Scroll to:
The role of chronic endometritis in the aspect of habitual miscarriage in patients with adenomyosis
https://doi.org/10.21886/2219-8075-2023-14-2-36-43
Abstract
Objective: to identify the presence of chronic endometritis in patients with adenomyosis and its cumulative role in the formation of reproductive losses.
Materials and methods: 101 women of reproductive age with grade II adenomyosis and habitual miscarriage were examined. The comparison group (group 2) consisted of 49 patients with grade II adenomyosis without a history of early reproductive losses. The control group (group 3) consisted of 50 healthy patients. In order to diagnose chronic endometritis, a comprehensive immunohistochemical study of the expression of specific immunological markers of chronic endometritis (natural killer CD56, B cells (CD20), plasma cells (CD138) and activated lymphocytes (HLA-DR) with antibodies and the determination of CD68 macrophages was carried out. Polymerase chain reaction in the “real-time” mode (PCR) was used to determine the types and amount of endometrial microbiota. Statistical data processing was carried out in the environment of the STATISTICA package.
Results: despite the absence of pathogenic and conditionally pathogenic microflora, according to the results of microbiological examination from the vagina and cervical canal of all examined patients, statistically significant differences in the detection frequencies of various microbiota taxa of crops from the uterine cavity were obtained. Patients with reproductive losses on the background of adenomyosis had microbiological and immunohistochemical signs of chronic endometritis of moderate and mild severity. The presence of chronic endometritis combined with adenomyosis contributes to the occurrence of pronounced endothelial dysfunctions, eventually leading to a violation of reproductive function in patients with a combination of HE and adenomyosis.
Conclusion: The similar symptoms of adenomyosis and CE, a reliable association of adenomyosis with CE in patients with habitual miscarriage requires the inclusion of immunohistochemical and microbiological examination of the endometrium in the volume of pre-gravidar examination, even if there are negative results of microbiological examination of the environment of the cervical canal.
For citations:
Kravtsova E.I., Lukoshkina I.N., Miroshnichenko L.B., Nicogda Y.V., Kravtsov I.I. The role of chronic endometritis in the aspect of habitual miscarriage in patients with adenomyosis. Medical Herald of the South of Russia. 2023;14(2):36-43. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-36-43
Introduction
In recent years, a number of domestic, national, and international investigations have been carried out proving the effect of adenomyosis on the course of spontaneous pregnancy or pregnancy resulting from the in vitro fertilization methods. In patients with adenomyosis, compared to the general population, there were noted significantly higher rates of preterm birth (41.7% versus 12.5%), hypertension disorders (25.6% versus 4.1%), premature discharge of amniotic fluid (19.4% versus 4.2%), low weight for gestational age (33.3% versus 5.4%), fetus malpresentation (27.8% versus 8.3%), cesarean section (58.3% versus 24.3%), and postpartum complications [1-5]. Tamura et al. [6], who conducted a multicenter retrospective investigation of pregnant women with adenomyosis in Japan, showed that adenomyosis in a patient was associated with habitual miscarriage, recurrent pregnancy loss, isthmic-cervical insufficiency, preeclampsia, and postpartum metritis. Nevertheless, according to a number of studies, some patients with adenomyosis with similar degrees of prevalence may have a physiological pregnancy and childbirth [7]. Known pathogenetic aspects of adenomyosis can partially explain the formation of gestation complications. In adenomyosis, there is excessive but abnormal vascularization of the endometrium, a pro-inflammatory shift in the cytokine cascade in the endometrium with activation of the local and systemic inflammatory response, increased production of prostaglandins, relative hyperestrogenism, molecular pathological changes in the endometrium, hypertrophy and hyperplasia of muscle fibers, which develops after the introduction of endometrial glands and stroma inside the myometrium [8]. All these changes at the early stages affect the interaction of the blastocyst with the endometrium and contribute to the development of chorion anomalies and disturbances in the modeling of spiral arteries with the formation of placental dysfunction. In the future, a disorder of the contractility of the uterus exacerbates the incidence of preterm labor and anomalies in labor. However, not in all patients with adenomyosis, the potential impact of the above pathological processes leads to early termination of pregnancy and causes reproductive losses.
