Scroll to:
Implantation zone as an echographic criterion identification of types of cicatricial and isthmic pregnancies in women with a scar on the uterus
https://doi.org/10.21886/2219-8075-2022-13-2-72-79
Abstract
Objective: the purpose of this publication is to present the establishment of the implantation zone of the fetal egg during ultrasound diagnostics at 5-7 weeks to differentiate the types of cicatricial and isthmian pregnancies in women with a scar on the uterus after cesarean section.
Materials and methods: the study included 11 women with low implantation of the fetal egg in the presence of a scar on the uterus. All underwent ultrasound with transabdominal and transvaginal approaches at 5–7 weeks of pregnancy to establish the place of implantation of the fetal egg relative to the scar on the uterus.
Results: according to the results of ultrasound evaluation of the implantation zone of the fetal egg in 5–7 weeks were differentiated: type 1 of scar pregnancy (1 case); type 2 of scar pregnancy (7 cases) and low implantation along the posterior wall of the uterus at the level of the scar intact from it (2 cases).
Conclusions: ultrasound at 5–7 weeks is a necessary method of examination in women with a scar on the uterus for the diagnosis of scar pregnancy. The establishment of the implantation zone of the fetal egg is a tool that allows differentiating the types of cicatricial and isthmic pregnancies.
Keywords
For citations:
Esetov M.A., Esetov A.M. Implantation zone as an echographic criterion identification of types of cicatricial and isthmic pregnancies in women with a scar on the uterus. Medical Herald of the South of Russia. 2022;13(2):72-79. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-72-79
Introduction
Scar pregnancy (SP) refers to uterine ectopic pregnancy, in which the fetal egg (FE) is implanted in the scar area after a cesarean section (CS) in the lower segment [1]. The frequency of SP is directly related to an increase in the CS frequency, accounting for approximately 0.15% of the number of operations (1 case per 800–2500 pregnancies) [2][3].
SP is a fundamentally high-risk pregnancy, often combined with abnormal placental attachment (spectrum of placental attachment abnormalities — SPAA). The association of developing SP with a high frequency of complications during the 1st-2nd trimesters of pregnancy has been noted. According to Calì et al., about 20% of women with this pathology needed surgery during the 1st trimester, 10% had an early rupture of the uterus and 15% had a hysterectomy. At the same time, among pregnancies progressing to the 3rd trimester, SPAA develops in about 3/4 of cases, mainly it is the placenta increta [4].
During the ultrasound examination, the diagnosis of SP is based on the following criteria proposed by Godin et al. [5]:
- empty uterine cavity, without contact with the fetal sac;
- clearly visible empty cervical canal without contact with the fetal sac;
- the presence of a FE in the projection of the uterus anterior wall at the level of the isthmus;
- absence or defect in the myometrial tissue between the bladder and the sac.
According to previously published data, ultrasound diagnostics of SP is the simplest up to seven weeks of pregnancy [1] and is solved in most cases by assessing the location of the FE relative to the scar on the uterus [6–10]. Subsequently, as the FE grows, after eight weeks for the diagnosis of SP, expert ultrasound is most often required using the Color Doppler Imaging (CDI) mode in order to visualize the exact location of placental attachment.
In 2000, Vial et al. [11] presented a description of two types of SP. The first type is when the implantation of the FE occurs on the scar with its subsequent growth into the isthmic part of the uterine cavity; the second is implantation directly into the scar on the uterus. According to the authors, pregnancy on the scar (type 1) involves the location of more than 50% of the FE in the uterine cavity, and pregnancy in the scar (niche) (type 2) more than 50% of the FE in the projection of the scar anteriorly from the uterine cavity.
These types may have different prognoses. Therefore, type 2 can manifest itself as an early rupture of the uterus already in the 2nd trimester and has a proven histopathological connection with the subsequent development of pathological attachment of the placenta, and therefore is considered a sonographic marker of this complication in the embryonic period [12]. At the same time, it was shown that SP implanted “on the scar” (type 1), in some cases, resulted in the development of less severe forms of SPAA, amenable to planned postpartum treatment [13].
All this shows that the low localization of the FE in women with a scar on the uterus after a CS in the lower segment requires a differential diagnosis of types of SP.
