Scroll to:
Clinical and biochemical phenotypes in cardiorespiratory comorbidity
https://doi.org/10.21886/2219-8075-2023-14-2-67-75
Abstract
Objective: to estimate the incidence of recurrent myocardial infarction, stroke, and mortality in patients with different phenotypes of myocardial infarction against the background of chronic obstructive pulmonary disease.
Materials and methods: 325 patients with myocardial infarction were examined: 195 patients with infarction against the background of chronic obstructive pulmonary disease and 130 patients without chronic obstructive pulmonary disease. We studied markers of endogenous intoxication: molecules of average mass, hematological indexes of intoxication, blood gas composition, apoptosis, protein peroxidation, lipid and antioxidant protection, inflammation and renal function. Statistical processing of the data was performed using SPSS 26.0 software package.
Results: A two-stage cluster analysis formed four clusters, which were labeled as «polymarker-retentive», «necrotic-inflammatory», «hypoxic-inflammatory», and a cluster with no endogenous intoxication syndrome. Among patients with myocardial infarction against the background of chronic obstructive pulmonary disease, the hypoxic-inflammatory phenotype prevailed — in 53.3 % of patients. Necrotic-inflammatory phenotype was noted in 8.2 % of patients, hypoxic-inflammatory — in 36.9% of patients, phenotype with the absence of endogenous intoxication syndrome — in 1.5 % of patients. Recurrent myocardial infarction, stroke and mortality constituted the combined endpoint. The highest incidence of the combined endpoint was observed in the polymarker-retentive infarct phenotype — in 37 (55.2 %) patients.
Conclusion: Vascular life-threatening and fatal events (recurrent myocardial infarction, stroke, death from cardiac causes) during 12-month follow-up are most typical for the polymarker-retentive phenotype. The clinical features of this phenotype were the frequent presence of Q-shaped MI, transmural myocardial damage, and the presence of complications in the acute period. COPD in these patients was characterized by a long course, high smoker's index, mostly 3rd degree bronchoobstruction, and frequent exacerbations. The results of this study allow for a personalized approach to the assessment of the annual prognosis in patients with acute myocardial infarction against COPD.
Keywords
For citations:
Prokofyeva T.V., Bashkina O.A., Polunina O.S., Sevostyanova I.V., Gritsenko E.L. Clinical and biochemical phenotypes in cardiorespiratory comorbidity. Medical Herald of the South of Russia. 2023;14(2):67-75. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-67-75
Introduction
Comorbidity is one of the major challenges facing modern healthcare systems, as a patient with two or more coexisting health conditions has now become a norm rather than an exception both in outpatient and inpatient clinical settings [1]. Therefore, the diagnosis and management of comorbid patients and the prediction of outcomes are taking on increasing importance [2].
One of the most common is cardiorespiratory comorbidity, and special attention is paid to coexisting myocardial infarction (MI) and chronic obstructive pulmonary disease (COPD) [3][4]. This comorbidity increases the probability of atypical clinical features of infarction and associated late diagnosis and damps the prognosis both in the acute period and after discharge [5–7]. This dictates the importance of identifying individuals at high risk of life-threatening vascular complications (recurrent MI, stroke) and fatal outcomes among comorbid MI patients, as well as necessitates the timely correction of modifiable predictors of adverse events.
To date, many authors have demonstrated that pathological conditions are characterized by endogenous intoxication syndrome (EIS). This syndrome is characterized by excessive accumulation of endogenous toxic substances due to hyperproduction or impaired excretion. The EIS increases the severity of the pathologic processes and worsens the prognosis [8][9]. Based on the diagnosis of endogenous intoxication, the clinical course and outcome of MI can be predicted for COPD patients.
A number of laboratory markers have been used to assess the EIS, which makes the investigations somewhat difficult. Phenotyping may become one of the solutions to this problem [10][11]. Identifying clinical and biochemical phenotypes and analyzing MI in COPD patients can help in predicting infarction outcomes.
The aim of the study was to evaluate the incidence of recurrent MI, stroke, and mortality in various phenotypes attributable to MI in patients with COPD.
