Scroll to:
Balneotherapy of epigastric pain syndrome: the meaning of motilin in the implementation of the therapeutic effect
https://doi.org/10.21886/2219-8075-2023-14-1-24-30
Abstract
Objective: to study the mechanism of action of low-mineralized sulfate-chloride-sodium mineral water on the clinical picture and motor function of the stomach in patients with epigastric pain syndrome using the regulatory peptide motilin as an example.
Materials and methods: 113 people of both sexes were examined, the average age was 22.3±0.21 years. The observation group (57 people) included individuals with functional dyspepsia in the variant of epigastric pain syndrome, the comparison group included practically healthy individuals (56 people). The questionnaire method was used according to the Gastrointestinal Symptom Rating Scale questionnaire; ELISA method for determining the concentration of motilin in the blood. Mineral water in a volume of 200 ml was taken once by both groups of patients, patients with EPS additionally received a course.
Results: in persons with epigastric pain syndrome, abdominal pain prevails. The course intake of mineral water provides a positive clinical trend, confirmed by a decrease in the syndromes of lesions of the upper gastrointestinal tract. A single and course intake of mineral water provides a stable increase in the level of motilin in functional dyspepsia.
Conclusion: patients suffering from functional dyspepsia in the variant of epigastric pain syndrome have persistent disorders of motilin secretion compared with healthy individuals. Stimulation of the stomach on a drinking test model showed inadequacy of changes in the level of this hormone. Both a single and a course intake of low-mineralized sulfate-chloride-sodium mineral water contributes to an increase in the concentration of motilin, which should be considered as a physical adaptogenic factor leading to a stable ordering of the body’s functional relationships.
For citations:
Shklyaev A.E., Kazarin D.D., Grigoreva O.A. Balneotherapy of epigastric pain syndrome: the meaning of motilin in the implementation of the therapeutic effect. Medical Herald of the South of Russia. 2023;14(1):24-30. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-1-24-30
Introduction
According to the Rome IV Criteria, functional dyspepsia (FD) may be defined as a set of symptoms, including pain and burning in the epigastric region (from 60 to 70%), a feeling of bloating after eating (80%), early satiety (from 60 to 70%), a feeling of overflow in the epigastric region (80%), nausea (60%), and vomiting (40%) during three or more (at least 6) months. At the same time, there is not any convincing evidence of the presence of organic pathology of the upper gastrointestinal tract, according to endoscopic and histological research methods. In addition, in order to exclude the presence of irritable bowel syndrome, there should not be any signs that the dyspepsia symptoms are relieved by defecation or are associated with stool disorders [1]. Only in 20–30%, an “organic” disease may be identified (in this case, it fully explains the presence of such symptoms) [2]. The clinical interpretation of dyspepsia is also extremely difficult, since it is impossible to convincingly distinguish the functional manifestations of the disease from the organic disorders [3]
FD is an extremely common problem in modern healthcare and it causes 4–5% of all patients’ referrals to general practitioners [4]. According to the data of the prospective international gastroenterological observational study DIGEST, a survey of more than 5500 people showed that about a third of people who did not have any established pathology from the gastrointestinal tract reported having symptoms of dyspepsia [5]. The severity of the FD clinical manifestations and the chronic course characteristic of the disease have a significant impact on patients’ life quality, and they are also associated with direct and indirect economic costs both for treatment and for social support measures during periods of temporary disability [6].
FD pathogenesis is the result of a complex interaction of a number of pathological factors, which include a violation of evacuation function, relaxation accommodation disorders [7], neuromuscular gastric dysfunction [8], visceral hypersensitivity to stretching and other factors (hydrochloric acid secretion, food intake, medications, etc.) [6][9][10]. In recent years, the phenomenon of visceral hypersensitivity has been studied with special attention. A number of studies clearly demonstrated an increased sensitivity to the distension of the proximal gastric segment in FD patients [11][12][13].