Adenomyosis and chronic endometritis have a number of common pathogenetic mechanisms of development and clinical manifestations. Among various proposed factors that contribute to a poor reproductive outcome in women with adenomyosis, persistent chronic endometritis may be an additional factor impairing the homeostasis and receptive environment of the endometrial bed. In this regard, noteworthy are the data claiming that chronic inflammation within endometriosis is the main cause of infertility and menstrual irregularities [9-12]. Based on these studies, which are devoted to the involvement of intrauterine microbial colonization in the mechanism of pregnancy rejection, the authors of the present paper undertook an investigation to identify the association of chronic endometritis with adenomyosis and their combined role in the formation of reproductive losses.
The purpose of the study was to identify the presence of chronic endometritis and its cumulative role in the formation of reproductive losses in patients with adenomyosis.
Materials and methods
In the course of a prospective cohort study, according to the main purpose, 101 women of reproductive age with habitual miscarriage associated with adenomyosis of the 2nd degree (group I) were examined at the Department of Obstetrics, Gynecology and Perinatology at the Clinic of the Federal State Budgetary Educational Institution of Higher Education "Kuban State Medical University" of the Ministry of Health of the Russian Federation and State Budgetary Healthcare Institution "Maternity Hospital", Krasnodar. The comparison group (group II) consisted of 49 patients with degree II adenomyosis without a history of early reproductive losses. The control group (group III) consisted of 50 healthy patients examined as part of pregravid preparation. Inclusion criteria were reproductive age (18–38 years), the presence of habitual miscarriage, adenomyosis confirmed by ultrasound and magnetic resonance imaging according to modern FIGO criteria (2018), as well as the results of a hysteroscopic examination.
Exclusion criteria were age less than 18 and over 45 years, other possible causes of recurrent pregnancy loss, somatic pathology critically complicating the course of pregnancy, history of acute and chronic metritis and salpingo-oophoritis, detection of sexually transmitted infections and pathogenic microflora during a microbiological examination of the contents of the vagina and cervical channel.
The patients included in the investigation signed voluntary informed consent in accordance with the Helsinki Declaration of the World Medical Association.
The patients were examined according to the generally accepted scheme including life history data, obstetric and gynecological history, complaints, and a general clinical and gynecological examination. To determine the types and amount of endometrial microbiota, real-time polymerase chain reaction (RT-PCR) was carried out, using the Femoflor-16 reagent kit; in addition, DNA of herpes simplex virus type 1 and 2 and cytomegalovirus was determined by RT-PCR methods using RPA (NPO in Russian) DNA technology kits (Russia). To exclude the possibility of contamination of samples from the uterine cavity with the microflora of the cervical canal, the contents of the uterine cavity were obtained with a double-lumen catheter guide using an intrauterine cytobrush (Uterobrush, Sweden). In order to diagnose chronic endometritis, a comprehensive immunohistochemical examination was carried out to determine the indicators, including the expression of specific immunological markers of chronic endometritis (natural killers CD56, B-cells (CD20), plasma cells (CD138) and activated lymphocytes (HLA-DR) with antibodies to CD56 (123C3, Roche-Ventana), CD138 ((B-A38) CD-138/syndecan-1, Roche-Ventana), CD20 ((L26, Roche-Ventana), HLA-DR (CR3/43 BioSystems), detection of CD68 macrophages using the Ventana BenchMark ULTRA immunohistostainer, monoclonal antibodies from Novocastra Lab. Ltd.). A sample of the endometrium was obtained by endometrial pipel biopsy with a "Paypel" type vacuum syringe; the investigation was carried out in the expected middle secretory phase of the menstrual cycle.
Statistical data processing was performed with the software package STATISTICA. The comparison of average values of indicators in groups of patients was carried out by the parametric t-test of Student and the non-parametric criteria of Krasker-Wallis, Kolmogorov-Smirnov, Mann-Whitney, and Wilcoxon. Numerical characteristics of indicators were calculated, including the number of women in groups (N), mean (M), and standard error of the mean (m). A comparison of nominal data was carried out using Pearson's χ2 test. To analyze the correlations between the indicators, the Spearman correlation coefficient was calculated. The statistical significance of the results in the analysis was assessed at p<0.001.