Materials and Methods
The study enrolled 325 MI patients treated at the Regional Vascular Center of Emperor Alexander II and Grand Duchess Maria Hospital in Astrakhan (2016–2019). Out of them, 195 COPD+MI patients were assigned to the main cohort, while the control group was 130 non-COPD MI patients. MI was diagnosed according to the criteria of the Fourth Universal Definition of Myocardial Infarction (2018) [12]. Coronary angiography was performed in all patients. The MI patients were managed according to the clinical guidelines [13][14]. The median age was 56.0 [ 52.0; 60.0] years. There were 189 males and 6 females among the COPD+MI patients. One hundred forty-six patients (74.9%) had Q-MI, and 49 (25.1%) were diagnosed with non-Q-MI. In 84 patients (43.1%), the MI was complicated (acute heart failure, cardiac arrhythmia, and conduction disorders).
COPD was diagnosed based on the clinical guidelines outlined in the Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, revised in 2020 [15]. The severity of wheezing in the COPD+MI patients was distributed as follows: grade II in 68 patients (34.9%), grade III in 88 patients (45.1%), and grade IV in 39 patients (20.0%). The smoking experience was 35 [ 30; 40] years with a pack-year index of 18.85 [ 16.0; 22.7].
The median age of the non-COPD MI patients was 56.0 [ 50.0; 62.0] years. There were 89 males and 41 females in this group. One hundred and one patients (77.7%) developed Q-MI, and 29 (22.3%) patients had non-Q-MI. Thirty-two (24.6%) patients experienced complications of acute MI.
This was a prospective cohort study. It was approved by the Local Independent Ethics Committee (Protocol No. 12 of January 18, 2016).
The patients were considered eligible if they had documented type I MI developed within 2 hours of the angina attack. The patients signed informed consent to participate in the study. The patients older than 65 years having any medical problem that could affect the study results (diabetes mellitus, hepatic and renal failure, malignancy) were excluded from the study.
All patients had routine clinical work-ups. In addition, special examinations were performed to determine EIS markers. They included direct mass spectrometry of middle molecules using a Varian Cary 50 Scan UV-Vis spectrophotometer (Australia), immunofluorescence staining for oxidative-stress markers in a Uniplan AIFR-01 enzyme-immunoassay analyzer (Picon), and flow cytofluorometry measuring apoptotic cell counts (Beckman Coulter Navios flow cytometer, USA).
The measurement results were analyzed using the SPSS program, version 26.0. Continuous variables were tested for their normality of distribution in groups and subgroups using the Kolmogorov-Smirnov test. Since the non-parametric distribution of data occurred in all cases, the values were evaluated as the median and interquartile range (Q1-Q3). To calculate statistical significance in three or more study groups, the Kruskal-Wallis test with Bonferroni correction was used, followed by post-hoc pairwise comparisons between the study groups. Statistical hypotheses were tested with a critical level of significance of 0.05. Pearson's χ2 test was used to compare categorical variables between the groups. The patients were clustered using a two-stage cluster analysis. In stepwise discriminant analysis, patient clustering was based on the stepwise selection of the predictor variables.
Results
The initial phase of the study involved the identification of EIS-specific markers and the clustering of the data array. Among the EIS markers, plasma, erythrocyte, and urine middle molecules, hematological toxicity indices (leukocytic index of intoxication, blood leukocyte shift index, neutrophil-to-lymphocyte ratio), saturation, circulating early apoptotic peripheral blood mononuclear cells, markers of lipid and protein peroxidation (malondialdehyde, protein peroxidation products) and antioxidant defense (total superoxide dismutase), inflammation (high-sensitivity C-reactive protein and lactoferrin), and renal function (urea, creatinine, glomerular filtration rate, proteinuria) were measured. Four clusters were formed by the two-stage cluster analysis of the laboratory results. They were designated as multiple marker retention, necrotic inflammatory, hypoxic inflammatory, and non-EIS clusters. The multiple marker retention type of the EIS was characterized by the highest markers specific for endogenous intoxication, apoptosis, inflammation, lipid and protein peroxidation and lower antioxidant protection, highest creatinine, and lower glomerular filtration rate. The obtained results indicated severe EIS with impaired endotoxin elimination. The proportion of this cluster accounted for 16.8%. The necrotic inflammatory and hypoxic inflammatory types of the EIS were associated with a moderate increase in the indices specific for endogenous intoxication. The fundamental difference between these clusters was the marker ratio of lipid and protein peroxidation and antioxidant defense. The necrotic inflammatory type of the EIS was characterized by higher markers of lipid and protein peroxidation and lower markers of antioxidant defense. This is specifically attributed to situations of acute stress, when an adaptive cascade has not been triggered yet. In the hypoxic inflammatory phenotype, markers of lipid and protein peroxidation were lower and markers of antioxidant defense were higher than in the necrotic inflammatory type of the EIS. This pattern is specific for chronic inflammatory processes, when antioxidant defense mechanisms become activated in response to the peroxidation activation. The necrotic inflammatory and hypoxic inflammatory clusters shared 11% and 40.7%, respectively. In the fourth cluster, all values remained within the reference ranges and were similar to the control group, and therefore this type was designated as non-EIS. This cluster accounted for 31.5%.
