Scroll to:
Microbiota of the vagina and myoma nodes in uterine myoma
https://doi.org/10.21886/2219-8075-2022-13-2-50-58
Abstract
Objective: to study the microbiota of the vagina, myomatous nodes, their bed in patients with fibroids.
Materials and methods: a comprehensive examination of 83 patients aged 26 to 50 years with diagnosed interstitial, interstitial-subserous uterine myoma was carried out. By age, all women are divided into 3 groups. Standard clinical and clinical laboratory studies, colposcopy, microbiological examination of the detachable posterior vaginal fornix, biopsy specimens of myomatous nodes and their bed, as well as morphological examination of biopsy specimens of myomatous nodes and their bed were performed.
Results: in the structure of clinical manifestations of uterine fibroids, pain syndrome was the leading one (p<0.05). The number of myoma nodes in patients varied from 1 to 22. The duration of the operation and the amount of blood loss did not differ significantly (p>0.05). According to the results of a bacteriological study of the detachable posterior vaginal fornix in patients in the 2nd group, a decrease in the frequency of detection and the number of lactobacilli was revealed compared to the 1st group, and in women in the 3rd group, these microorganisms were absent. Among the anaerobic microorganisms in the 1st and 2nd groups, Eubacterium spp. dominated, in the 3rd — Peptostreptococcus spp. In patients of group 3, the frequency of detection of Bacteroides spp. was significantly increased (p<0.05). Among the aerobic spectrum of microorganisms in all groups, coagulasenegative staphylococci predominated. In a bacteriological study of biopsy specimens, the absence of growth of microorganisms in myomatous nodes was observed in 7.2% of cases, in the tissue of the bed of myomatous nodes in 17.7%. The microbiota of myomatous nodes and their bed in most cases was represented by anaerobic taxa. According to the results of a morphological study of biopsy specimens, no inflammatory reaction of tissues was detected.
Conclusions: In women with uterine myoma of different age groups, multidirectional changes in the vaginal microbiota were revealed. In most cases, the myomatous node (92.8%) and its bed (82.3%) are not sterile with the dominance of anaerobic microbiota taxa. Identified significant correlations in the loci «vagina – myomatous node – myomatous node bed» indicate their relationship. The detection of various taxa of microorganisms in the myomatous node and its bed, according to morphological studies, is not associated with the presence of infectious and inflammatory processes in the tissues.
Keywords
For citations:
Nikitina E.S., Rymashevsky A.N., Naboka Y.L., Rymashevsky M.A., Gudima I.A., Svirava E.G. Microbiota of the vagina and myoma nodes in uterine myoma. Medical Herald of the South of Russia. 2022;13(2):50-58. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-50-58
Introduction
Uterine fibroids (UFs) are the dominant pelvic tumor in all countries [1–9]. The etiology and pathogenesis of this pathology are not only the subject of close study but also a wide field for discussion. A number of studies proved the importance of sex hormones influencing the growth of myomatous nodes (MNs) [10–19]. Moreover, the role of estrogens and progesterone in the development of UFs is almost equivalent; however, there is variability in both clinical symptoms and the course of the disease [10]. In particular, UFs in one and the same patient are often characterized by different rates of growth and/or regression in the presence of common effects of sex hormones [20]. However, the role of angiogenesis and vascularization, which control tumor growth, cannot be ignored [21]. The authors also discuss the importance of progenitor cells, the presence of which can lead to UF manifestation, in particular, stem cell (SSC) verification in myometrium [22].
At present, the role of the microbial factor in the development of UFs is understudied. A few works on this issue [23] suggest that infectious and inflammatory processes of the pelvic organs and uterine annexes may be the triggers of this pathology in some cases. Morphological examination of uterine preparations obtained from patients with a confirmed diagnosis of "endometritis" verifies the "rudiments" of MNs located around the inflammatory zone. DNA from chlamydia, mycoplasmas, or their combinations was detected in the biopsy specimens of MNs by PCR. Various disorders [24] of the vaginal microbiocenosis may affect the development of UFs. However, there are almost no data on the MN microbiota and/or microbiome. It can be assumed that they have their own, unique microbial patterns, the presence of which does not determine the development of an inflammatory process in the uterus.
The study aimed to evaluate the microbiota of the vagina, MNs, and their bed in patients with fibroids.