In addition, with a low-lying FE, differentiation of cases of low implantation of the FE along the posterior wall of the uterus (intact from the scar), the so-called isthmic pregnancy (which does not have an unambiguous prognosis and in some cases ends with the birth of a viable fetus without complications for the mother) is required.
Kangning Li and Qing Dai in 2020 [14] showed that a direct sign for the differentiation of the types of SP and isthmic pregnancy could be the establishment of the implantation site (development of the hairy chorion) of the FE in the presence of a scar on the uterus.
The purpose of the study was to present the identification of the implantation zone of the FE during the ultrasound diagnostics at the 5th-7th weeks, to differentiate the types of scar and isthmic pregnancy in women with a scar on the uterus after a CS.
Materials and methods
During 2008–2022, 1,351 ultrasounds were performed at the 5th-9th weeks of pregnancy. Ultrasound was performed both at the request of patients and to clarify the diagnosis when referring patients with various problems. VolusonI and E8 devices (GE Medical systems, Kretz Ultrasound, Zipf, Austria) with convexic (RAB 2-5 MHz) and transvaginal (RIC 5–7 and 6–12 MHz) sensors were used.
While ultrasounding women with the uterus scar at the 5th-7th weeks, the following sequence was used in order to assess the location of the FE: studies began with transabdominal ultrasound followed by a transition to transvaginal scanning. In all cases, archiving of images was carried out.
At the first stage, the formation of a group with low implantation was carried out. For this purpose, the sign of Timor-Tritsch et al. [15] was used, who proposed to evaluate the location of the FE relative to the middle of the uterus. In order to do this, the distance from the external pharynx to the bottom of the uterus is divided in half by a perpendicular line (“mid-uterus line”). The second line is carried out through the middle of the FE (“FE line”) parallel to the first line (Figure 1). The location of the “FE line” below the “mid-uterus line” is a criterion for low implantation and high risk of developing SP and SPAA. In cases of difficulty in drawing a line from the cervix to the bottom, the localization of the FE in the lower third of the uterine cavity was used as a criterion for low implantation. In order to do this, the uterine cavity from the inner pharynx to the bottom of the uterine cavity is divided into three equal segments (Figure 2).
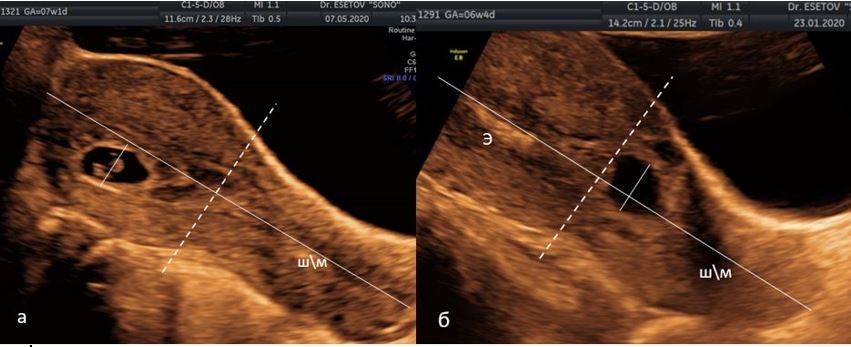
Рисунок 1 (a, б). Метод оценки низкого расположения плодного яйца (ПЯ) по I.E. Timor-Tritsch и соавт. [15]. На сагиттальном срезе матки сплошная линия, проведенная по эндометрию от наружного зева до дна, и перпендикуляры через середину этой линии (пунктирная линия - «линия середины матки») и через середину ПЯ.
а) середина ПЯ расположена выше «линии середины матки»;
б) середина ПЯ расположена ниже «линии середины матки». ШМ — шейка матки, Э — эндометрий.
Figure 1. Method for assessing the low location of the fetal egg (FE) according to I.E. Timor-Tritsch et al. [15]. On the sagittal section of the uterus, a solid line drawn along the endometrium from the external os to the bottom, and perpendiculars through the middle of this line (the dotted line is the “line of the middle of the uterus”) and through the middle of the FE.
a) the middle of the FE is located above the "line of the middle of the uterus";
b) the middle of the FE is located below the "line of the middle of the uterus." CMM - cervix, E - endometrium.