For phenotyping the MI patients, the authors compared clinical and instrumental findings and medical history between the clusters in the group of COPD and non-COPD patients. The comparison results are presented in the table below.
Table 1
Cluster comparison in the group of patients with MI + COPD (n=195)
|
Indicator |
The first cluster (n=72) |
The second cluster (n=16) |
The third cluster (n=104) |
The fourth cluster (n=3) |
P |
|
Age, full years |
52 [ 48–59] |
52 [ 47–54] |
50 [ 47–54] |
54 [ 51.5–57.5] |
P=0.303 |
|
Male gender, n (%) |
71 (98.6) |
16 (100) |
99 (95.2) |
3 (100) |
P=0.575 |
|
Presence of the Q tooth |
66 (91.7) |
10 (62.5) |
69 (66.3) |
1 (33.3) |
P<0.001 P1–4=0.003 |
|
ST elevation, n (%) |
60 (83.3) |
8 (50) |
59 (56.7) |
1 (33.3) |
P<0.001 P2–3=0.012 P2–4=0.001 |
|
Multiple coronary artery lesions, n (%) |
65 (90.3) |
10 (62.5) |
69 (66.3) |
1 (33.3) |
P<0.001 P1–2=0.006 |
|
Presence of complications |
72 (100) |
8 (50) |
53 (51) |
0 (0) |
P<0.001 |
|
Localization of MI, n (%) i.21.0 Acute transmural anterior wall i.21.1 Acute transmural lower wall i.21.2 Acute transmural of other specified localizations i.21.4 Acute subendocardial |
30 (41.7)
18 (25.0)
5 (6.9)
19 (26.4) |
13 (81.3)
0
0
3 (18.7) |
44 (42.3)
21 (20.2)
13 (12.5)
26 (25.0) |
1 (25.0)
1 (25.0)
0
4 (50.0) |
|
|
Clinical variant of MI, n (%) Typical Abdominal Cerebral Arrhythmic Asthmatic |
55 (76.4) 1 (1.4) 5 (7.0) 5 (7.0) 6 (8.2) |
14 (87.6) 0 1 (6.2) 1 (6.2) 0 |
90 (86.5) 0 0 4 (3.8) 10 (9.6) |
3 (100) 0 0 0 0 |
P=0.16 |
|
Killip, n (%) I II III IV |
43 (59.7) 23 (31.9) 2 (2.8) 4 (5.6) |
8 (50.0) 6 (37.4) 1 (6.3) 1 (6.3) |
74 (71.2) 22 (21.2) 3 (2.9) 5 (4.7) |
3 (100) 0 0 0 |
P=0.515 |
|
Presence of tachycardia, n (%) |
66 (91.7) |
7 (43.8) |
57 (54.8) |
0 |
P<0.001 P1–2<0.001 P1–3<0.001 |
|
Presence of rhythm and conduction abnormalities, n (%) |
32 (44.4) |
5 (31.3) |
27 (26.0) |
0 |
P=0.032 P1–3=0.021 |
|
EF according to Echo-CS, % |
49 [ 41.5–53.5] |
52 [ 48–56] P1=0.029 |
49 [ 45–53] P1=0.906 P2=0.225 |
55 [ 53–57] P1=0.19 P2=1.0 P3=0.445 |
P=0.009
|
|
Duration of COPD, years |
7 [ 5–10] |
4 [ 3–5] P1=0.005 |
5 [ 4–7] P1=0.001 P2=1.0 |
5 [ 4.5–6] P1=1.0 P2=1.0 P3=1.0 |
P<0.001 |
|
Number of cigarettes smoked per day, n |
13 [ 10–15] |
10 [ 10–10] P1=0.004 |
10 [ 10–10] P1<0.001 P2=1.0 |
10 [ 10–10] P1=0.326 P2=1.0 P3=1.0 |
P<0.001 |
|
Smoking history, years |
35 [ 30–40] |
35 [ 33–37] |
35 [ 30–40] |
35 [ 31.5–39] |
P=0.631 |
|
Index pack/year |
22.3 [ 17.5–30.0] |
17.5 [ 16.8–18.5] P1=0.013 |
