealed. A "method of antenatal prediction of the severity of cerebral disorders in newborns" was proposed. Conclusions: The obtained results make it possible to predict the risk of hypoxic-ischemic damage to the central nervous system in newborns antenatally using hemodynamic parameters in the mother-placenta-fetus system at 36 weeks of gestation.
Keywords
For citations:
Berezhanskaya S.B., Abduragimova M.К. Hypoxic-ischemic brain damage of the fetus and newborn with hemodynamic disorders in the “mother-placenta-fetus” system. Medical Herald of the South of Russia. 2022;13(4):88-99. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-4-88-99
Introduction
The problem of hypoxic-ischemic injury of the central nervous system (CNS) in neonates remains relevant, because annually, this pathology affects about one million infants worldwide and is associated with long-term cognitive, neurosensory, and motor defects [1][2]. According to statistics from the Russian Ministry of Health, there has been a more than twofold increase in neonatal encephalopathy since 2000, with perinatal hypoxia being the dominant factor in the formation of the pathology [3][4].
The etiopathogenesis of hypoxic-ischemic cerebral disorders includes antenatal events, starting with altered immune and endocrine adaptation of the mother to pregnancy, invasion of trophoblast cells, and the initial stages of placental formation, which is recognized as a temporary but indispensable organ for the continuation of gestation and, perhaps, the most significant organ in human life [5][6][7].
The interest in the placenta increased as knowledge accumulated, opening up new problems and requiring new and more complex solutions. The latter is related to the polyfunctionality of the placenta and the development of changes associated with hypoxia and hemodynamic disorders, leading to maternal and fetal vascular malperfusion, neuroimmune interactions and non-infectious inflammation, insufficient placental protective functions, and several others. It all creates conditions of prolonged stress, chronic hypoxia, poor nutrition, and gas exchange, which slows the growth of the placenta itself and the development of the fetus.
Most researchers point out that the major factor of chronic antenatal fetal hypoxia is placental insufficiency [8][9][10]. It is caused by pathomorphological changes in the maternal and/or fetal part of the placenta. They are reflected in the indicators of the hemodynamics of the uterine-placental-fetal complex, which are leveled because of the activation of compensatory mechanisms of protection in the initial stages. At the same time, disorders of other functional capabilities of the placenta, that arise after and/or in parallel, aggravate the degree of severity of placental insufficiency and reduce the ability to protect and compensate for the effects of negative factors, that the brain is sensitive to [6][7][11].
The study aimed to identify the dependence of the severity of cerebral disorders in newborns and children in the first year of life on the indicators of blood flow in the uteroplacental complex.
Materials and Methods
A clinical observational, analytical, combined study was conducted partly as a longitudinal type and partly as a case-control type. The study was approved by the ethical committee of Rostov State Medical University and conducted in accordance with the international standard Guideline for Good Clinical Practice (GCP).
The study included 184 full-term newborns delivered in the maternity hospital who were examined and treated in the departments of neonatal pathology and pediatric departments 1 and 2 of the Scientific Research Institute of Obstetrics and Pediatrics in 2018–2021. The main group consisted of children with cerebral ischemia of degrees II and III of severity and its complications (Group II, n=78; Group III, n=42), who met the following criteria: (a) presence of antenatal risk factors for hypoxic-ischemic CNS lesions (maternal age and health status, a history of complicated obstetric and gynecological pathology in the present pregnancy and delivery); (b) child's condition at birth (Apgar score ≤ 7, psychomotor status in points by the scales of Zhurb and Mastyukova ≤ 23 in the early neonatal period [12]).
Exclusion criteria were: children from twins, those with congenital malformations, hereditary pathology, intrauterine infections, hemolytic disease of the newborn, those born to women with a history of hemostasis disorders, repeated cases of spontaneous abortion, women with anemia of degrees 2–3 before and during pregnancy, type I and II diabetes, degree II and III obesity, children who received blood transfusions.
The control group included 64 full-term neonates without signs of CNS lesions (Group I) in the early neonatal period. Fourteen children in this group had manifested neurological symptoms by the end of the neonatal period; therefore, they were included in Group II, increasing the number of children to 92. Thus, one month after the start of the observation, the number of children in Group I was 50.
All children underwent general clinical examination, which included CBC and urine tests, biochemical blood assay, electrocardiography and echocardiography, abdominal ultrasonography, including the functional status of the biliary system and kidneys, and an examination by an ophthalmologist.
Assessment of neurological status in the early and late neonatal period, at 3, 6, and 12 months of life, was performed based on syndromes development; the severity of the course of the disease was determined according to the "classification of perinatal lesions of the nervous system in newborns" and their consequences [13] and the severity criteria according to Barashnev [14].