Рисунок 2. Эхограмма верхних 2/3 полости матки (красный овал) и перешеек матки, сагиттальное сканирование.
Figure 2. Sonogram of the upper 2/3 of the uterine cavity (red oval) and the isthmus of the uterus, sagittal scanning
In patients with a scar on the uterus and low localization of the FE, the implantation zone of the FE (the side of the development of the hairy chorion) was established. The implantation site was established according to the recommendations of Abdallah et al. [16]. On the sagittal section, the implantation site is determined in the form of a hyperechoic thickening of the FE wall from the side opposite to the direction of displacement of the uterine cavity, which was considered as the initial site of formation and development of the placenta. It has also been shown that the side of the FE adjacent to the vitelline sac is the side of placenta development [3]. An additional criterion may be the presence of peritrophoblastic blood flow in the implantation zone, which is characterized by high velocity (20 cm/s) and low resistance (pulsation index (PI) < 1) [17].
Additionally, the “crossover sign” (COS) (“sign of intersection”) [18] and the thickness of the myometrium above the FE were evaluated. The thickness of the myometrium was measured between the anterior contour of the FE and the serous lining of the uterus. The absence of myometrium was considered a sign of cesarean scar pregnancy (CSP). The thickness of less than 5 mm was estimated as increasing the probability of the presence of the CSP.
Results
In total, during this period, 10 cases of low FE location were diagnosed in women with a scar on the uterus at the time of the 5th-7.5th weeks of pregnancy.
The average age of the patients was 27 years (from 19 to 41 years), 6 women had a history of two CSs, 3 had 1 CS, and 1 had 3 CSs.
With ultrasound at 5–7 weeks of pregnancy with low FE implantation in women with a scar on the uterus, the following categories were evaluated:
- scar implantation (SP) — 1 case;
- CSP — 7 cases;
- low implantation along the back wall of the uterus at the level of the scar intact from it — 2 cases.
In all these cases of CSP, the zone of development of the fleecy chorion was along the anterior and anteroinferior wall (Figure 3). At the same time, in one case, the FE was located intramurally (Figure 3a), and in 5 cases, when localized in the scar, a characteristic wedge-shaped pole of the FE was noted, identical to the shape of the niche (Figure 3b).
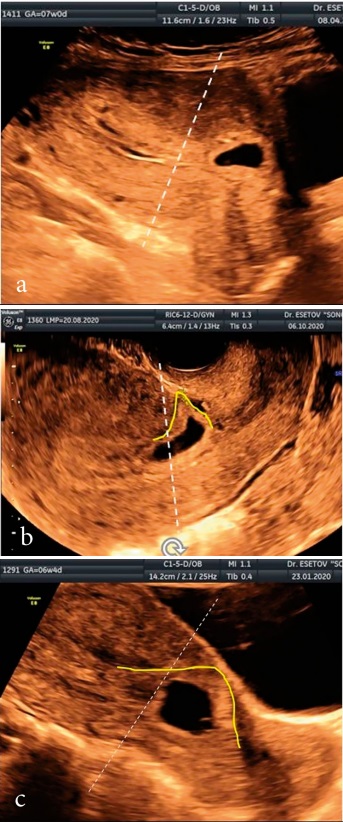
Рисунок 3 (a–c). Разновидности беременностей в рубце матке: а) интрамуральная локализация ПЯ в рубце; b) клиновидная форма ПЯ, расположенного в рубце; c) расположение ПЯ в проекции рубца; жёлтая линия — зона имплантации ПЯ.
Figure 3. Varieties of pregnancies in the uterine rumen: a) intramural localization of the FE in the rumen; b) wedge-shaped FE located in the scar; c) the location of the FE in the projection of the scar; yellow line — PU implantation zone.
In 6 cases with CSP, pregnancies were terminated in a gynecological hospital. One patient fell out of our supervision.
In the 1st case, the low FE localization was regarded as SP. The implantation zone of the joint was located along the anterior wall at the level of the formed scar (Figure 4) without direct insertion into it. The thickness of the myometrium above the FE was 8 mm. Upon further observation, the pregnancy turned out to be undeveloped.