17.8 [ 15.5–21.6] P1<0.001 P2=1.0 |
17.5 [ 15.8–19.5] P1=0.685 P2=1.0 P3=1.0 |
P<0.001 |
|
Degree of bronchial obstruction, % |
3 [ 2–4] |
3 [ 2–3] |
3 [ 2–3] |
3 [ 3–3] |
P=0.058 |
|
VFE1, % |
44.0 [ 26.5–54.5] |
47.5 [ 43.5–54.0] |
45.0 [ 39.0–55.0] |
45.0 [ 41.0–46.0] |
P=0.09 |
|
CAT test results |
18.5 [ 14–33] |
16 [ 13.5–19] P1=0.266 |
18 [ 13–19] P1=0.036 P2=1.0 |
22 [ 20–22.5] P1=1.0 P2=0.833 P3=1.0 |
P=0.017 |
|
Results of the mMRS test |
2 [ 2–4] |
2 [ 1–2] P1=0.01 |
2 [ 1–2] P1<0.001 P2=1.0 |
2 [ 1.5–2] P1=0.544 P2=1.0 P3=1.0 |
P<0.001 |
|
High frequency of exacerbations, n (%) |
55 (76.4) |
13 (81.3) |
82 (78.8) |
0 (0) |
P=0.966 |
|
COPD Group, n (%) A B C D |
17 (23.6) 8 (11.1) 16 (22.2) 31 (40.8) |
3 (18.8) 7 (43.8) 6 (37.5) 0 (0) |
22 (21.2) 32 (30.8) 42 (40.4) 8 (7.7) |
0 (0) 2 (66.7) 1 (33.3) 0 (0) |
P<0.001 |
|
COPD phenotype, n (%) Emphysematous Bronchitic Mixed |
17 (23.6) 20 (27.8) 35 (48.6) |
4 (25.0) 3 (18.8) 9 (56.3) |
28 (26.9) 35 (33.7) 41 (39.4) |
0 (0) 1 (33.3) 2 (66.7) |
P=0.736 |
Note: P is the level of statistical significance when comparing 4 groups (Kruskal-Wallis test), P1 is the level of statistical significance with cluster 1, P2 is the level of statistical significance of differences with cluster 2, P3 is the level of statistical significance of differences with cluster 3. Differences are statistically significant at P<0.05.
The multiple marker retention phenotype was predominant among middle-aged males. Out of them, 83.3% had ST-elevation MI, and in 91.7%, ECG showed pathological Q-waves. This phenotype was associated with multivessel coronary artery disease (90.3%) and complications in the acute period (100%). Anterior transmural MI was the most common finding. Although MI cases with angina attacks prevailed, it should be noted that all clinical variants could occur in this phenotype. For 91.7% of individuals, tachycardia was reported, and 44.4% had other cardiac arrhythmias and conduction disorders.
The affected patients were found to have the longest history of COPD, the highest number of cigarettes smoked per day and the pack-year index. Among the clinical features, wheezing grade 3, COPD group D, and mixed COPD phenotype were highlighted. The majority of COPD individuals (76.4%) experienced frequent exacerbations.
Phenotype-specific laboratory findings of endogenous intoxication, apoptosis, inflammation, oxidative stress, and hypoxia were the highest possible. There were laboratory signs of renal impairment suggesting endotoxin hyperproduction and impaired elimination.