Materials and Methods
The study was conducted based on the facilities of the Gynecology Department, Clinical Diagnostic Laboratory, Morphological Department of the Central Scientific-Research Laboratory, Departments of Obstetrics and Gynecology No. 1 and Microbiology and Virology No. 1 of RostMSU of the Ministry of Health of the Russian Federation in the period from 2016 to 2019. The study was approved by the local independent ethics committee (protocol No. 19/16 of October 4, 2016). The study design included a comprehensive examination of 83 women with diagnosed UFs aged 26 to 50 years old (mean age 36.6±0.8 years old).
Inclusion criteria were UFs diagnosed by bimanual and ultrasound examinations; interstitial, interstitial-subserosal localization of MNs; MNs ≥5 cm in size; the presence of menses; a patient's written consent to participate in the study.
Exclusion criteria were taking hormonal (within 6 months before the study) and antibacterial drugs (within 1 month before the study), pelvic cancer, and a patient's refusal to participate in the study.
Patients were divided by age into three groups. Group I (n=19) included patients aged 26–34 years old, Group II (n=48) –35–44 years old, and Group III (n=16) – 45-50 years old.
The office evaluation of the patients included standard clinical and clinical-laboratory examinations and simple and extended colposcopy.
According to Guideline 4.2.2039-05 (2006), the vaginal discharge from the posterior vaginal fornix (VDPVF) was taken from each patient 24 hours before the operation. Intraoperatively, biopsy specimens of MNs and their beds (MNBs) were also taken for microbiological examination. VDPVF specimens were taken with a sterile swab (Sterile Pure Viscose Swab w/Polypropylene Stick), placed in a medium (Hiculture Transport Swabs w/Alternative Thioglycollate Medium), and transported to the laboratory (30–60 min). The cultural study was performed according to the corresponding technique [25] using nutrient media for both aerobic (MacConkey Agar, HiCrome Klebsiella Selective Agar Base, HiCrome Candida Differential Agar, HiCrome Enterococci Agar, HiCrome Aureus Agar Base, Blood Agar Base, Streptococcus Selection Agar) and anaerobic (dense nutrient media Bifidobacterium Agar, MRS Agar, Anaerobic Agar, Schaedler Agar, Bacteroides Bile Esculinum Agar, liquid – Shaedler Broth and semi-liquid Blaurock nutrient media) microbiota taxa. Cultivation was performed under aerobic (t -37 ºC, 24–48 h) and anaerobic (AnaeroHiGas Pak, t ‑37 ºC, 48–72 h) conditions. Identification of the isolated microorganisms was performed according to common methods.
Intraoperatively, biopsy samples (0.5×0.5 cm) of MNs and MNBs were taken into Eppendorf tubes containing thioglycolic buffer (1 ml) and transported to the laboratory for not more than 60 minutes. Then, the test material was homogenized (Becton Picrinson Mediamachine System). Bacteriological examination was performed on nutrient media for aerobic and anaerobic microorganisms described above under appropriate cultivation conditions. Identification of microorganisms isolated from biopsy specimens was also performed according to generally accepted methods.
The morphological examination was performed by the traditional method with the preparation of paraffin sections of the studied material (MNs, MNBs) and stained with hematoxylin-eosin and Van Gieson’s picrofuchsin. A microscope Micros (Austria) at 300× magnification was used for a microscopic study of the preparations.
A statistical analysis of the results was performed using the statistical processing and visualization environment R ver 3.2 (R Foundation for Statistical Computing, Vienna, Austria). The quantitative characteristics of microorganisms verified at different loci were presented as median and lower and upper quartiles (the Kruskal-Wallis test was used for comparison). Pearson’s c2 and Fisher’s tests were used to compare the reliability of differences. Spearman’s rank correlation coefficient was used to assess the closeness of the relationship between certain attributes.
Results
Pain syndrome dominated in the structure of clinical manifestations of UFs, which was more frequently (p<0.05) registered in Group III patients (Figure 1). In the same group, dysuric disorders were detected in more than 1/3 of cases (p<0.05).