The interaction of hormonal regulators of the gastrointestinal tract also plays a significant role in the formation of FD. For example, relaxation of the proximal gastric segment is provided by cholecystokinin, secretin, vasoactive intestinal polypeptide, gastrin, somatostatin, dopamine, gastrin-releasing peptide, glucagon, and bombesin, at a time when the prokinetic hormone motilin increases the muscle tone [14]. The physiological effect of motilin suggests an important role of violations of its secretion in the epigastric pain syndrome (EPS) formation in the case of FD. There is data concerning the gastric mucosa hypersensitivity of FD patients to hydrochloric acid with a simultaneous increase in its concentration (in some patients) [15]. In combination with visceral hypersensitivity, it significantly contributes to EPS manifestations — pain and/or “burning” in the epigastric region. Being a motor regulator, motilin promotes the physiological emptying of the stomach on the one hand, and on the other hand, it stimulates the HCl-independent secretion of pepsin by the main cells of the gastric mucosa [16]. Thus, the insufficient regulatory action of motilin can contribute to the stagnation of food in the gastric lumen, delayed protein breakdown, a concordant increase in HCl concentration, and the appearance or intensification of the EPS symptoms.
Treatment of FD patients is based on the traditional plan for patients with gastrointestinal pathology. However, in addition to the medicine therapy, an important role is assigned to lifestyle modification, dietary recommendations, and, if necessary, correction of psychoemotional status [6]. As for the medicine therapy, preference is given to antisecretory medicines in the case of EPS, to prokinetics — in the formation of postprandial distress syndrome [17]. One of the promising options for improving the effectiveness of FD therapy at the present time is the use of therapeutic table mineral waters (MWs) [18]. Various studies carried out by a number of authors have confirmed the clinical effectiveness of using MWs in the treatment of chronic diseases of the gastroduodenal zone [19][20][21].
The purpose of the study was to explore the mechanism of the low-mineralized sulfate-chloride-sodium mineral water impact on the clinical picture and motor gastric function in patients suffering from EPS by the example of the regulatory peptide motilin.
Materials and methods
The study included 113 people (two groups of patients); the average patient’s age was 22.3±0.21 years old. The observation group (57 people) included persons suffering from FD in the form of EPS diagnosed according to Rome IV criteria; the comparison group included practically healthy persons (56 people). The severity of symptoms of upper gastrointestinal tract lesions was determined according to the Gastrointestinal Symptom Rating Scale (GSRS) questionnaire. The motilin concentration in the blood of patients was determined using a set of reagents for enzyme immunoassay (Cloud-Clone Corp., USA) on an enzyme immunoassay analyzer Stat Fax-2100 (USA). The drinking test was carried out in the morning on an empty stomach as follows: the patients drank non-carbonated drinking water at room temperature until they felt full saturation with fixing the volume of water taken.
The patients of the observation group took non-carbonated low-mineralized sulfate-chloride-sodium MW (M 1.5–2.7 g/dm3) with a weakly alkaline reaction of the aqueous medium (pH 8.9±0.5) of the sanatorium “Metallurg” (Izhevsk, the Udmurtian Republic) (Figure 1, Table 1).
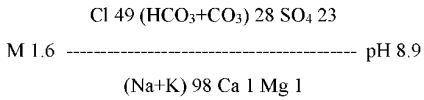
Figure 1. Balneological formula of mineral water of the sanatorium “Metallurg”
Table 1
Chemical composition of drinking mineral water of the sanatorium “Metallurg” (mg/l)
|
Cations |
Anions |
Non-dissociated molecules |
|
Na+K – 501.4 |
Cl – 390.5±0.8 |
Boric acid – 49.4±2.4 |
|
Ca – 5.0±0.1 |
HCO3 – 329.4±9.9 |
Silicic acid – 5.6±1.1 |
|
Mg – 3.0±0.1 |
SO4 – 243.3±8.2 |
|
|
NH4 – 0.4±0.1 |
CO3 – 48.0±5.9 |
|
|
F – 3.8±0.1 |
||
|
Br – 1.1±0.9 |
||
|
Sum of cations – 509.8 |
Sum of anions – 1017.0 |
This particular study is a four-stage prospective research. In the first stage, the authors conducted a questionnaire using the GSRS questionnaire, which made it possible to diagnose EPS as a variant of FD, to exclude the presence of esophagus, stomach, and duodenum organic pathologies by means of esophagogastroduodenoscopy, and to form patients groups. The initial motilin concentration in the blood serum was also measured at this stage.