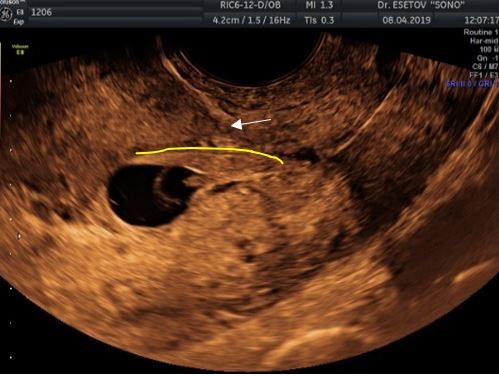
Рисунок 4. Случай БНР. Эхограмма зоны имплантации ПЯ по передней стенке (жёлтая линия) на уровне сформированного рубца на матке (стрелка).
Figure 4. BNR case. Sonogram of the FE implantation zone along the anterior wall (yellow line) at the level of the formed scar on the uterus (arrow).
In 2 cases, an isthmic pregnancy was determined with a low location of the FE. In these cases, the localization of the zone of development of the hairy chorion was located along the posterior wall of the uterus intact from the scar (Figure 5). An additional assessment of blood flow showed its peritrophoblastic variant in the implantation zone along the posterior wall of the uterus (Figure 5b). The thickness of the myometrium above the FE was 3 and 12 mm. In one case, the pregnancy later turned out to be undeveloped, in the other, the pregnancy was terminated at the request of the patient.
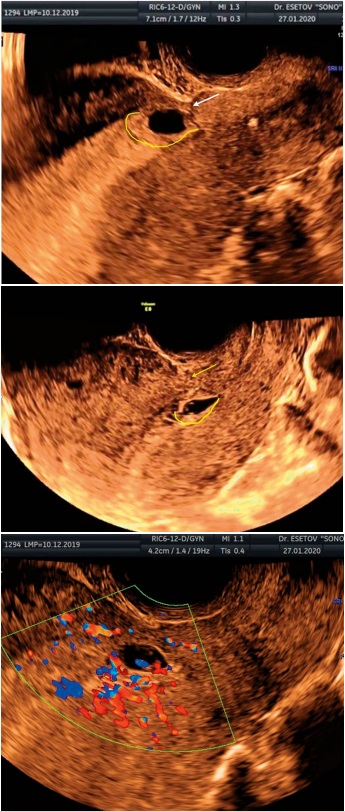
Рисунок 5 (а, b). Истмическая беременность, с расположением зоны имплантации ПЯ (жёлтая линия) по задней стенке полости матки, интактно от рубца (стрелка); c) перитрофобластический кровоток в зоне имплантации ПЯ по задней стенке матки.
Figure 5. (a,b). Isthmic pregnancy, with the location of the implantation zone of the FE (yellow line) along the posterior wall of the uterine cavity, intact from the scar (arrow); c) peritrophoblastic blood flow in the area of implantation of the FE along the posterior wall of the uterus.
Discussion
In the international literature, the term “Cesarean scar pregnancy” is used to describe both types of pregnancy associated with a scar on the uterus after CS: “pregnancy on the scar” (type 1), and “pregnancy in the scar” (type 2). The authors of this study consider it appropriate to use the term “scar pregnancy” as a variant combining both types.
Ultrasound diagnosis of SP in the early stages is the simplest, but prenatal imaging cannot fully predict the clinical outcome. On the one hand, it is believed that in cases of SP diagnosis, termination of pregnancy is currently the most reasonable option. On the other hand, it has been shown that, despite the maternal morbidity associated with prolongation of SP, the majority (~75%) of pregnancies progressing to term lead to live birth [13]. In 2017, Agten et al. [13] showed that out of 6 cases of SP (type 1), 5 ended with a CS with normal placentation and only in 1 case, a hysterectomy was performed for placenta accreta.
Based on these studies, the main task of ultrasound in early pregnancy in women with a scar on the uterus is to try to differentiate risks based on the establishment of the 1st and the 2nd SP types. In addition, low localization of the FE in women with a scar on the uterus may also be associated with an isthmic pregnancy intact from the scar.
In a number of studies, it has been convincingly shown that a high correlation with the development of SP and SPAA has a low position of the FE in the presence of a scar on the uterus [6, 10].