Results
The age diapason of the patients ranged from 25 to 38 years (32.8±3.9 years, with M0=30 years). All the groups were similar in age. The mean age of patients in group I was 32.5±4.1 years with M0=32 years, in group II — 32.8±1.9 years with M0=32 years (p=0.14; χ2=8.641). Height and weight indicators in the cohort of the examined patients were marked in the range from 48 to 86.6 kg, height from 154 to 178 cm, the body mass index from 19.2 to 34.5 kg/m2. No significant differences, according to these indicators, were found in the groups (p=0.01; χ2=9.654).
Among the somatic diseases in patients with adenomyosis, iron deficiency anemia (IDA) prevailed (52.6%), and 12.6% of cases had anemia of the 2nd degree. The number of identified patients with IDA in the control group was significantly less (10%), p=0.04.
The most common symptom of adenomyosis was heavy menstrual bleeding (HMB) with spotting before and after menstruation. This symptomatology was noted in 94.2% of patients in group I and in 93% of patients in group II; in the control group, HMB was not observed (χ2=26.2, p<0.001, χ2=24.2, p<0.001).
Dysmenorrhea from the menarche moment was noted in 86.5% of patients in group I, predominantly of moderate severity (47.3%). In group II, dysmenorrhea was detected in 79.6%, mostly mild (65.3%). In the control group, the incidence of dysmenorrhea accounted for 16% (χ2=7.86, p<0.05) and was assessed by patients as mild.
As already noted, patients with a history of acute and chronic metroendometritis and salpingo-oophoritis were deliberately excluded from the study. Of the gynecological infectious diseases in patients with a history of adenomyosis, recurrent vaginitis (26.3%) and sexually transmitted infections including chlamydia, Mycoplasma genitalium, trichomonas, and ureaplasma were registered in 28.4%. In the control group, these diseases were encountered significantly less (χ2 =26.3, p<0.001).
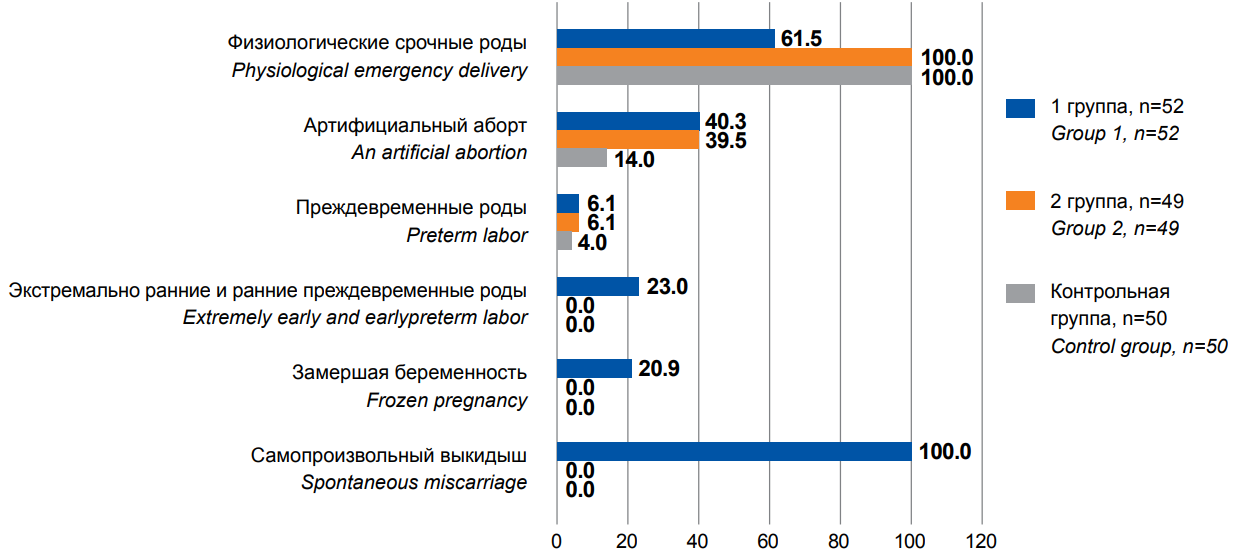
Figure 1. Obstetric anamnesis in the examined patients, %.