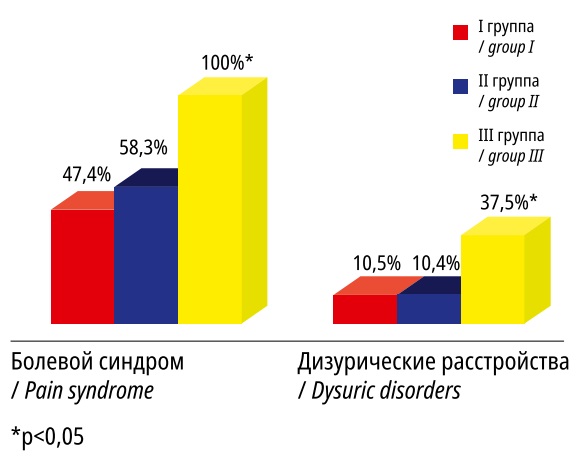
Рисунок 1. Значимые отличия в клинических проявлениях миомы матки у пациенток I–III групп
Figure 1. Significant differences in the clinical manifestations of uterine fibroids in patients of groups I–III.
The number of MNs in the patients of the studied groups ranged from min 1 to max 22. The mean numbers were 2.4±0.5 in Group I, 5.2±0.8 in Group II, and 3.3±0.6 in Group III. Multiple UFs were verified in 42.1% of the patients in Group I, 70.8% – in Group II, 81.3% – in Group III. In single UFs, the mean node size was 10.9±0.7 cm; in multiple UFs, the mean minimum node size was 2.3±0.5 cm; the mean maximum node size was 9.7±0.7 cm. There were no significant differences in the duration of operations and amount of blood loss in the studied groups (p>0.05).
The main symbionts of this biotope (lactobacilli) were absent in Group III patients during the bacteriological study of VDPVF. The detection rate (p<0.05) and quantitative characteristics (p>0.05) of these microorganisms were lower in Group II compared to those in Group I (Table 1). The narrower spectrum of microorganisms verified in the VDPVF in Group III, with the absence of not only lactobacilli but also bifidobacteria, peptococci, and yeast-like fungi of the Candida genus, is noteworthy.
The dominating cluster of anaerobic microorganisms in Groups I and II was Eubacterium spp., and in Group III, Peptostreptococcus spp. The rate of detection of Bacteroides spp. was significantly higher (p<0.05) in Group III compared to the results obtained in Groups I and II. Among the aerobic component of the vaginal microbiocenosis, coagulase-negative staphylococci (CNS) dominated in all groups with a 100.0% detection in Group III. Streptococcus spp. was significantly more frequently (p<0.05) verified in this group (81.3%) compared with the same figures in Groups I and II (42.1% and 50.0%, respectively). Candida yeast-like fungi were absent in the vaginal biotope in Group III but their detection rate was significantly (p<0.05) higher in Group I. E. coli was detected only in patients from Group II. Eubacterium spp., Peptostreptococcus spp., and CNS were the most stable associates of the vaginal biotope in the patients of the studied groups.
The following circumstance is noteworthy in the analysis of quantitative indices: Group II patients had significantly higher (p<0.05) mean values (median) for Peptostreptococcus spp., Bacteroides spp., Candida spp., and Group III patients had lower (p<0.05) Corynebacterium spp. compared to similar parameters in other groups.
Таблица / Table 1
Микробный спектр влагалища при миоме матки у пациенток I–III групп
Microbial spectrum of the vagina with uterine myoma in patients of groups I–III
|
Микроорганизмы Microorganisms |
I группа I group |
II группа II group |
III группа III group |
|||
|
Частота обнаружения Detection frequency (%) |
Концентрация Concentration (lg КОЕ/т) |
Частота обнаружения Detection frequency (%) |
Концентрация Concentration (lg КОЕ/т) |
Частота обнаружения Detection frequency (%) |
Концентрация Concentration (lg КОЕ/т) |
|
|
Lactobacillus spp. |
42,1* |
3,4 [ 3; 6] |
16,7 |
2,5 [ 2; 3] |
0 |
0 |
|
Eubacterium spp. |
94,7 |
6,2 [ 5; 8] |
91,7 |
5,8 [ 3; 8] |
25,0* |
5,7 [ 3; 8] |
|
Peptostreptococcus spp. |
84,2 |
3,9 [ 2; 7] |
66,7* |
5,1* [ 3; 7] |
75,0 |
4,2 [ 2; 8] |
|
Peptococcus spp. |
57,9* |
5,3 [ 3;7,5] |
20,8 |
4,8 [ 3; 6] |
0 |
0 |
|
Propionibacterium spp. |
42,1* |
4,1 [ 2; 6] |
29,2 |
4,8 [ 2,5;6] |
31,3 |
5,2* [ 3; 7] |
|
Bacteroides spp. |
15,8 |
3,0 [ 2; 4] |
4,2 |
6,0* [ 4; 7] |
56,3* |
3,0 [ 2; 4] |
|
Bifidobacterium spp. |
10,5 |
2,5 [ 2; 3] |
20,8* |
2,0 [ 2;3,5] |
0 |
0 |
|
КОС |
73,7 |
3,3 [ 2; 6] |
70,8 |
2,8 [ 2; 4] |
100,0* |
2,9 [ 2; 5] |
|
Streptococcus spp. |
42,1 |
2,1 [ 2; 3] |
50,0 |
3,5 [ 2; 6] |
81,3* |
4,0 [ 3; 5] |
|
Corynebacterium spp. |
36,8 |
4,0 [ 3; 5] |
45,8 |
3,4 [ 2,5;6,5] |
31,3* |
2,4* [ 2; 4] |
|
Candida spp. |
26,3* |
5,2 [ 3,5;7] |
4,2 |
7,0* [ 6; 8] |
0 |
0 |
|
E.сoli |
0 |
0 |
20,8 |
3,8 [ 2; 6] |
0 |
0 |
Note: *p<0.05 — significant difference between groups, mean values are presented in the table as Median [Lower quartile; Upper quartile]; the comparison was carried out using the Kruskal-Wallis test.