Imaging techniques were used to detect and assess the severity of brain lesions: ultrasound examination of the brain through the anterior fontanel (on the apparatus Siemens Acusonantares (USA) using a PH4-1 phased multifrequency sector sensor with a frequency range of 1–9 MHz and VIVID 3PRO (USA) sector sensors with a frequency range of 7.5 MHz (for newborns and children under 3 months old) and 5 MHz (from 3 months and older)) and MRI of cerebral structures (when indicated) were performed. Cerebral blood flow was studied by ultrasound Doppler sonography (on Aloka-SSD-1400 (Japan) by duplex scanning through anterior fontanel with a 5 MHz microconvex sensor; Multi-DopRT2 version DWL2.55a (DWL Elektronische Systeme GmbH, Germany) by spectral transcranial Dopplerography through the temporal acoustic window with a 2 MHz frequency pulse mode sensor). Electroencephalographic research was carried out on EEGA21/26 Encephalan-131-03 (NPKF Medikom-MTD, Taganrog). Computer EEG video monitoring was performed when indicated.
To identify the most significant antenatal predictors of fetal and neonatal hypoxic-ischemic brain injury in the groups of examined children, a maternal record review was performed to reveal hemodynamic parameters in the "mother-placenta-fetus" system obtained during obstetric monitoring at 12–13, 20–21, 28–32, and 36–40 weeks of gestation.
Comprehensive B-mode echographic examination of pregnant women and Doppler imaging (color Doppler mapping, pulsed-wave Dopplerometry) of uterine and umbilical arteries, and the middle cerebral artery were performed using convex and volumetric sensors at 2–6 mHz and 3.5–5.0 mHz and Voluson E8Expert (GE System, USA).
Statistical analysis was performed with the software packages MS Excel 2019 (Microsoft, USA) and Statistica version 12.5, (IBM, USA), SPSS27.001. Quantitative data were described using median (Me), lower and upper quartiles (Q1;Q3) because they did not comply with the normal distribution law. Categorical data were described with absolute values and percentages.
To compare intergroup differences, the authors used the nonparametric Kruskal-Wallis test for independent samplings. Nonparametric correlation analysis was performed with Spearman's test (r). The differences were considered statistically significant at p<0.05.
Results
Group I included healthy newborns delivered by healthy women without gestational or delivery complications who had an Apgar score of 8–9 and had a quantitative score of 27–29 by the Zhurb and Mastyukova scale during the observation period (up to 1 year of life).
The examined children in clinical Groups II and III were primarily delivered by women with a history of obstetric and gynecological complications during pregnancy and labor. The most frequent complications of pregnancy pathology included a threat of spontaneous abortion and miscarriage in the second and third trimesters, arterial hypertension, compensated feto-placental insufficiency, premature placental maturation, and impaired uterine-placental hemodynamics at weeks 28–32 and 36–40, verified by Doppler scanning. These complications led to the development of perinatal hypoxia of medium and severe degrees.
Most babies in Group II (60.3%) were born with an Apgar score of 6 to 8, and by the 5th minute, the score was typical for healthy infants (7 to 8 points), which in some observations did not correspond to the severity of neurologic symptoms developed later; 33.3% of babies were born with mild asphyxia, and 21.8% were born with moderate asphyxia. In Group III, already at minute 1, the condition of the prevailing majority was assessed as severe (64.9%), and one in five of them was born in an extremely severe condition (12% of the total number in the group), which usually persisted by minute 5 (Table 1).
Table 1.
Assessment of newborns on the Apgar scale at 1 and 5 minutes of life (Me [Q1;Q3])
|
Score in points |
Groups of children |
|||||
|
Group I, n=64 |
Group II, n=78 |
Group III, n=42 |
||||
|
1' |
5' |
1' |
5' |
1' |
5' |
|
|
Ме (Q1;Q3) |
8 [ 7;8] |
9 [ 8;9] |
7 [ 6;8] |
8 [ 7;8] |
5 [ 3;6] |
6 [ 5;6] |
Note: When analyzing the indicators, significant differences are determined between groups I and II, I and III, II and III at the 1st and 5th minutes (p= 0.002 at the 1st minute between groups I and II, in other cases p= 0.0001).
There were significant group differences in the structure of neurological disorders in the first weeks of life. As the severity of hypoxic-ischemic brain injury increased, the symptoms of inhibition of unconditioned reflex activity and muscle tone disorders became more pronounced and stable. In the early neonatal period, suppression syndrome and seizure syndrome were diagnosed more frequently in Group III patients compared to those in Group II (33.3; 40.5%; and 9.0; 28.6%) as manifestations of a more pronounced lesion. At the same time, the incidence rate of the pre-excitation syndrome was over two-fold higher in the group with light hypoxia (44.9%; 19.0%). At the same time, the majority of the children with a moderately severe hypoxic-ischemic brain injury had a decrease in the severity of symptoms accompanied by a high incidence rate of muscle hypotonia (42.3%) or hypertonicity (44.9%), pyramidal insufficiency (56.4%), and vegetovisceral disturbances (69.2%) in the neonatal period. In the group of children with severe brain damage, symptoms of muscle hypotonia (81.0%) and pyramidal insufficiency (76.2%), associated with a persisting syndrome of suppression, were noted more frequently for 2–3 weeks.