As the simplest method of diagnosing SP, it is proposed to conduct transvaginal ultrasound (TVU) in the 5th-7th weeks of pregnancy in women with a scar on the uterus [1, 6, 10, 18]. According to Rotas et al. [1], the method of TVU in the diagnosis of CSP proved to be effective with a sensitivity of 84.6% (95% TO; 0.763–0.905).
The authors of this study believe that the assessment of the location of the FE relative to the middle of the uterine cavity more correctly allows for transabdominal ultrasound (TAU), which is usually the beginning of the study. With this method, the cervix and the body of the uterus are in the same plane. TVU is used for a detailed targeted assessment of the area of surgery, the contour of the uterus and bladder and determining the place of the FE implantation. Moreover, for a qualitative study, moderate filling of the bladder is necessary. In case of TVU, which is performed with an emptied bladder, when the uterus is in hyperanteflexia, the assessment of the middle of the uterus according to the method of Timor‐Tritsch et al. [15] is not always correctly feasible. In such cases, the authors of this study made a conclusion about the low location of the PE by the location of the PE in the lower third of the uterine cavity.
The recommendations of ultrasound at the 5th-7th weeks are due to the fact that only in these terms, it is possible to diagnose SP by the FE localization. During these periods, it is possible to obtain a picture of such signs of CSP as intramural localization of the FE in the scar and the wedge-shaped shape of the FE corresponding to the shape of the niche into which it is implanted. After 7 weeks, with growth, the FE begins to occupy the uterine cavity and gradually changes shape, which can lead to an incorrect assessment of its location. All this after 8 weeks requires the use of an expert assessment using the CDK mode to visualize the exact place of attachment of the placenta in relation to the scar and assess the signs of SPAA [4].
A low FE implantation in the presence of a scar to the uterus requires differentiation of the 1st and 2nd SP types and low implanted uterine pregnancy (isthmic pregnancy), intact from the scar.
Intramural FE localization and the wedge-shaped shape, indicating implantation in the uterine scar, are visual signs of the 2nd SP type (CSP). However, in the absence of this picture, the low localization of the FE in the presence of a uterine scar requires an assessment of additional echographic signs of differential diagnosis.
There are works showing the effectiveness of a number of signs (COS, thickness of the myometrium above the FE) for ultrasonic differentiation of types of SP [19]. However, as experience shows, the assessment of these signs is not always possible with ultrasound. The drawing of a straight line along the endometrium recommended by the authors is not always practically possible, especially with an empty bladder. In addition, these signs do not subdivide cases of the 1st SP type and isthmic pregnancy with implantation along the posterior wall of the uterus.
So, Table 1 shows the criteria for the thickness of the myometrium above the FE as a sign for assessing the possible development of SPAA.
Таблица / Table 1
Эхографическая оценка толщины миометрия над ПЯ, как критерия приращения плаценты
Sonographic assessment of the thickness of the myometrium over the PU as a criterion for placenta accreta
|
Авторы Authors |
Толщина миометрия Myometrial thickness |
|
|
Критерий приращения, мм Increment criterion, mm |
Нет приращения, мм No increment, mm |
|
|
E. Moschos et al. [20] |
≤ 3 — всегда / always |
≥ 5 |
|
I. E. Timor-Tritsch, A. Monteagudo [15] |
≤ 1-3 — признак БРМ / sign of BRM |
|
|
A. Kaelin Agten et al. [13] |
≤ 2— всегда / always |
≥ 4 |
|
Li K., Dai Q. [14] |
2,35 — умеренное диагностическое значение / moderate diagnostic value |
|
|
G. Cali b et al. [4] |
≤ 5 высокая частота / high frequency |
|
|
O. Osser et al. [21] |
Менее 2,2 мм составляет 14%, 23% и 43% при первом, втором или третьем КС соответственно. Поэтому толщина остаточного миометрия не является хорошим показателем. Less than 2.2 mm is 14%, 23% and 43% at the first, second or third CS, respectively. Therefore, the thickness of the residual myometrium is not a good indicator. |
|
The data presented in the table demonstrate the absence of a single value of the thickness of the myometrium as a prognostic criterion. In addition, practice shows the difficulty of accurately measuring the thickness of the myometrium during ultrasound. Therefore, it seems real to accept as a prognostic value for CSP only the absence of the myometrium above the FE.