The bacteriological study of MNs (size ≥5 cm) in patients in the three groups did not reveal microbial growth in 7.2% of cases, which, in the authors’ opinion, is not related to the sterility of these loci but to the lack of technical possibilities of culturing. Microorganisms from MNs were isolated in monovariants (20.8%), as well as in bacterial associations – two-component (57.1%) and three-component (22.1%) ones.
It should be noted that the MN microbiota was primarily represented by anaerobic taxa (Figure 2). The exception was E.coli that were detected in both MNs and VDPVF in Group II. Peptococcus spp. (68.4%) dominated in the studied locus in Group I, Peptococcus spp. and Peptostreptococcus spp. – in Group II (45.8% and 41.7%, respectively), and Bacteroides spp. – in Group III (56.3%). Propionibacterium spp. were absent in MNs in Group III patients, but they were recorded in VDPVF (31.3%). Veillonella spp. were absent in the VDPVF in the study groups, but they were isolated from the MNs in Groups I and II.
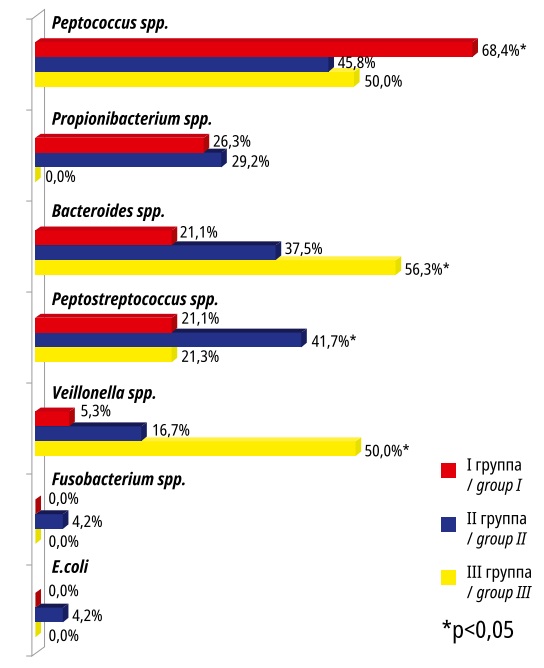
Рисунок 2. Микробные паттерны миоматозных узлов в исследуемых группах
Figure 2. Microbial patterns of myomatous nodes in the studied groups
The quantitative characteristics of MN infestation (median) did not differ significantly (p>0.05) in the studied groups, with a mean value (lg 2.0±0.5 CFU/biopsy specimen) and an interquartile range of 2.0 [lower quartile] to 3.0 [upper quartile].
The bacteriological study of MNBs did not reveal any microbial growth in 17.7%; monovariants were registered in 26.6%, 2-component bacterial associations – in 43.0%, and 3-component or more – in 12.7%. Taxonomic characteristics of microorganisms isolated from MNBs were similar to those isolated from MNs but with some variations in the frequency of detection of certain microbial taxa (Figure 3).