In 69.2% and 71.4% of children in Groups II and III, respectively, a syndrome of vegeto-visceral dysfunction was diagnosed, which was associated with a variety of polymorphic symptoms, primarily on the skin: pronounced red or white dermographism, "marbled" pattern, periorbital or perioral cyanosis, acrocyanosis, and general hyperhidrosis of palms and feet. Functional disorders of the gastrointestinal tract manifested as dyskinesia of the hypomotor or hypermotor type, spasm or excessive relaxation of sphincters of the digestive system, which led to impaired motility of the gastrointestinal tract, frequent posseting, and unstable stool. Vegeto-visceral disorders also included episodes of rapid breathing up to 55–60 per minute, tachy or bradycardia, and thermoregulatory disorders in the form of non-infectious subfebrility and febrility.
Follow-up observation revealed that in 15.2% of children in Group II, neurological symptoms completely regressed by the age of one year. The persisting clinical manifestations by the end of the first year of life transformed in most cases into a syndrome of minimal cerebral dysfunction, which was observed in almost every third patient and more frequently combined with disorders of the autonomic nervous system (33.7%), hyperactivity and hyperexcitability syndrome (21.8%).
The syndrome of minimal cerebral dysfunction of the residual-organic genesis was registered in Group III by the first year of life only in 19.0% of children; significantly more frequently in various combinations with delayed pre-speech and motor development, hyperactivity and hyperexcitability syndrome, hemoliquorodynamic impairments, and autonomic nervous system disorder.
An increase in the lesion severity led to an increase in the occurrence rate of severe forms of motor development, with the formation of various forms of infantile cerebral palsy (spastic tetraparesis (1 and 4 children, respectively), hemiparesis (2 and 3); only in Group III, spastic diplegia was registered in one child and its atonic-astatic form – in two children) by 1–1.5 years of age. Symptomatic epilepsy and partial paroxysms with subsequent generalization debuted in the first year of life in 14.3% of children from Group III and were more frequently combined with spastic tetraparesis (9.5%) (Table 2).
Table 2.
Manifestations of cerebral disorders at the age of one year in children with moderate to severe lesions
|
Neurological manifestations |
Group II, n=92 |
Group III, n=42 |
|
Absence of neurological symptoms |
14 (15.2%) |
- |
|
Minimal brain dysfunction syndrome |
26 (28.3%) |
8 (19.0%) |
|
Delayed pre-speech development |
24 (26.1%) |
25 (59.5%) |
|
Disorder of the autonomic nervous system |
31 (33.7%) |
27 (64.3%) |
|
Hyperactivity and hyperexcitability |
20 (21.8%) |
16 (38.1%) |
|
Syndrome of hemolyquorodynamic disorders |
24 (26.1%) |
18 (42.9%) |
|
Motor development disorders |
38 (41.3%) |
31 (73.8%) |
|
Tempo delay of motor development |
28 (30.4%) |
17 (40.5%) |
|
Cerebral palsy: - spastic tetraparesis |
1(1.1%) |
4 (9.5%) |
|
- hemiparesis |
2 (2.2%) |
3 (7.1%) |
|
- spastic diplegia |
- |
1 (2.4%) |
|
- atonic-astatic form |
- |
2 (4.8%) |
|
Symptomatic epilepsy |
- |
6 (14.3%) |
|
Psychomotor development in points* |
23 [ 21;24.5] |
16 [ 13;18] |
Note: * – quantitative assessments of psychomotor development on a 30-point scale (Zhurba L.B., Mastyukova E.A., 1981).
The generally recognized opinion about the persisting rate of perinatal hypoxic-ischemic brain lesions in the fetus and newborn, the revealed high rate of severe cerebral disorders and their complications, including those leading to disabilities, in some cases, the inconsistency of the severity of lesions with the relatively "favorable" course of pregnancy and labor determined the feasibility of an in-depth study of hemodynamic abnormalities in the "mother-placenta-fetus" system as one of the significant antenatal predictors of hypoxic-ischemic brain lesions.
The analysis of blood flow parameters in the uterine-placental-fetal complex vessels (Table 3) revealed that the dynamics of uterine and fetal blood flow parameters in the mothers from clinical Group I corresponded to the reference percentile tables (Medvedev, 1996–2016).
It was determined that uterine Pi values in the first trimester were more than half (by 66.1 and 65.7% for AUD and AUS, respectively) higher than those before labor. Asymmetry of right and left values was registered: Pi AUD values were lower than those in the left one in the vast majority of cases.
Table 3.