In group I, the main pathology of the reproductive process in all patients was habitual miscarriage and/or pregnancy loss in the form of early and extremely early preterm birth with an unfavorable outcome for the fetus. Nevertheless, in some patients of group I (61.5%), the first birth completed safely. In group II, reproductive losses were not registered in history. The rate of late preterm birth in groups I and II did not differ significantly, amounting to 6.1% (Figure 1).
In group I, there was a significantly greater number of postpartum complications (80.8% (χ2=24.0, p<0.001)), accompanied by curettage of the uterine cavity and artificial abortions after the first birth 40.3% (χ2=16.0, p<0.001) 0.001). The first pregnancy was interrupted by 10 (19.2%) women (χ2=42.1, p<0.001). Patients in the control group (group III) did not have a history of reproductive losses, and artificial abortions after the first birth amounted to 14%, which significantly differed from the results of both groups I and II (χ2=12.2, and χ2=10.8, p<0.001, respectively). According to the literature, a high rate of intrauterine interventions and medical termination of pregnancy can be attributed to factors for adenomyosis development [3].
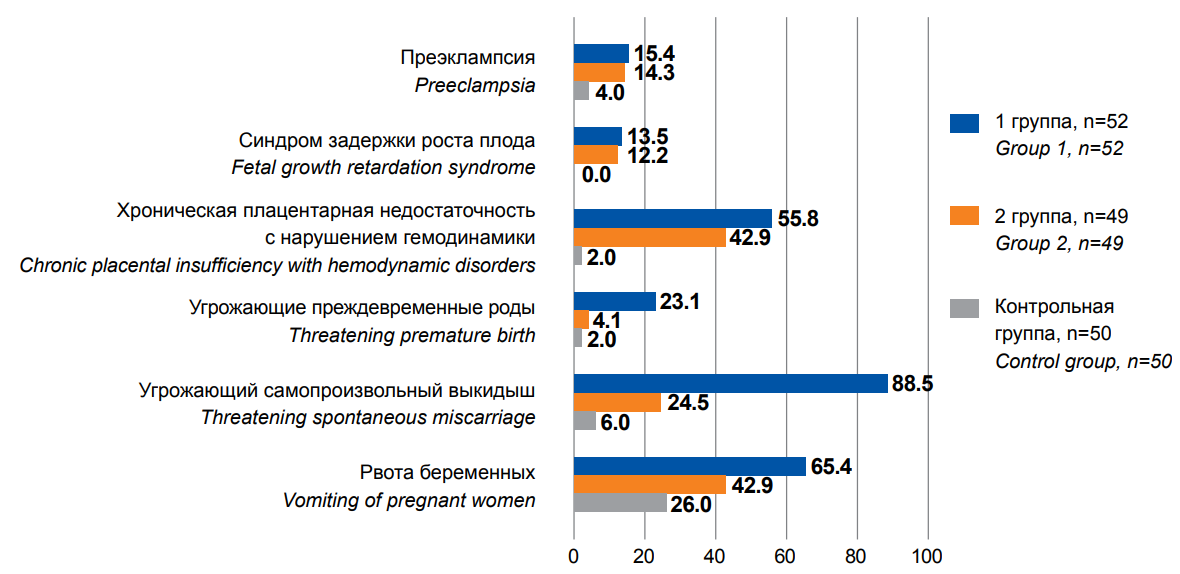
Figure 2. Pregnancy complications in the anamnesis in the examined patients, %
Despite the fact of successfully completed pregnancies in the anamnesis, in patients with adenomyosis in groups I and II, some or other complications in gestation were revealed significantly more often. Mild vomiting as a condition determining the mother's maladjustment to a progressive pregnancy [13] was observed in more than half (65.4%) of patients in group I and in 42.9% of patients in group II. Moderate vomiting and severe vomiting were revealed in 5 (8.2%) patients of group I (Figure 2). In the control group, the rate of mild vomiting was significantly lower and amounted to 26% (χ2=16.6, p<0.001). Threatening miscarriage aggravated the pregnancy course in 88.5% of pregnant women in group I and 24.5% of pregnant women in group II. Threatening preterm birth in group I was marked significantly more often, amounting to 23.1% (χ2=26.3, p<0.001). On the contrary, chronic placental insufficiency with hemodynamic disturbances was observed in 55.8% of patients in group I and in 42.9% of patients in group II, which was significantly comparable (χ2=1.68, p=0.195). Placental insufficiency led to intrauterine growth retardation in 13.5% of patients in group I and in 12.2% of patients in group II (χ2=1.23, p=0.165). Attention is drawn to the fact that in both clinical groups, an increased incidence of moderate preeclampsia (15.4 and 14.3%, respectively, groups I and II) was revealed without intergroup differences (χ2=0.06, p=0.79); its rate exceeded the general population values (2–8%) and significantly differed (χ2=28.6, p<0.001) from the control group (4%) (Figure 2).