Given the ambiguity of the echographic evaluation of these indirect signs, Li and Dai [14] showed that a direct sign of various types of SP and isthmic pregnancy can be the determination of the zone of development of the hairy chorion relative to the scar on the uterus. In all the considered cases, ultrasound allowed assessing the implantation zone of the FE and differentiating the types of SP and isthmic pregnancy. In cases assessed by the authors of the study as the 2nd SP type, the implantation zone was located along the anterior and anterior walls of the uterus. In one case, the absence of the implantation zone in the scar allowed talking about the presence of the 1st SP type (pregnancy on the scar). In two cases, only visualization of the implantation zone along the posterior wall, intact from the scar, allowed regarding them as cases of isthmic pregnancy.
Based on the literature data and this study observations, the following ultrasound CSP signs (type 2) can be distinguished at the 5th-7th weeks: empty upper 2\3 uterine cavities; intramural localization of the FE in the scar area; wedge-shaped anterior pole of the FE filling the scar niche; missing myometrial layer between the FE and the bladder; implantation zone along the anterior and the middle wall of the uterus. In this form, the most likely is the development of acute symptoms, such as bleeding or rupture of the uterus, already in the first half of pregnancy.
It is more difficult to consult women with CSP when the implantation zone of the joint is located above the formed scar. On the one hand, Timor‐Tritsch [22] notes that the decision to continue or terminate pregnancy can be made depending on the residual thickness of the myometrium between the placenta and the bladder. On the other hand, according to the results of the study by Calí et al., the low localization of FE in the presence of a scar on the uterus, even as an isolated sign, showed high sensitivity in predicting a severe degree of SPAA and an unfavorable surgical outcome [19].
There are also unpredictable cases of isthmic pregnancy with FE implantation along the posterior wall of the uterus, intact from the scar, in which the probability of pregnancy development before the terms of a viable fetus is described, without signs of abnormal attachment of the placenta.
Conclusion
The ultrasound SP diagnosis is valid at the 5th-7th weeks. During this period, a low FE location in women with a uterus scar can be considered an SP sign. The evaluation of the implantation zone is a sign that allows diagnosing and differentiating both types of SP and isthmian pregnancy by means of ultrasound. Mandatory echography at the 5th-7th weeks in pregnant women with a CS history is necessary to optimize pregnancy management and improve outcomes.
References
1. Esetov M.A., Esetov A.M. Jehografija v jem-brional'nom periode. Beremennost' v rubce na matke. Moscow: Izdatel'skij dom Vidar-M, 2020. (In Russ.)
2. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-81. DOI: 10.1097/01.AOG.0000218690.24494.ce.
3. McKenna DA, Poder L, Goldman M, Goldstein RB. Role of sonography in the recognition, assessment, and treatment of cesarean scar ectopic pregnancies. J Ultrasound Med. 2008;27(5):779-83. DOI: 10.7863/jum.2008.27.5.779.
4. Calì G, Timor-Tritsch IE, Palacios-jaraquemada j, Monteaugudo A, Buca D, et al. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(2):169-175. DOI: 10.1002/uog.17568.
5. Godin PA, Bassil S, Donnez j. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997;67(2):398-400. DOI: 10.1016/S0015-0282(97)81930-9.
6. Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121(2):171-81; discussion 181-2. DOI: 10.1111/1471-0528.12557.
7. Sarkisov S.Eh., Demidov A.V., Belousov D.M., Romanovskaya O.A. Cesarean scar ectopic pregnancy ultrasound diagnostics. Ultrasound and functional diagnostics. 2009;(2):36–42. (In Russ.). eLIBRARY ID: 12962363
8. Chechneva M.A., Panov A.E., Fedorov A.A., Blagina E.I. Vozmozhnosti ul'trazvukovoj diagnostiki i vedenija beremennosti pri nalichii rubca na matke. SonoAce Ultrasound. 2015;(27): 3–10. (In Russ.).
9. D'Antonio F, Palacios-jaraquemada j, Lim PS, Forlani F, Lanzone A, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47(3):290-301. DOI: 10.1002/uog.14950.