Indicators of dopplerometry of the uterine and fetoplacental complex at different gestation periods (Mе [Q1;Q3])
|
Gestational age |
Groups |
Pi AUD5 |
Pi AUS |
Pi AUM |
Pi ACM |
|
12-13 weeks |
I |
1.65 |
1.75 |
- |
- |
|
II |
1.72 |
1.88 |
- |
- |
|
|
III |
1.84 |
1.97 |
- |
- |
|
|
р |
0.435 |
0.450 |
|||
|
20-21 weeks |
I |
1.21 |
1.37 |
1.25 |
1.43 |
|
II |
1.46 |
1.57 |
1.44 |
1.63 |
|
|
III |
1.66 |
1.62 |
1.53 |
1.63 |
|
|
р |
0.173 |
0.770 |
0.182 |
0.231 |
|
|
28-32 weeks |
I |
0.54 |
0.64 |
0.93 |
1.87 |
|
II |
0.70 |
0.77 |
1.03 |
1.87 |
|
|
III |
0.72 |
0.86 |
1.18 |
1.91 |
|
|
р |
0.095 |
0.059 |
0.758 |
0.955 |
|
|
36-40 weeks |
I |
0.56 |
0.60 |
0.90 |
1.75 |
|
II |
0.68 |
0.70 |
0.90 |
1.78 |
|
|
III |
0.72 |
0.84 |
1.10 |
1.88 |
|
|
р |
0.001 |
0.009 |
0.082 |
0.056 |
Note: Pi – pulsation index; AUD – right uterine artery; AUS – left uterine artery; AUM – umbilical artery; ACM – middle cerebral artery; p – significance level of differences; p - comparison using the Fisher criterion (bilateral); pI – statistically significant differences compared to group I.
The dynamics of hemodynamic indices in the mothers of children with hypoxic-ischemic brain damage of moderate severity at different gestational periods had their peculiarities. First, starting from the first trimester of pregnancy, the uterine blood flow parameters were higher than those of Group I mothers. Second, there was symmetry in the numerical values of blood flow on the right and left.
It was determined that the Doppler parameters in Group III and II mothers indicated higher mean values compared to the reference values. There were statistically significant differences in the blood flow velocities in the right (AUD) and left (AUS) uterine arteries at 36–40 weeks versus control (p=0.003; p=0.02). In every sixth woman, a symmetry in AUD and AUS was recorded with values 15–20% higher than those in the control group. At the same time, although asymmetry of Pi AUD and AUS was preserved in every fifth woman, there was a significant increase in Pi AUS values on the side where the placenta was located.
The analysis of individual Pi AUM and ACM values in Groups II and III before labor revealed a significant excess of Pi AUM over reference values (in 12.8% and 14.3%, respectively), although the median feto-placental blood flow values were within the reference intervals. At the same time, the opposite dynamics of Pi ACM before labor were recorded in 10.2 and 11.9%, respectively.
The authors compared the numerical values of the studied indices and identified the number and structure of uterine-placental hemodynamic disturbances in order to determine the occurrence rate of their localizations.
The authors revealed that in the second trimester of pregnancy, there was a high detection rate of uterine-placental hemodynamic disturbances in Groups II and III (47.4% and 61.9%) and sporadic events in the healthy group (4.7%) (Table 4).
Table 4.
Uteroplacental hemodynamics disturbances according to Dopplerometry at 28-32 weeks in mothers of the examined groups
|
Localization of disturbances |
Group I, n=64 |
Group II, n=78 |
Group III, n=42 |
|||
|
abs. |
% |
abs. |
% |
abs. |
% |
|
|
AUD, AUS |
3 |
4.7 |
23 |
29.5 |
16 |
38.1 |
|
AUD, AUS, AUM |
- |
- |
11 |
14.1 |
7 |
16.7 |
|
AUM |
- |
- |
3 |
3.8 |
3 |
7.1 |
|
Without violations |
61 |
95.3 |
41 |
52.6 |
16 |
38.1 |
Note: % are given in relation to the number of mothers in the group of examined children; AUD, AUS – right and left uterine arteries; AUM – umbilical arteries.
Changes in the uterine arteries (29.5% and 38.1%) dominated the pattern of hemodynamic abnormalities in Groups II and III, but both groups recorded a fairly large number of changes in only one uterine artery (15.4 of 29.5%; 16.7 of 38.1%). Blood flow changes occurred more frequently in the AUS (10.3 versus 5.1% in Group II; 11.9 versus 4.8% in Group III), which can be explained by anatomical peculiarities during pregnancy – dextroposition of the enlarged uterus, which leads to the changes in the angle of the uterine artery and increased resistance in it [15].
The analysis of the overall rate of hemodynamic abnormalities in the mothers of the examined cohort revealed that at 28–32 weeks' gestation in Group III, uteroplacental hemodynamic abnormalities were more frequently detected, being statistically significantly different from the healthy group (p=0.017).