The necrotic inflammatory phenotype was represented by middle-aged males (100%). Half of them had ST elevation on admission and complications in the acute period of MI (50% each). Q-MI and multivessel disease were slightly more frequent (62.5% each). The transmural MI dominated (81.3%). Among the patients with this phenotype, angina attacks were the most frequent (87.6% of cases). Cardiac arrhythmia, including tachycardia, occurred in less than half of patients (43.8 and 31.3%, respectively).
The duration of COPD in patients with this phenotype was relatively short — from 4 to 7 years with a median of 5 years, the pack-year index was moderate (17.8 [16.8–18.5]), and wheezing grade 2–3 was observed. COPD groups B and C with the mixed phenotype were the most common.
In the necrotic inflammatory phenotype, the clinical pathology showed moderate endogenous intoxication, apoptosis, inflammation, and imbalance of pro- and antioxidants with an increase in pro-oxidants and low levels of antioxidants, which were specific features of the acute process.
The hypoxic inflammatory phenotype was mainly represented by middle-aged male patients (95.2%). Such characteristics as ST elevation on admission, pathological Q-waves on ECG, MI-associated complications, and multivessel disease were not significant, as they affected only half of the cases or slightly more. The above-mentioned signs have demonstrated that this phenotype was similar to the necrotic inflammatory group. It should be noted that along with the clinical symptoms, such as angina attacks (86.5%), asthma associated with a risk for MI (9.6%) was more common with this phenotype than with the others.
The duration of COPD in this phenotype was the least long, the pack-year index was moderate (17.5 [ 15.5–21.6]), and wheezing grade 2‒3 was also observed. The group mainly included the patients with COPD groups B and C, bronchitic and mixed phenotype.
Laboratory findings in this phenotype were as follows: moderate endogenous intoxication as shown by middle molecule levels, mild apoptosis, and imbalance of pro- and antioxidants with a relatively small increase in markers of lipid and protein peroxidation and a moderate decrease in markers of antioxidant defense specific for a chronic inflammatory process with antioxidant defense activation.
The non-EIS phenotype was generally represented by middle-aged males. Two-thirds of cases experienced non-Q and non-ST elevation MI along with single-vessel coronary artery disease, if any. The acute period of MI was uncomplicated. The phenotype was described by the typical lesion locations and clinical signs; no cardiac arrhythmias, including tachycardia, were reported.
The COPD was short in duration (5 [ 4, 5–6] years), with the patients having a moderate smoking history and pack-year index. Among the patients, those with wheezing grade 3 prevailed. FEV1, CAT/mMRS scores were indicative of satisfactory well-being and good quality of life. The clinical course was noteworthy — COPD exacerbations were rare in all patients. Group B patients and mixed COPD phenotype prevailed (66.7%).
There were no laboratory signs of endogenous intoxication, inflammation, hypoxia, and renal impairment. The ratio of markers of lipid and protein peroxidation and markers of antioxidant defense was well-balanced.
In the next phase of the study, the incidence of post-MI outcomes during the 12-month follow-up was analyzed. Within the one-year follow-up, 30 patients were censored (19 from the COPD+MI group, 11 from the non-COPD group). Thus, post-MI outcomes were evaluable in 176 COPD patients and 119 non-COPD patients. As follows from Table 2, 36 fatal outcomes related to cardiovascular system pathology (20.5%) were reported among the COPD patients. The difference with the non-COPD patients with 13 fatal cases (10.9%) was statistically significant (P = 0.038). The risk of a fatal outcome in the COPD patients was 2.1 times higher vs. non-COPD (95% CI 1.06–4.14). When it comes to the MI/stroke recurrence and non-cardiac mortality rates, the subgroups differences were not statistically significant (P = 0.051, P = 0.325, and P = 0.418, respectively), although they were more frequent among comorbid patients.