Microbiological examination revealed microflora in the endometrium in 96% of patients. In this respect, 87.8% of patients of group II and 90% of patients of the control group had predominantly normal microflora, represented by Lactobacillus spp. (χ2=0.03, p=0.85). In group I, the rate of detection of Lactobacillus spp. was significantly lower and amounted to 38.5% (χ2=25.6, p<0.001). The total bacterial mass (TBM) in patients of the control group averaged 107.9 GE/ml (genomic equivalents per milliliter of biomaterial) with a relative amount of lactobacilli of 107.3 GE/ml, which corresponded to -0.1(log) of the TBM. In patients of group II, TBM averaged 107.2 GE/ml with a relative amount of lactobacilli of 106.9 GE/ml, which corresponded to -0.1(log) of TBM. In patients of group I, the TBM averaged 106.7 GE/v, the absolute number of lactobacilli was 103.3 GE/ml, and the relative number of lactobacilli averaged -2.3 (Log) of the TBM.
The detection rate of other microorganisms in group II and the control group was minimal, amounting to no more than 4–8%. Gardnerella vaginalis, Mobiluncus spp., Corynebacterium spp., and Eubacterium spp. dominated among the obligate anaerobic organisms. These microorganisms were detected in patients in an amount not exceeding 104 CFU/ml (colony-forming units). In patients of group II and the control group, no Atopobium vaginae, mycoplasmas, and viruses were detected.
Among patients of group II (4.1%), facultative anaerobic microorganisms were revealed in an amount not exceeding 104 CFU/ml and were represented by Enterobacteriaceae spp. (12.2% of patients), Staphylococcus spp. (4.1% of patients), and Candida spp. (4.1% of patients). Pathogenic microorganisms and examined viruses were not detected in patients of group III. In general, according to the state of microbial insemination, patients of group II and the control group did not have significant differences (χ2=0.04, p=0.95).
In patients of group I, the detection rate of opportunistic and pathogenic microorganisms was significantly higher than in patients without reproductive problems (group II) and the control group, p<0.001. In most cases, facultative anaerobic microflora prevailed in an amount exceeding 104 CFU/ml and was represented by Enterobacteriaceae spp. (38.5%), Streptococcus spp. (15.4%), and Staphylococcus spp. (36.5%) (Table 1).
Table 1
Detection rate of microorganisms in the endometrium among the examined groups, %
|
group I, n=52 |
group II, n=49 |
group III (control), n=50 |
||||
|
absolute values |
% |
absolute values |
% |
absolute values |
% |
|
|
Lactobacillus spp. |
20 |
38.5 |
43 |
87.8 |
45 |
90.0 |
|
Enterobacteriaceae spp. |
24 |
46.2 |
6 |
12.2 |
5 |
10.0 |
|
Eubacterium spp. |
23 |
44.2 |
3 |
6.1 |
2 |
4.0 |
|
Staphylococcus spp. |
19 |
36.5 |
2 |
4.1 |
4 |
8.0 |
|
Streptococcus spp. |
8 |
15.4 |
0 |
0.0 |
0 |
0.0 |
|
Gardnerella vaginalis + Prevotella bivia + Porphyromonas spp. |
11 |
21.2 |
4 |
3.0 |
2 |
4.0 |
|
Sneathia spp. + Leptotrichia spp. + Fusobacterium spp. |
3 |
5.8 |
3 |
6.1 |
0 |
0.0 |
|
Megasphaera spp. + Veillonella spp. + Dialister spp. |
2 |
3.8 |
1 |
2.0 |
0 |
0.0 |
|
Lachnobacterium spp. + Clostridium spp. |
9 |
17.3 |
0 |
0.0 |
0 |
0.0 |
|
Mobiluncus spp. + Corynebacterium spp. |
9 |
17.3 |
0 |
0.0 |
0 |
0.0 |
|
Peptostreptococcus spp. |
3 |
5.8 |
0 |
0.0 |
0 |
0.0 |
|
Atopobium vaginaе |
14 |
26.9 |
0 |
0.0 |
0 |
0.0 |
|
Candida spp. |
9 |
17.3 |
2 |
4.1 |
2 |
4.0 |
|
Mycoplasma hominis |
8 |
15.4 |
0 |
0.0 |
0 |
0.0 |
|
Ureaplasma (urealyticum + parvum) |
7 |
13.5 |
0 |
0.0 |
0 |
0.0 |
|
Mycoplasma genitalium |
3 |
5.8 |
0 |
0.0 |
0 |
0.0 |
|
Chlamydia trachomatis |
3 |
5.8 |
0 |
0.0 |
0 |
0.0 |
|
Вирус простого герпеса 1/2 тип Herpes simplex virus type 1/2 |
10 |
19.2 |
0 |
0.0 |
0 |