Hemodynamic changes at 36–40 weeks in Groups II and III were detected in nearly every second woman (41.0% and 42.9%), which was significantly higher than in the healthy group (p=0.007 for each group). The distribution of uteroplacental hemodynamic abnormalities according to the localization of changes is presented in Table 5.
Table 5.
Uteroplacental hemodynamics disturbances according to Dopplerometry at 36-40 weeks in mothers of the examined groups
|
Localization of disturbances |
Group I, n=64 |
Group II, n=78 |
Group III, n=42 |
|||
|
abs. |
% |
abs. |
% |
abs. |
% |
|
|
AUD, AUS |
5 |
7.8 |
19 |
24.4 |
12 |
28.6 |
|
AUD, AUS, AUM |
1 |
1.6 |
9 |
11.5 |
4 |
9.5 |
|
AUM |
- |
- |
4 |
5.1 |
2 |
4.7 |
|
Without violations |
58 |
90.6 |
46 |
59.0 |
24 |
57.1 |
Note: % are given in relation to the number of mothers in the group of examined children; AUD, AUS – right and left uterine arteries; AUM – umbilical arteries.
Doppler imaging during pregnancy in 6 women of Group I identified non-critical uteroplacental hemodynamic disturbances, with 4.7% of the changes affecting one of the uterine arteries and 3.1% of both (in one case, there was a combination of uterine and fetal-placental blood flow disturbances). Relatively healthy children born to mothers with such complications were assigned to the group with threatening conditions.
In Group II, abnormalities of the uteroplacental complex were more frequently registered, they were detected in every fifth woman. Moreover, combined changes in uterine and umbilical arteries were observed in 11.9% of patients. An isolated increase in Pi AUM was detected in 5.1% of women, which was primarily determined by pathology of the umbilical cord.
In Group III, uterine-placental hemodynamics disturbances were detected in half of the women, and uterine-placental blood flow disorders were noted in almost every third woman. Isolated changes in one of the uterine arteries were detected in 11.9% of women with predominant lesions of the left artery, and in both uterine arteries in 16.7%. Combined impairment of uterine and fetal-placental blood flow, which did not reach critical values, was detected only in 9.5% of cases, and isolated increased blood flow velocity in umbilical arteries was detected in 4.7% of women.
Obviously, fetal hemodynamics (as an integrative index of fetal condition) depends on the interplay of many factors, some of which may be unknown to date. The authors analyzed Pi AUM in the third trimester of pregnancy and before delivery depending on PiAUD and AUS parameters (Figs. 1–3).
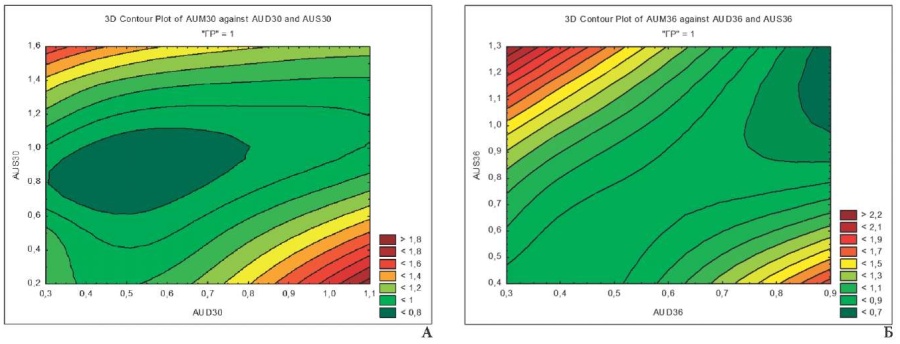
Fig. 1. Relationship of Pi AUD, AUX and AUX at 28-32 (A) and 36-40 (B) weeks of gestation in group I mothers
Fig. 1 demonstrates optimal Pi AUM values (0.7–1.07) over a larger area, which is associated with normal uterine-placental blood flow in mothers of this group. Level-line maps change their shape in the dynamics of pregnancy, which is associated with a decrease in Pi AUD and AUS by the time of delivery to increase blood flow and decrease uterine peak systolic velocity, redistributing total uterine blood flow in favor of the descending branch of the uterine artery responsible for blood supply of the cervix [16].
In the group of children with moderate CNS lesions, the pattern of the effect of hemodynamics in the uterine artery system on the fetus can be clearly seen (Fig. 2 A, B). It should be noted that increased blood flow in the umbilical arteries was observed at extremely high and low AUD and AUS velocities. The deviation of one of the parameters to one side or the other led to an increase in Pi AUM, which reflects the importance of adequate hemodynamics in both uterine vessels. The three-dimensional planar projection at 36–40 weeks' gestation reflects the predominant influence of AUD, associated with the differences in the diameter of the right and left uterine arteries and veins, and uterine artery index of the right and left uterine halves in favor of the vessels on the right. Probably, the morphological asymmetry of the uterine vascular bed is related to the development of the organ from the paired Müllerian ducts [17].