Table 2
Outcomes of the 12-month follow-up of patients with MI and MI+COPD
|
Outcomes |
MI, n=119 |
MI+COPD, n=176 |
P |
OR; 95% CI |
|
Lethality from cardiac causes, n (%) |
13 (10.9) |
36 (20.5) |
P=0.038 |
2.1; 1.0–-4.15 Cramer 0.126 |
|
Recurrent MI with a nonfatal outcome, n (%) |
7 (5.9) |
23 (13.1) |
P=0.051 |
|
|
Stroke, n (%) |
2 (1.7%) |
8 (4.5%) |
P=0.325 |
|
|
Lethality from noncardiac causes, n (%) |
4 (3.4%) |
11 (6.3%) |
P=0.418 |
|
Due to the etiopathogenetic correlation, the outcomes, such as recurrent MI, stroke, and vascular mortality, could be combined into a composite endpoint (CEP).
The CEP incidence for different phenotypes is illustrated below.
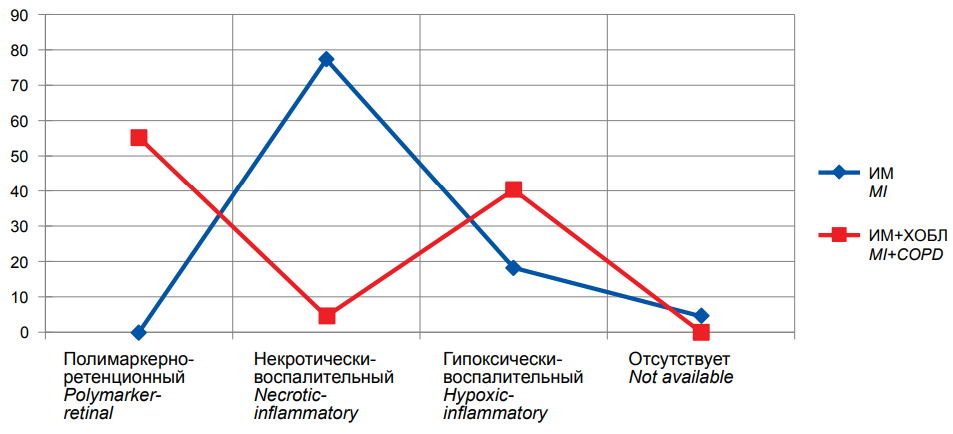
Figure. Frequency of Combined Endpoint (Recurrent MI, Stroke, Vascular Mortality) in COPD patients at 12-month follow-up (n=176).
The figure demonstrates that the COPD+MI group was mainly presented by the multiple marker retention phenotype with the greatest CEP incidence: it was achieved in 37 (55.2%) people. The incidence rates were 3 (4.5%) patients with the necrotic inflammatory phenotype, and 27 (40.3%) patients with the hypoxic inflammatory phenotype; no cases were reported among those with the non-EIS phenotype.
Discussion
The study results suggest the effect of comorbidities on the post-infarction period in MI patients. The 12-month follow-up has demonstrated that the COPD+MI patients had a higher incidence of life-threatening conditions, such as recurrent MI and stroke, and the risk of a fatal outcome was statistically significantly higher than in the non-COPD patients. The obtained data are consistent with the results from Shishkina’s study (2020), where high comorbidity (Charlson Comorbidity Index > 4) was considered a significant predictor of one-year mortality in young and middle-aged MI patients. Other predictors included LVEF ≤ 40% at discharge, Killip II (acute heart failure) or higher, heart rate > 100 bpm on admission, cardiogenic shock, post-infarction cardiosclerosis, neutrophil-to-lymphocyte ratio ≥ 4.52, mitral regurgitation, hemoglobin < 130 g/L [16].
Barabash et al. (2017) have also made an attempt to identify the main risk factors for recurrent MI. These were older age, low physical activity, multifocal atherosclerosis, undereducation, and inadequate social security [17].
According to Mitkovskaya et al. (2015), the probability of an adverse outcome (recurrent MI or death) within one year after a large-focal MI significantly increases with arterial hypertension, elevated serum triglycerides, multivessel coronary artery disease, high left ventricular end-systolic dimension, and depression [18].
In the study by Novikova et al. (2017), arterial hypertension, diabetes mellitus, and low compliance have become significant risk factors for recurrent MI [19].