10. Timor-Tritsch IE, D'Antonio F, Calí G, Palacios-jaraquemada j, Meyer j, Monteagudo A. Early first-trimester transvaginal ultrasound is indicated in pregnancy after previous Cesarean delivery: should it be mandatory? Ultrasound Obstet Gynecol. 2019;54(2):156-163. DOI: 10.1002/uog.20225.
11. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6):592-3. DOI: 10.1046/j.1469-0705.2000.00300-2.x.
12. Timor-Tritsch IE, Monteagudo A, Cali G, Palacios-jaraquemada jM, Maymon R, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43(4):383-95. DOI: 10.1002/uog.13282.
13. Kaelin Agten A, Cali G, Monteagudo A, Oviedo j, Ramos j, Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted «on the scar» versus «in the niche». Am J Obstet Gynecol. 2017;216(5):510.e1-510.e6. DOI: 10.1016/j.ajog.2017.01.019.
14. Li K, Dai Q. Differential Diagnosis of Cesarean Scar Pregnancies and Other Pregnancies Implanted in the Lower Uterus by Ultrasound Parameters. Biomed Res Int. 2020;2020:8904507. DOI: 10.1155/2020/8904507.
15. Timor-Tritsch IE, Monteagudo A, Cali G, El Refaey H, Kaelin Agten A, Arslan AA. Easy sonographic differential diagnosis between intrauterine pregnancy and cesarean delivery scar pregnancy in the early first trimester. Am J Obstet Gynecol. 2016;215(2):225.e1-7. DOI: 10.1016/j.ajog.2016.02.028.
16. Abdallah Y, Naji O, Saso S, Pexsters A, Stalder C, et al. Ultrasound assessment of the peri-implantation uterus: a review. Ultrasound Obstet Gynecol. 2012;39(6):612-9. DOI: 10.1002/uog.10098.
17. jurkovic D, jauniaux E, Kurjak A, Hustin j, Campbell S, Nicolaides KH. Transvaginal color Doppler assessment of the uteroplacental circulation in early pregnancy. Obstet Gynecol. 1991;77(3):365-9. PMID: 1992400.
18. Cali G, Forlani F, Timor-Tritsch IE, Palacios-jaraquemada j, Minneci G, D'Antonio F. Natural history of Cesarean scar pregnancy on prenatal ultrasound: the crossover sign. Ultrasound Obstet Gynecol. 2017;50(1):100-104. doi: 10.1002/uog.16216.
19. Calí G, Timor-Tritsch IE, Forlani F, Palacios-jaraquemada j, Monteagudo A, et al. Value of first-trimester ultrasound in prediction of third-trimester sonographic stage of placenta accreta spectrum disorder and surgical outcome. Ultrasound Obstet Gynecol. 2020;55(4):450-459. DOI: 10.1002/uog.21939.
20. Moschos E, wells CE, Twickler DM. Biometric sonographic findings of abnormally adherent trophoblastic implantations on cesarean delivery scars. J Ultrasound Med. 2014;33(3):475-81. DOI: 10.7863/ultra.33.3.475.
21. Osser OV, jokubkiene L, Valentin L. Cesarean section scar defects: agreement between transvaginal sonographic findings with and without saline contrast enhancement. Ultrasound Obstet Gynecol. 2010;35(1):75-83. DOI: 10.1002/uog.7496.
22. Timor-Tritsch IE. Cesarean scar pregnancy: a therapeutic dilemma. Ultrasound Obstet Gynecol. 2021;57(1):32-33. DOI: 10.1002/uog.23549.
About the Authors
M. A. EsetovRussian Federation
Murad A. Esetov, Dr. Med. Sci., Associate Professor, Associate Professor of the Department of Radiation Diagnostics and Radiation Therapy with the improvement of Doctors
Makhachkala
A. M. Esetov
Russian Federation
Azedin M. Esetov, Cand. Med. Sci., Associate Professor of the Department of Obstetrics and Gynecology, Faculty of Medicine
Makhachkala
Review
For citations:
Esetov M.A., Esetov A.M. Implantation zone as an echographic criterion identification of types of cicatricial and isthmic pregnancies in women with a scar on the uterus. Medical Herald of the South of Russia. 2022;13(2):72-79. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-72-79







