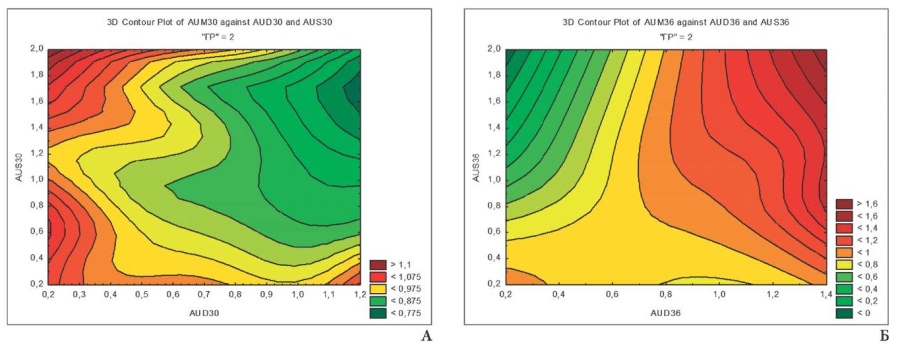
Fig. 2. Relationship of Pi AUD, AUX and AUX at 28-32 (A) and 36-40 (B) weeks of gestation in group II mothers
Similar tendencies in the relationship of Pi AUD, AUS, and AUM were observed in Group III. Overall, there was a "narrow" corridor of normal AUM blood flow velocity at different gestational periods with a predominant influence of AUD (Fig. 3A, B).
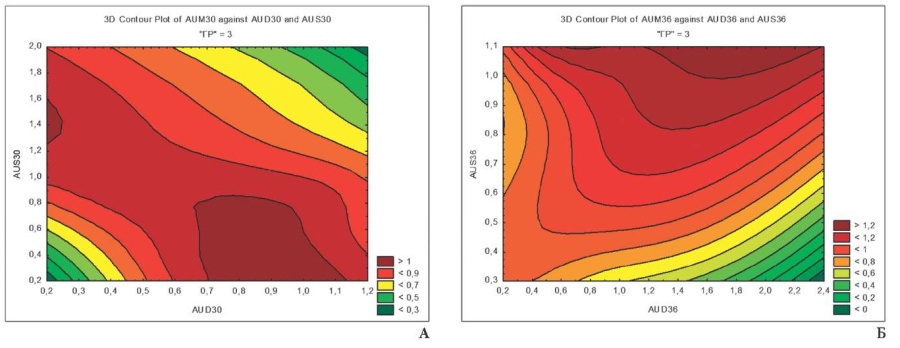
Fig. 3. Relationship of Pi AUD, AUX and AUX at 28-32 (A) and 36-40 (B) weeks of gestation in group III mothers
It is noteworthy that elevated AUM blood flow velocity was registered nearly on the total projection area, since the numerical values of the analyzed parameters in the groups of patients with severe conditions exceeded those in Groups I and II.
Discussion
A retrospective analysis of maternal documentation with emphasis on feto-placental vascular abnormalities in the gestational dynamics of the mothers of 184 full-term newborns was performed to identify predictors allowing for antenatal diagnostics and prediction of hypoxic-ischemic brain damage development in newborns.
In the healthy group, uterine and fetal blood flow parameters had characteristic regular dynamic features. They reflected a gradual decrease in AUD and AUS blood flow velocity curves in the dynamics of pregnancy. It was associated with a decrease in total peripheral vascular resistance in the maternal placental reserve capillaries.
Asymmetry of Pi AUD and AUS with a predominant increase in pulsation index in the left uterine artery was determined by the prevalence of right-sided and ambilateral placental location in the vast majority of pregnant women [18][19].
The decrease in Pi AUM in the dynamics of physiological pregnancy reflected the process of placental adaptation to fetal growth. In the third trimester, it is manifested by a steady decrease in the resistance of blood vessels to supply the significantly increasing oxygen requirements of the fetus.
The analysis of Doppler parameters in children with severe and moderate lesions revealed elevated Pi values in the utero-feto-placental vessels. Aggravation of the severity of the condition led to an increase in the median Pi AUD, AUS, AUM, and ASM along with an increase in the interquartile range. There were statistically significant intergroup differences in Pi AUD and AUS at 36-40 weeks in the severe cerebral impairment group compared with controls (p=0.003; p=0.02).
The probability of influence and hence dependence on several factors of uterine and feto-placental hemodynamic parameters was assumed, which determined the associations between the studied parameters, which could be clearly seen in the three-dimensional images. Level-line maps recorded a clear relationship between Pi AUD, AUS, and AUM values in the second and third trimesters of pregnancy. They also reflected the influence of the blood flow intensity in the uterine arteries on the fetal blood flow with the predominant influence of AUD (Figs. 1–3).