0.0 |
|
Цитомегаловирус Cytomegalovirus |
5 |
9.6 |
0 |
0.0 |
0 |
0.0 |
Obligate anaerobic microflora in 15.3% of patients in an amount exceeding 104 CFU/ml was represented by Gardnerella vaginalis (group I, 21.2% of patients), Mobiluncus spp./Corynebacterium spp. (17.3% of patients), and Eubacterium spp. (44.2% of patients).
Atopobium vaginae was detected in 26.9% of patients in group I. Candida spp., Mycoplasma hominis, and Ureaplasma (urealyticum + parvum) were found on average in 14.5% of patients. In 3 (5.8%) patients of group I, Chlamydia trachomatis was revealed in the endometrium, while Herpes simplex virus 1/2 type was detected in 10 (19.2%) patients, and Cytomegalovirus was found in 5 (9.6%) patients.
The data of the present investigation confirm the fact of the non-sterility of the uterine cavity with predominant contamination of the endometrium with lactobacilli in healthy patients (group III) and patients with adenomyosis in the absence of reproductive losses (group II). The endometrial microbiota in patients with adenomyosis and reproductive problems (group I) was characterized by a significant increase in the detection rate of obligate and facultative anaerobic opportunistic and pathogenic organisms, including Chlamydia trachomatis, Herpes simplex virus, and Cytomegalovirus.
The results of morphological investigations showed that patients of group I had an endometrium of an inferior stage of secretion with a reduced number of pinopodia. When comparing with the immunohistochemistry data, which revealed that the expression of PgR and ER receptors in the glands and stroma was increased relative to medium secretion, the morphological picture corresponds to the early secretion phase, while leukemia inhibitory factor (LIF) expression is reduced by 40%. In group II, desynchronization of the secretory phase was observed in half of the patients, but the expression of LIF was within normal reference values. Almost half of the patients of group I (44.2%) and only 5 (10.2%) patients of group II had a stroma of uneven density with intermittent incoherent edematous areas and more compact cytogenic areas; there were foci of fibrosis, microhemorrhages, while spiral arteries, which walls were thickened in some places, reached the surface. This generally indicates a focal developmental, delay as well as reduced endometrial receptivity, probably, of combined genesis stipulated by hormonal and inflammatory pathogenetic constituents (Table 2).
Table 2
Results of immunohistochemical examination of endometrial biopsies
|
Immunohistochemical study data |
group I, n=52 |
group II, n=49 |
||
|
absolute values |
% |
absolute values |
% |
|
|
Desynchronization of the menstrual cycle phase |
52 |
100.0 |
24 |
49.0 |
|
Synchronized phase of the menstrual cycle |
0 |
0.0 |
25 |
51.0 |
|
Chronic endometritis with an autoimmune component |
34 |
65.4 |
8 |
16.3 |
|
Chronic endometritis without an autoimmune component |
18 |
34.6 |
1 |
2.0 |
The obtained data of the histological examination are confirmed by immunohistochemical parameters in all patients of group I, attesting to chronic endometritis of moderate severity. In 65.4% of patients of group I, an autoimmune component of the inflammatory process was detected, while in 34.6% of patients, an autoimmune component was not observed. Analysis of the results of immunohistochemical investigation of the endometrium showed a significant increase in the number of CD68+ macrophages in patients of group I, 5.9±1.4 versus 2.1±0.8 in group II (χ2=12.3, p<0.001), with a maximum in patients with viral-bacterial infection. In group II, mild chronic endometritis (CE) was verified in 9 patients (18.3%), while the majority of them (16.3%) had an autoimmune component (χ2=27.5, p<0.001) (Table 3).