Although the hypoxic inflammatory phenotype was predominant among the COPD+MI patients, the most frequent life-threatening vascular events (recurrent MI, stroke), as well as fatal outcomes, were reported in the multiple marker retention phenotype. This seems quite logical, since it is the multiple marker retention phenotype that is characterized by the highest biochemical markers of endogenous intoxication, renal impairment, multivessel coronary artery disease, ST elevation and pathological Q-wave on ECG, transmural myocardial damage, and clinical signs of complications in the acute period. The role of renal impairment as a predictor of recurrent MI was also highlighted by Gorbunova [20]. According to the author, other significant predictors included diabetes mellitus and multifocal atherosclerosis [20].
In this study, the patients with the multiple marker retention phenotype had long-term COPD, the highest pack-year index, wheezing grade 3 prevailing, and frequent exacerbations.
Conclusion
Based on the results, it may be concluded that the identification of clinical and biochemical phenotypes is the most substantiated for predicting late life-threatening vascular complications (recurrent MI, stroke) and fatal outcomes in COPD patients during the 12-month follow-up. Recurrent MI, stroke, and cardiac mortality rates have been reported most frequently in the multiple marker retention phenotype. The study results suggest a personalized approach to the one-year prognosis of MI in patients with COPD.
References
1. Oganov R. G., Denisov I. N., Simanenkov V. I., Bakulin I. G., Bakulina N. V., et al. Comorbidities in practice. Clinical guidelines. Cardiovascular Therapy and Prevention. 2017; 16 (6): 5-56. (In Russ.) doi: 10.15829/1728-8800-2017-6-5-56
2. Drapkina O. M., Shutov A. M., Efremova E. V. Comorbidity, multimorbidity, dual diagnosis — synonyms or different terms? Cardiovascular Therapy and Prevention. 2019; 18 (2): 65-69. (In Russ.) doi: 10.15829/1728-8800-2019-2-65-69
3. Kozlova I. V., Ryabova A. Yu., Osadchuk M. A., Dvoretskiy L. I., Shapovalova T. G. Approaches to the treatment of exacerbation of chronic obstructive pulmonary disease with comorbid hypertension. Pulmonologiya. 2021; 31 (4): 439-445. (In Russ.) doi: 10.18093/0869-0189-2021-31-4-439-445
4. Kanorsky S. G., Mamedov M. N. O. Revascularization of coronary and peripheral arteries in diabetes mellitus: a cardiologist's view. International Journal of Heart and Vascular Diseases. 2021; 9 (21): 4-13. (In Russ.) doi: 10.24412/2311-1623-2021-31-4-13
5. Kuzmichev B. Yu., Voronina L. P., Tarasochkina D. S., Polunina O. S., Prokofieva T. V., et al. Hyperhomocysteinemia as a risk factor for a complicated course of myocardial infarction against the background of the chronic obstructive pulmonary disease. Astrakhan medical journal. 2019; 14 (3): 79-87. (In Russ.) URL: https://cyberleninka.ru/article/n/gipergomotsisteinemiya-kak-faktor-riska-oslozhnennogo-techeniya-infarkta-miokarda-na-fone-hronicheskoy-obstruktivnoy-bolezni-legkih
6. Grigoryeva N. Y., Maiorova M. V., Korolyova M. E., Samolyuk M. O. Comorbidity and polymorbidity of the patient with chronic obstructive pulmonary disease and cardiovascular diseases. Terapevticheskii arkhiv. 2019; 91 (1): 43-47. (In Russ.) doi: 10.26442/00403660.2019.01.000027
7. Chaulin A. M., Duplyakov D. V. Comorbidity in chronic obstructive pulmonary disease and cardiovascular disease. Cardiovascular Therapy and Prevention. 2021; 20 (3): 2539. (In Russ.) doi: 10.15829/1728-8800-2021-2539
8. Pashina E. V., Zolotavina M. L. A complex of biochemical indices in the assessment of the formation of stages of endogenous intoxication in a cell. Modern problems of science and education. 2019; 6: 200. (In Russ.). eLIBRARY ID: 42406067
9. Zolotavina M. L., Pashina E. V. Modern methodological problems of assessing endogenous intoxication. Science and World. 2014; 11 (15): 38-41. (In Russ.). eLIBRARY ID: 22544170
10. Alekseeva Y. V., Vyshlov E. V., Pavlyukova E. N., Ussov V. Yu., Markov V. A., Ryabov V. V. Impact of microvascular injury various types on function of left ventricular in patients with primary myocardial infarction with ST segment elevation. Kardiologiia. 2021; 61 (5): 23-31. doi: 10.18087/cardio.2021.5.n1500
11. Mustafina S. V., Vinter D. A., Rymar O. D., Scherbakova L. V., Gafarov V. V., et al. Obesity phenotypes and the risk of myocardial infarction: a prospective cohort study. Russian Journal of Cardiology. 2019;(6): 109-114. (In Russ.) doi: 10.15829/1560-4071-2019-6-109-114
12. Thygesen K., Alpert J. S., Jaffe A. S., Chaitman B. R., Bax J. J., et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018; 138 (20): e618-e651. Erratum in: Circulation. 2018; 138 (20): e652. PMID: 30571511. doi: 10.1161/CIR.0000000000000617.