Along with extremely high Pi AUM and ACM values in Groups II and III, low Pi ACM values were detected before labor in 10.2 and 11.9% of women, respectively, indicating severe fetal blood flow abnormalities requiring emergency delivery.
It is important to note that 21.4% of the mothers in clinical Group III had episodes of null and retrograde blood flow in the second and third trimesters. It indicates severe vasospasm of the small vessels of the maternal and fetal placenta, causing a significant increase in total peripheral vascular resistance.
Correlation analysis with Spearman's test identified reliable (p<0.05) direct and inverse correlations of moderate and weak strength between Pi AUD, AUS, AUM, and ACM indices at 36–40 weeks of gestation, as well as with Pi ACM at birth. The observed situation is quite natural, since hemodynamics, as an integrative index, reflects the functional state of the feto-placental complex without a clear determination of the etiology of the pathological process.
One should pay attention to the direct associations of average strength between blood flow indices in the group of children with severe conditions: between Pi AUD values at 36–40 weeks and AUM at the same term and at birth (r=0.37, p<0.05 and r=0.42, p<0.05, respectively), between Pi AUM and AUM values at 36–40 weeks (r=0.39, p<0.05), between Pi AUM values at 36–40 weeks and at birth (r=0.35, p<0.05).
The profound significance of Doppler indices in assessing the functional system "mother-placenta-fetus" is confirmed by the results of the multifactorial analysis "Classification trees", which helped develop a "method for antenatal prediction of the severity of cerebral disturbances in newborns" depending on the corresponding values of hemodynamic indices. The Pi value in AUD (100.0%) was of maximum importance in deciding for the division of the study groups by severity already at 36 weeks of gestation, which is quite logical. The role of AUS and ACM blood flow indices, as model factors for predicting the probability of hypoxic-ischemic brain damage, was reduced at least two-fold (49.1; 40.2%) in the examined cohort of children. The systems of inequalities, obtained using this method, allowed for predicting hypoxic-ischemic brain damage already intrauterine at 36 weeks with a high degree of sensitivity (92.0%) and specificity (90.5%).
Conclusions
- Persistent cerebral hemodynamic abnormalities in the fetus correlated significantly with changes in blood flow parameters in the uterine-placental-fetal complex starting from the early stages of pregnancy. The most pronounced correlations were observed in patients with elevated blood flow velocity curves in the right uterine artery.
- The analysis of the blood flow patterns in the "mother-placenta-fetus" system depending on the condition of the children at birth and in the dynamics of the first year of life provides the establishment of the most significant hemodynamic parameters. Newborns from mothers with persistent disorders of uteroplacental hemodynamics and, in case of unstable changes, with elevated values of Pi AUD and ASM are at high risk of developing moderate to severe hypoxic-ischemic lesions of the CNS.
- The hemodynamic changes detected at 36 weeks' gestation indicate placental hypoxia and fetal ischemic cerebral disturbances that persist and/or aggravate at birth. For this reason, a "method for antenatal prediction of the severity of cerebral disturbances in newborns" was developed using multifactorial analysis, based on comparing the values of pulse indices in the uterine, umbilical, and middle cerebral arteries. It will allow the specialists to establish the nature and extent of cerebral pathology in the neonatal period.
Conclusion
Fetal hypoxia occurs and intensifies because of the progressive deterioration of hemodynamics of the feto-placental complex, accompanied by decreased transport of oxygen and nutrients through the placenta, leads to decompensated hypoxia and acidosis, and depends on the severity of placental insufficiency.
These hemodynamic changes begin with the implantation of the trophoblast and lead to profound progressive changes at the cellular level and disorders of intercellular interactions, which lead to the formation of cerebral pathology of varying severity with the possibility of prolongation to severe postnatal brain damage with polymorphic clinical manifestations.
Authors contribution:
S.B. Berezhanskaya — research design development;
S.B. Berezhanskaya, M.K. Abduragimova — obtaining and analysis of the data;
M.K. Abduragimova — writing the text of the manuscript; review of publications on the topic of the article.
Conflict of interest. Authors declare no conflict of interest.
References
1. Ovsjannikov D.Ju., Krsheminskaja I.V., Bojcova E.V.; Ovsjannikova D.Ju., ed. Perinatal'naja asfiksija, gipoksicheski-ishemicheskaja jencefalopatija i ih posledstvija. Moscow: RUDN; 2018. (In Russ.).