In the second stage, patients of both groups underwent a drinking test, after which the content of blood motilin was determined. The third stage included a single intake of MW from the sanatorium “Metallurg” (200 ml), followed by the immediate measuring of the motilin concentration. At last, only the patients suffering from EPS took part in the fourth stage. They were drinking MW from the sanatorium “Metallurg” (20–25 °C, 60 minutes before meal, 200 ml and 3 times a day) during three weeks, after which they underwent repeated questioning according to the GSRS questionnaire and a study of the motilin concentration in the blood serum.
The statistical analysis was carried out using the Statistica 13.0 package. The authors of the study determined the reliability of differences in the analyzed features by the nonparametric Mann-Whitney U-test for independent samples and the nonparametric Wilcoxon T-test for related samples since part of the results obtained did not obey the law of normal distribution, according to the Kolmogorov-Smirnov criterion. The results were considered reliable at p<0.05.
Results
The analysis of clinical symptoms according to the GSRS Quality of Life Questionnaire (Table 2) revealed the prevalence of abdominal pain syndrome in patients suffering from EPS (4.33±0.23 points) both in the general structure of gastroenterological manifestations and in comparison with healthy individuals (p=0.000).
Table 2
Quality of life in patients with EPS according to the GSRS questionnaire, scores (M±m)
|
GSRS scales |
Healthy |
Patients with abdominal pain syndrome |
|||
|
Before treatment |
After treatment |
p |
p* |
||
|
Abdominal pain |
2.33±0.18 |
4.33±0.23 |
2.22±0.13 |
0.000 |
0.000 |
|
Reflux syndrome |
1.80±0.12 |
1.89±0.11 |
1.81±0.11 |
0.395 |
0.000 |
|
Diarrheal syndrome |
1.54±0.14 |
1.67±0.13 |
1.70±0.13 |
0.900 |
0.157 |
|
Intestinal syndrome |
2.02±0.10 |
2.17±0.93 |
1.92±0.10 |
0.246 |
0.000 |
|
Constipation syndrome |
1.39±0.09 |
1.11±0.02 |
1.10±0.03 |
0.058 |
0.228 |
|
Sum scale |
9.07±0.41 |
11.17±0.46 |
8.70±0.30 |
0.001 |
0.000 |
Note: p — the significance of differences in quality of life indicators in patients with abdominal pain syndrome and healthy persons; p* — the significance of differences in the dynamics of quality of life indicators in patients with abdominal pain syndrome during treatment.
According to the other scales of the GSRS questionnaire, there were no significantly significant differences between the groups of patients who participated in this particular study. It should also be noted that the severity of symptoms of gastrointestinal disorders, with the exception of abdominal pain syndrome, was almost the same in both patient groups. The differences with the character of a tendency to statistical reliability were obtained for constipation syndrome, and in the case of patients suffering from EPS, its severity was slightly lower than in healthy individuals.
The course intake of the tested MW provided positive dynamics of clinical symptoms in the examined patients suffering from EPS. The severity of abdominal pain syndrome (by 48.04%, p=0.000), reflux syndrome (by 4.23%, p = 0.000), and dyspeptic syndrome (by 11.5%, p=0.000) was evidently decreased, which led to a significant decrease in the overall score of the GSRS questionnaire by 22.11%. This parameter indicates an increase in the patients’ life quality during the treatment.
The volume of liquid taken during the drinking test in the group of patients suffering from EPS was 1066.67±70.15 ml, which is 34.37% more than in the group of healthy individuals (700.0±67.76 ml) (p=0.000).