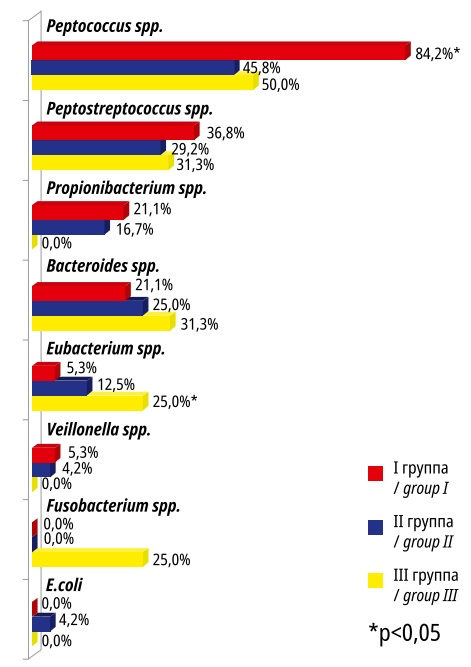
Рисунок 3. Микробиота ложа миоматозных узлов в исследуемых группах
Figure 3. Microbiota of the bed of myomatous nodes in the studied groups
Peptococcus spp. dominated in the MNB specimens in the studied groups. The rate of Eubacterium spp. detection was significantly (p<0.05) higher in Group III compared to Groups I and II and there were no Propionibacterium spp. and Veillonella spp. The presence of E. coli was similarly recorded only in Group II.
The bacterial content in MNB (median) samples in the studied groups did not differ significantly (p>0.05), being lg 2.4±0.6 CFU/biopsy specimen.
The correlation associations (CAs) between microorganisms verified in the loci "vagina – MN", "vagina – MNB" were studied; the significant values are presented in Table 2. The obtained CAs were direct in most cases, and only in Group III, it was reverse for Eubacterium spp. and Peptostreptococcus spp., which indirectly indicates the interrelation between the studied loci. The question arises if the fact of detection of microorganisms in the MN and its bed is associated with an infectious and inflammatory process in the tissues. To answer this question, the authors carried out a morphological study of the biopsy specimens, which proved the absence of an inflammatory tissue reaction (Figures 4, 5).
Таблица/ Table 2
Корреляционные связи в биотопах «влагалище – миоматозный узел», «влагалище – ложе миоматозного узла»
Correlations in biotopes "vagina – myomatous node", "vagina – bed of myomatous node"
|
Микроорганизмы Microorganisms |
I группа I group |
II группа II group |
III группа III group |
||||||||||
|
r1 |
p1 |
r2 |
p2 |
r1 |
p1 |
r2 |
p2 |
r1 |
p1 |
r2 |
p2 |
||
|
E.coli |
- |
- |
- |
- |
0,79 |
<0,001 |
0,79 |
<0,001 |
- |
- |
- |
- |
|
|
Eubacterium spp. |
- |
- |
0,86 |
<0,001 |
0,66 |
<0,001 |
0,57 |
<0,001 |
-0,34 |
>0,05 |
0,05 |
>0,05 |
|
|
Peptostreptococcus spp. |
0,63 |
<0,01 |
0,59 |
<0,01 |
0,4 |
<0,01 |
0,46 |
<0,001 |
-0,07 |
>0,05 |
-0,07 |
>0,05 |
|
|
Peptococcus spp. |
0,69 |
<0,01 |
0,49 |
<0,05 |
0,28 |
>0,05 |
0,39 |
<0,01 |
- |
- |
- |
- |
|
|
Propionibacterium spp. |
0,8 |
<0,001 |
0,62 |
<0,01 |
0,24 |
>0,05 |
0,38 |
<0,01 |
- |
- |
- |
- |
|
|
Bacteroides spp. |
0,88 |
<0,001 |
0,41 |
>0,05 |
0,54 |
<0,001 |
0,78 |
<0,001 |
0,3 |
>0,05 |
0,71 |
<0,01 |
|
Note: r1, p1 - indicators for the locus "vagina - myomatous node", r2, p2 - indicators for the locus "vagina - bed of the myomatous node"
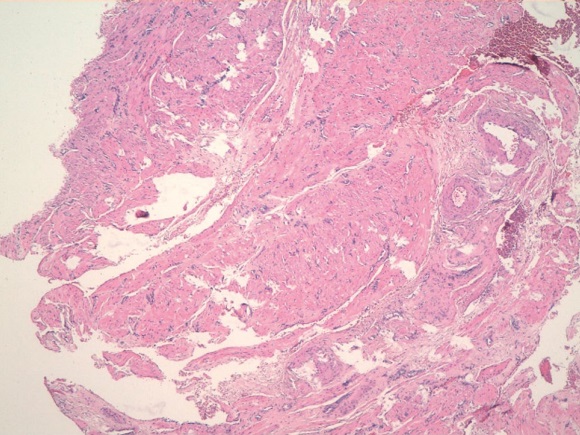
Рисунок 4. Миоматозный узел. Клеточная лейомиома. Опухоль представлена пучками разнонаправленно идущих гладкомышечных волокон. Окраска гематоксилином-эозином. Ув.100
Figure 4. Myomatous node. Cellular leiomyoma. The tumor is represented by bundles of multidirectional smooth muscle fibers. Stained with hematoxylin-eosin. Magnification 100
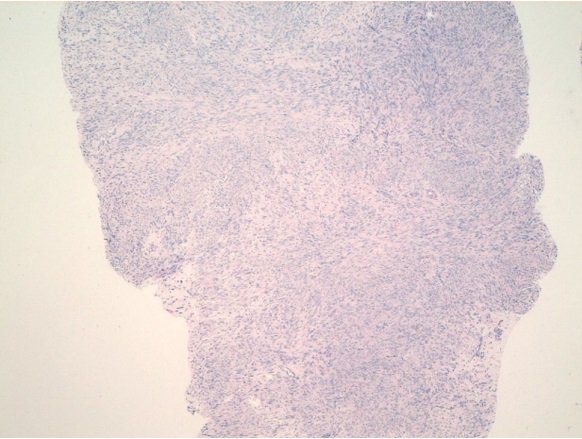
Рисунок 5. Ложе узла. Пласты мышечных волокон, расположенных в циркулярном и косом направлении. Между мышечной тканью грубоволокнистая соединительная ткань, крупные толстостенные артерии. Окраска гематоксилином-эозином. Ув.100