13. Acute myocardial infarction with ST-segment elevation electrocardiogram. Clinical guidelines. Ministry of Health of the Russian Federation, Society of Emergency Cardiology Specialists. 2016. (In Russ.).
14. Diagnosis and treatment of patients with acute coronary syndrome without ST-segment elevation electrocardiogram. Clinical guidelines. Ministry of Health of the Russian Federation, Society of Emergency Cardiology Specialists. 2015. (In Russ.).
15. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020.
16. Shishkina E. A., Khlynova O. V., Cheremnykh A. B. Prediction of posthospital mortality in young and middle-aged myocardial infarction patients. doctor.ru. 2020; 19 (5): 24-29. (In Russ.). doi: 10.31550/1727-2378-2020-19-5-24-29
17. Barbarash O. L., Sedykh D. Y., Gorbunova E. V. The main factors determining the risk of recurrent myocardial infarction. Heart: a journal for practicing physicians. 2017; 16 (1): 10-50. (In Russ.). URL: https://scardio.ru/ratings/uploads/2122.pdf?688681396
18. Mitkovskaya N. P., Pinchuk A. F., Pavlovich T. P., Statkevich T. V., Balysh E. M. Prediction of adverse outcomes in patients with postinfarction cardiosclerosis. Cardiology in Belarus. 2015; 5 (42): 44-50. (In Russ.). eLIBRARY ID: 25134637
19. Novikova R. A., Alekseichik S. E., Goncharik T. A., Alekseichik D. S., Sankovich E. V. Recurrent myocardial infarction, causes of its development, diagnostic difficulties and prevention. Emergency Medicine. 2017; 6 (2): 229-234. (In Russ.). eLIBRARY ID: 29120607
20. Gorbunova N., Sedikh D., Brukhanova I., Krestova O., Vedernikova A. Recurrent myocardial infarction: risk factors and prevention. Physician. 2017; 9: 84-86. (In Russ.). eLIBRARY ID: 30040056
About the Authors
T. V. ProkofyevaRussian Federation
Tatyana V. Prokofyeva, M. D., associate professor
Department of Internal Medicine
Department of Pediatrics
Astrakhan
O. A. Bashkina
Russian Federation
Olga Alexandrovna Bashkina, M. D., Ph. D., Head of Department
Department of Pediatrics
Astrakhan
O. S. Polunina
Russian Federation
Olga Sergeyevna Polunina, M.D., Doctor of medical sciences, Professor, Head of the Department
Pediatrics Faculty
Department of Internal Medicine
Astrakhan
I. V. Sevostyanova
Russian Federation
Irina V. Sevostyanova, M. D., Associate professor
Department of Internal Medicine
Astrakhan
E. L. Gritsenko
Russian Federation
Elena L. Gritsenko, anesthesiologist-resuscitator
Department of Cardiology № 2
Astrakhan
Review
For citations:
Prokofyeva T.V., Bashkina O.A., Polunina O.S., Sevostyanova I.V., Gritsenko E.L. Clinical and biochemical phenotypes in cardiorespiratory comorbidity. Medical Herald of the South of Russia. 2023;14(2):67-75. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-67-75







