In order to study the pathogenetic and sanogenetic mechanisms of EPS, the dynamics of motilin concentration in blood serum in both examined groups were analyzed as well (Table 3).
Table 3
Motilin concentration in blood (M±m)
|
Groups |
Motilin concentration, pg/ml |
|||
|
Before treatment |
After the drinking test |
After a single dose of mineral water |
After the course treatment of mineral water |
|
|
Patients with abdominal pain syndrome (n=57) |
8780.67±102.94 |
9060.33±98.33 |
9226.05±65.29 |
9426.33±60.99 |
|
Healthy (n=56) |
9665.64±106.94 |
9536.07±58.98 |
8564.11±467.92 |
- |
The initial motilin level in the examined patients suffering from EPS was statistically significantly lower than in healthy patients (p=0.000). After a drinking test, an increase in the motilin concentration by 3.18% was detected in the group of EPS patients, which does not reach the level of reliability (p=0.491). This parameter confirms the reaction inadequacy of the regulatory system of the upper gastrointestinal tract. After a single intake of MW from the sanatorium “Metallurg” (200 ml), a significant increase in the motilin level in the blood serum was revealed by 5.08% (p=0.001). In the case of EPS, the course MW balneotherapy on the case of the sanatorium “Metallurg” provided a steady increase in the motilinemia level (by 7.35%, p= 0.001) compared to the baseline. In the group of healthy individuals, a statistically significant decrease in the motilin concentration of the blood serum was obtained immediately after the drinking test by 1.34% (p=0.04). After a single intake of MW, no significant change in the concentration of motilin could be detected (p=0.352).
Discussion
The data obtained from the examined patients on the GSRS questionnaire with the predominance of the result on the abdominal pain scale correspond to the classical clinical picture of the EPS. The relatively small value on the constipation syndrome scale in this category of patients can be probably explained by the sitophobia manifestations. Due to pain in the epigastric region after eating, the patients limit the amount of the food consumed, while increasing the multiplicity of its receptions, which leads to the stimulation of the gastrocolic reflex. The positive dynamics of clinical symptoms in patients suffering from EPS during the course of balneotherapy, accompanied by an increase in their quality of life, necessitated the disclosure of the sanogenesis mechanisms.
The results of the drinking test demonstrated a greater tolerance of EPS patients to water stress. In the authors’ opinion, this happens due to a change in the accommodative gastric capacity and the inadequacy of the phases of the migrating motor complex associated with the pathology of motilin secretion (initially reduced level). The decrease in the motilin concentration in the blood serum of healthy individuals after a drinking test is obviously related to the methodology of this particular study. Since blood sampling was carried out immediately after the test, these dynamics reflect stage I and the beginning of stage II of the migrating motor complex, whereas the content of motilin undulates only in phase III [22].
The absence of a significant change in the motilin concentration after a single intake of MW in a group of healthy individuals is apparently due to the insignificance of the contact effect of low-mineralized MW with preserved mechanisms of humoral regulation. At the same time in the case of EPS patients, there is a statistically significant increase in the motilin level. Course treatment with low-mineralized sulfate-chloride-sodium MW of EPS patients was accompanied by a persistent increase in the motilin concentration. The high content of sodium cations in combination with chlorine and sulfate anions suggests a stimulating effect of the used MW on enzymatic secretion in the gastrointestinal tract, as well as the formation of intestinal regulatory peptides, which include motilin. Its sanogenetic effect is complemented by Cl ions, which enhance the peristaltic activity of smooth myocytes of the digestive system. The average content of Ca ions in MW provides an antispasmodic effect, and its slightly alkaline reaction reduces the acidity in the gastric lumen [23].