Table 3
Immunohistochemical criteria of chronic endometritis identified in clinical groups
|
Groups |
|
|
|
|
|
|
CD56 |
CD138 |
CD20 |
HLA-DR |
CD 68 (+) |
|
|
group I, n=52 |
23.3±12.5 |
2.5±1.8 |
4.4±1.2 |
3.1±1.1 |
5.9±1.4 |
|
group II, n=49 |
12.8±5.2* |
2.06±1.2 |
3.5±1.3* |
1.9±0.9* |
2.1±0.8* |
|
Significance level of differences Χ2 |
15.4 |
9.8 |
9.6 |
10.2 |
12.3 |
|
p |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
Discussion
A pregravid examination of 101 patients with adenomyosis of degree II revealed a number of factors associated with the problem of habitual miscarriage and early reproductive losses. Despite the absence of pathogenic microflora according to the results of the microbiological examination of the contents of the vagina and cervical canal, all patients with reproductive losses, associated with adenomyosis, had immunohistochemical signs of chronic endometritis of moderate and mild severity accompanied by detecting facultative anaerobic, obligate anaerobic and viral invasion in the endometrium.
The predisposing factor in the penetration of microbial agents into the epithelial and stromal lining of the uterine mucosa in these patients was apparently an increased rate of intrauterine interventions. Patients with adenomyosis without a history of reproductive losses were mostly negative for morphological and immunohistochemical signs of chronic endometritis or had immunohistochemical signs of autoimmune chronic endometritis of mild severity in the absence of pathogenic and conditionally pathogenic microflora in the endometrium. Meanwhile, the pregnancy course in these patients, although not accompanied by fatal losses, was distinguished by a significant increase in the rate of gestational complications, complications of labor activity and the postpartum period. Obviously, the well-known activation of the macrophage link at adenomyosis [14], leading to the formation of primary and secondary placental dysfunction, is enhanced by interaction with bacterial endotoxins, cell wall proteins, and viruses followed by the development of a hyperexcessive pro-inflammatory cytokine response. Thus, chronic endometritis combined with adenomyosis contributes to the emergence of pronounced endothelial dysfunctions, eventually leading to impaired reproductive function in these patients (that is, with a combination of chronic endometritis and adenomyosis).
Conclusion
Pregravid preparation of patients with adenomyosis demands an individual approach depending on the obstetric and gynecological history of the patients. The presence of similar symptoms of adenomyosis and chronic endometritis and a significant association of adenomyosis with chronic endometritis in patients with habitual pregnancy losses demands the inclusion of immunohistochemical and microbiological investigations of the endometrium in the pregravid examination.