2. Solevåg AL, Schmölzer GM, Cheung PY. Novel interventions to reduce oxidative-stress related brain injury in neonatal asphyxia. Free Radic Biol Med. 2019;142:113-122. doi: 10.1016/j.freeradbiomed.2019.04.028
3. Afanasyeva N.V., Strizhakov A.N. Outcomes of pregnancy and labor in fetoplacental insufficiency of various severity. Gynecology, obstetrics and perinatology. 2004;3(2):7–13. (In Russ.). eLIBRARY ID: 9899698
4. Krasnorutckaja O.N., Ledneva V.S. Clinical and biochemical indices in diagnosis of developmental disorders in children with consequences of perinatal lesion of the nervous system. Pediatria. 2018;97(3):175–179. https://doi.org/10.24110/0031-403X-2018-97-3-175-179
5. Shallie PD, Naicker T. The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. Int J Dev Neurosci. 2019;73:41-49. doi: 10.1016/j.ijdevneu.2019.01.003
6. Rosenfeld CS. The placenta-brain-axis. J Neurosci Res. 2021;99(1):271-283. doi: 10.1002/jnr.24603
7. Ortega MA, Fraile-Martínez O, García-Montero C, Sáez MA, Álvarez-Mon MA, et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells. 2022;11(3):568. doi: 10.3390/cells11030568
8. Istomina N.G., Makarovskaya E.A., Baranov A.N., Revako P.P. Diagnosis of fetal hypoxia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021;6:29-33 (in Russ.) https://dx.doi.org/10.18565/aig.2021.6.29-33
9. Pavlova NG, Bezhenar’ VF, Bolshakova MV, Pastushenkov VL, Yakovleva AA, Karev VE. Expression of hypoxia-inducible factor (HIF-1α) in the placenta and fetal brain tissue in chronic placental insufficiency under experimental conditions. Russian Journal of Human Reproduction. 2022;28(1):36‑44. (In Russ.). https://doi.org/10.17116/repro20222801136
10. Wardinger JE, Ambati S. Placental Insufficiency [Electronic resource]. Treasure Island (FL): StatPearls Publishing, 2022.
11. Redline RW. Placental pathology: Pathways leading to or associated with perinatal brain injury in experimental neurology, special issue: Placental mediated mechanisms of perinatal brain injury. Exp Neurol. 2022;347:113917. doi: 10.1016/j.expneurol.2021.113917
12. Zhurba L.T., Mastjukova E.M. Narushenie psihomotornogo razvitija detej pervogo goda zhizni. Moscow: Medicina; 1981. (In Russ.).
13. Burkova A.S., Volodin N.N., Zhurba L.T., Medvedev M.I., Rogatkin S.O., Timonina O.V. Classification of perinatal damages to the nervous system and their outcomes in first-year infants (manual of the Russian association perinatologiests). Clinical practice in pediatrics. 2006; 1(5):38–70. (In Russ.) eLIBRARY ID: 9552949
14. Barashnev Ju.I. Perinal'naja nevrologija. Izd. 2 -e, dop. Moscow: Triada–H; 2011. (In Russ.).
15. Kaptilnyi V.A. Prognostic significance of isolated disorders in the uteroplacental perfusion in pregnancy. V.F. Snegirev archives of obstetrics and gynecology. 2015;2(2):19-25. (In Russ.) eLIBRARY ID: 34088558
16. Chekhonatskaya M.L., Rogozhina I.E., Yannaeva N.E. Change characteristics blood flow uterine before labor. Saratov journal of medical scientific research. 2008;2(20):67-70. (In Russ.) eLIBRARY ID: 10426678
17. San'kova I.V., Kaplunova O.A., Chaplygina E.V. Asymmetry of the uterine vessels. Journal of Anatomy and Histopathology. 2017;6(4):42-46. (In Russ.) https://doi.org/10.18499/2225-7357-2017-6-4-42-46
18. Vasileva V.V., Botasheva T.L., Khloponina A.V., Pelipenko I.G., Shubitidze M.G. Placenta migration in dependence on the center-peripheral asymmetry of the mother-placenta-fetus function system. Sovremennye problemy nauki i obrazovanija. 2018;1:68. (In Russ.). eLIBRARY ID: 32473066
19. Palieva N.V., Botasheva T.L., Khloponina A.V., Zavodnov O.P., Zheleznyakova E.V., Ganikovskaya Y.V. Effect of morpho-functional asymmetries of the mother - placenta - fetus system on metabolic homeostasis during pregnancy. The Bulletin of the Adyghe State University. 2018;4(231):63–70. (In Russ.). eLIBRARY ID: 37024359
About the Authors
S. B. BerezhanskayaRussian Federation
Sofia B. Berezhanskaya - Dr.Med.Sci., Professor, Chief Researcher of the Pediatric Department of Scientific Research Institute of Obstetrics and Pediatrics, Scientific Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
M. К. Abduragimova
Russian Federation
Marina K. Abduragimova - pediatrician of the pediatric department №2, Scientific Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
Rostov-on-Don
Competing Interests:
Authors declares no conflict of interest
Review
For citations:
Berezhanskaya S.B., Abduragimova M.К. Hypoxic-ischemic brain damage of the fetus and newborn with hemodynamic disorders in the “mother-placenta-fetus” system. Medical Herald of the South of Russia. 2022;13(4):88-99. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-4-88-99







