Conclusion
During the study, it was confirmed that patients suffering from FD, the EPS variant, had persistent motilin secretion disorders compared with healthy individuals. Physiological gastric stimulation on a drinking test model demonstrated the inadequacy of changes in the level of this hormone in the case of EPS. Both methods of single and course intake of low-mineralized sulfate-chloride-sodium MW contribute to an increase in the motilin concentration. A single dose contributes to a slightly more pronounced increase in the initial motilin concentration, which is due to the physiological stress-induced effect of MWs during internal intake. The course intake should be considered a physical adaptogenic factor leading to a stable ordering of the functional relationships of the body, in particular, the humoral regulation of the gastrointestinal tract [24]. This provides positive dynamics of the clinical picture and improves the quality of life of patients suffering from EPS.
References
1. Madisch A., Andresen V., Enck P., Labenz J., Frieling T., Schemann M. The Diagnosis and Treatment of Functional Dyspepsia. Dtsch Arztebl Int. 2018;115(13):222-232. doi:10.3238/arztebl.2018.0222.
2. Stanghellini V., Chan F.K., Hasler W.L., Malagelada J.R., Suzuki H., Tack J., Talley N.J. Gastroduodenal disorders. Gastroenterology. 2016;150(6): 1380-1392. doi: 10.1053/j.gastro.2016.02.011.
3. Moayyedi P., Talley N.J., Fennerty M.B., Vakil N. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295(13):1566-76. doi:10.1001/jama.295.13.1566.
4. Baranov S.A., Nechaev V.M., Shulpekova Yu.O., Supryaga I.V., Kurbatova A.A. Functional dyspepsia and its treatment methods. Rheumatology Science and Practice. 2020;58(1):87-90. (In Russ.) doi: 10.14412/1995-4484-2020-87-90.
5. Frieling T., Schemann M., C. Pehl C. Das Reizdarmsyndrom – eine Fehlbezeichnung? Z Gastroenterol 2011;49(5):577-578. doi: 10.1055/s-0031-1273323.
6. Dicheva D.T., Subbotina Yu.S., Bektemirova L.G., Andreev D.N. Functional dyspepsia: from pathogenesis to therapeutic aspects. Meditsinskiy sovet = Medical Council. 2019;(3):18-25. (In Russ.) doi: 10.21518/2079-701X-2019-3-18-25.
7. Sheptulin A.A., Kurbatova A.A. New Rome-IV criteria of the functional dyspepsia (review). Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2016;26(4):124-128. (In Russ.) doi: 10.22416/1382-4376-2016-26-4-124-128.
8. Harer K.N., Hasler W.L. Functional Dyspepsia: A Review of the Symptoms, Evaluation, and Treatment Options. Gastroenterol Hepatol (N Y). 2020;16(2):66-74. PMID: 34035704.
9. Shklyaev A.E., Galikhanova Yu.I., Bessonov A.G., Maximov K.V. The correction of psychoemotional status in young people with overweight with increased consumption of mineral water. Experimental and Clinical Gastroenterology. 2020;9:18-23. (In Russ.) doi: 10.31146/1682-8658-ecg-181-9-18-23.
10. Shklyaev A.E., Kazarin D.D. Motilin and cholecystokinin in functional dyspepsia: unity and struggle of opposites. Health, demography, ecology of finno-ugric peoples 2022;2:36-41. (in Russ.).
11. Vandenberghe J., Vos R., Persoons P., Demyttenaere K., Janssens J., Tack J. Dyspeptic patients with visceral hypersensitivity: sensitisation of pain specific or multimodal pathways? Gut. 2005;54(7):914-919. doi: 10.1136/gut.2004.052605.
12. Tack J., Caenepeel P., Fischler B., Piessevaux H., Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121(3):526-535. doi: 10.1053/gast.2001.27180.
13. Shklyaev A.E., Maksimov K.V., Grigorieva O.A. MRI diagnostics of functional dyspepsia. Digital Diagnostics. 2021;2(1S):12-13. (In Russ.). doi:10.17816/DD20211s12
14. Kareva E.N., Serebrova S.Yu. Challenges in drug treatment of gastric motility disorders. Experimental and Clinical Gastroenterology. 2017;(7):167-183. (In Russ.). eLIBRARY ID: 30507338.