References
1. Gabidullina R. I., Kuptsova A. I., Koshelnikova E. A., Nuhbala F. R., Bagirli R. R., et al. Adenomyosis: clinical aspects, impact on fertility and pregnancy outcome. Gynecology. 2020; 22 (4): 55-61. doi: 10.26442/20795696.2020.4.200264
2. Kravtsova E. I., Kutsenko I. I., Avakimyan A. A. Features of the course of pregnancy, childbirth and postpartum period in patients with adenomyosis. Medical Herald of the South of Russia. 2020; 11 (1): 41-45. (In Russ.) doi: 10.21886/2219-8075-2020-11-1-41-45
3. Mochimaru A., Aoki S., Oba MS, Kurasawa K., Takahashi T., Hirahara F. Adverse pregnancy outcomes associated with adenomyosis with uterine enlargement. J Obstet Gynaecol Res. 2015; 41 (4): 529-33. doi: 10.1111/jog.12604
4. Liu X. Y., Zhang Y., Wei Y., Li R., Zhao Y. Y. [Perinatal outcome of pregnant women with adenomyosis]. Zhonghua Fu Chan Ke Za Zhi. 2020; 55 (11): 743-748. (In Chinese). doi: 10.3760/cma.j.cn112141-20200810-00630
5. Shinohara S., Okuda Y., Hirata S., Suzuki K. Adenomyosis as a Potential Risk Factor for Adverse Pregnancy Outcomes: A Multicenter Case-Control Study. Tohoku J Exp Med. 2020; 251 (3): 231-239. doi: 10.1620/tjem.251.231
6. Tamura H., Kishi H., Kitade M., Asai-Sato M., Tanaka A., et al. Complications and outcomes of pregnant women with adenomyosis in Japan. Reprod Med Biol. 2017; 16 (4): 330-336. doi: 10.1002/rmb2.12050
7. Rafi J., Pathiraja P. D. M., Gelson E., Brown R., Alleemudder D. Obstetric and perinatal outcomes in women with endometriosis. Obstet Gynecol. 2022; 24 (4): 242-250. doi: 10.1111/TOG.12831
8. Tapilskaya N. I., Gaydukov S. N., Shanina T. B. Adenomyosis as a separate phenotype of endometrial dysfunction. Effective pharmacotherapy. 2015; (5): 62-68. (In Russ.) eLIBRARY ID: 23109304
9. Unanyan A. L., Sidorova I. S., Kogan E. A., Belogubova S. Yu., Demura T. A., et al. Endometriosis, adenomyosis, chronic endometritis: clinical and pathogenetic relationships and reproductive failures. Obstetrics and gynecology. 2018;(10): 136-40. (In Russ.) doi: 10.18565/AIG.2018.10.136-140
10. Takebayashi A., Kimura F., Kishi Y., Ishida M., Takahashi A., et al. The association between endometriosis and chronic endometritis. PLoS One. 2014; 9 (2): e88354. doi: 10.1371/journal.pone.0088354
11. Orazov M. R., Radzinsky V. E., Volkova S. V., Khamoshina M. B., Mikhaleva L. M., et al. Chronic endometritis in women with endometriosis-associated infertility. Gynecology. 2020; 22 (3): 15–20. (In Russ.) doi: 10.26442/20795696.2020.3.200174
12. Khan K. N., Fujishita A., Ogawa K., Koshiba A., Mori T., et al. Occurrence of chronic endometritis in different types of human adenomyosis. Reprod Med Biol. 2021; 21 (1): e12421. doi: 10.1002/rmb2.12421
13. Lipatov I. S., Tuzikov Yu. V., Kutuzova O. A., Prikhod'ko A. V., Frolova N. A., Ryabova S. A. Clinical and pathogenetic variants of maladaptation to pregnancy at early stages of gestation. Obstetrics, Gynecology and Reproduction. 2017; 11 (1): 5-13. (In Russ.) doi: 10.17749/2313-7347.2017.11.1.005-013
14. Serdyukov S. V., Bochkareva D. S., Ogorodnik A. S. Immunological aspects of the occurrence and progression of endometriosis. Bulletin of the Volgograd State Medical University. 2019; 4 (72): 15-20. (In Russ.) doi: 10.19163/1994-9480-2019-4(72)-15-20
About the Authors
E. I. KravtsovaRussian Federation
Elena I. Kravtsova, Cand. Sci. (Med.), Associate Professor
Department of Obstetrics, Gynecology and Perinatology
Krasnodar
I. N. Lukoshkina
Russian Federation
Irina N. Lukoshkina, Cand. Sci. (Med.), associate Professor
Department of obstetrics, gynecology and Perinatology
Krasnodar
L. B. Miroshnichenko
Russian Federation
Lyudmila B. Miroshnichenko, a gynecologist
Krasnodar
Y. V. Nicogda
Russian Federation
Yulia V. Nicogda, Cand. Sci. (Med.), Assistant
Department of Obstetrics, Gynecology and Perinatology
Krasnodar
I. I. Kravtsov
Russian Federation
Igor I. Kravtsov, a gynecologist
Krasnodar
Review
For citations:
Kravtsova E.I., Lukoshkina I.N., Miroshnichenko L.B., Nicogda Y.V., Kravtsov I.I. The role of chronic endometritis in the aspect of habitual miscarriage in patients with adenomyosis. Medical Herald of the South of Russia. 2023;14(2):36-43. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-36-43







