15. Ivashkin V.T., Mayev I.V., Sheptulin A.A., Lapina T.L., Trukhmanov A.S. et al. Diagnosis and treatment of the functional dyspepsia: clinical guidelines of the Russian Gastroenterological Association. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2017;27(1):50-61. (In Russ.). doi: 10.22416/1382-4376-2017-27-1-50-61.
16. Kuznetsova E.I., Rymareva E.A., Dicheva D.T., Andreev D.N.Gastroesophageal reflux disease and diabetes mellitus: pathophysiological mechanisms of comorbidity. Consilium Medicum. 2019;21(8):23–28. (In Russ.). doi:10.26442/20751753.2019.8.190497.
17. Lazebnik L.B., Alexeenko S.A., Lyalukova E.A., Samsonov A.A., Bordin D.S. Recommendations on management of primary care patients with symptoms of dyspepsia. Experimental and Clinical Gastroenterology. 2018;(5):4-18. (In Russ.). eLIBRARY ID: 35606979.
18. Lazebnik L.B., Simanenkov V.I., Tikhonov S.V., Lishchuk N.B. Clinical study of the efficacy of natural mineral water “Borjomi” in patients with functional dyspepsia. Experimental and Clinical Gastroenterology. 2016;(11):26-30. (In Russ.). eLIBRARY ID: 27218521.
19. Gorbunov A. Yu., Tronina D.V. Change in the hormonal status in cholelithiasis in the process of treatment with mineral water "Uvinskaya". Experimental and Clinical Gastroenterology. 2017;8(144):75-78. (In Russ.). eLIBRARY ID: 29970261
20. Shklyaev A.E., Kazarin D.D., Bazhenov E.L., Gorbunov Yu.V. Morphological aspects of the gastric mucosa during eradication therapy in patients with type 2 diabetes. Health, demography, ecology of finno-ugric peoples. 2020;1:46-49. (In Russ.). eLIBRARY ID: 42879139.
21. Shklyaev A.E., Semenovykh E.A., Maksimov K.V. Management of postprandial distress syndrome in a young patient with the course application of still mineral water “Uvinskaya”. Experimental and Clinical Gastroenterology. 2020;(9):89-93. (In Russ.). doi: 10.31146/1682-8658-ecg-181-9-89-93.
22. Cimmerman Ja.S., Vladimirskij E.V., Rybolovlev E.V. Fizioterapija i kurortnye lechebnye faktory v gastrojenterologii. Perm; 2006 (In Russ.).
23. Ito 3., Sekiguchi T. Fiziologija i patofiziologija ZhKT. M: Medicina; 1989 (In Russ.).
24. Ljubchik V.N., Buglak V.N., Kaladze N.N. Lechebnoe primenenie mineral'nyh natural'nyh pit'evyh vod respubliki Krym. Simferopol; 2016 (In Russ).
About the Authors
A. E. ShklyaevRussian Federation
Aleksey E. Shklyaev, Dr. Sci. (Med.), Professor, Professor of Department of Faculty Therapy with Courses of Endocrinology and Hematology, Rector
Izhevsk
Competing Interests:
Authors declares no conflict of interest.
D. D. Kazarin
Russian Federation
Daniil D. Kazarin, assistant of Department of Faculty Therapy with Courses of Endocrinology and Hematology, Rector
Izhevsk
Competing Interests:
Authors declares no conflict of interest.
O. A. Grigoreva
Russian Federation
Olga A. Grigoreva, postgraduate of Department of Faculty Therapy with Courses of Endocrinology and Hematology
Izhevsk
Competing Interests:
Authors declares no conflict of interest.
Review
For citations:
Shklyaev A.E., Kazarin D.D., Grigoreva O.A. Balneotherapy of epigastric pain syndrome: the meaning of motilin in the implementation of the therapeutic effect. Medical Herald of the South of Russia. 2023;14(1):24-30. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-1-24-30







