Figure 5. Knot bed. Layers of muscle fibers located in the circular and oblique direction. Between the muscle tissue coarse fibrous connective tissue, large thick-walled arteries. Stained with hematoxylin-eosin. Magnification 100
It should be noted that Group I, II, and III patients had no infectious-inflammatory complications in the postoperative period.
Discussion
Patients in the study groups had multidirectional data on the detection rate of various taxa of the vaginal microbiota, in particular lactobacilli. These microorganisms were absent in patients from Group III who were in the age range of 45–50 years old, i.e. in the premenopause, but whose cluster of anaerobic microorganisms had dominating Bacteroides spp., which agrees with the data published by Muhleisen and Herbst-Kralovetz (2016) [26]. Despite the fact that lactobacilli primarily dominate the vaginal biotope and perform a number of positive functions [27, 28], their detection rates were reduced in patients with UFs in Groups I and II. The vaginal microbiota is very plastic and any pathological condition, in particular UFs, changes the compositions of microbial communities in women of different age groups.
To date, there are data on the microbiota and/or microbiome of the uterus, placenta, fallopian tubes, and ovaries [29–31]. The bacterial taxa that prevail in different types of vaginal microbiota were described [27, 28]. However, the microbiota of MNs remains understudied. The present study only slightly uncovers this intriguing and interesting issue, since the obtained data indicate that MNs and their beds are non-sterile, and there are no signs of an infectious and inflammatory process in this cohort of subjects. However, the scenario of infectious-inflammatory complications can unfold along a different vector, when normal symbionts of the vaginal biotope, MN, and MNB, under certain conditions can be the manifestants of infectious-inflammatory complications.
Further studies should be aimed at expanding the cohort of subjects of different ages with UFs, including pregnant women with this pathology, by isolating patients, if any, with various infectious-inflammatory complications in the postoperative period, and conducting a comprehensive comparative analysis of the microbiota of the studied loci in the groups with and without complications.
Conclusion
- In women with UFs of different age groups, multidirectional changes in the vaginal microbiota were revealed.
- In the majority of cases, the UF (92.8%) and its bed (82.3%) are not sterile with the dominance of anaerobic microbiota taxa. The detected significant correlations in the loci "vagina – MN – MNB" testify to their interrelation.
- The detection of different microbial taxa in the UF and its bed, according to morphological studies, is not associated with the presence of infectious and inflammatory processes in the tissues.
References
1. Goldenberg RL, Klebanoff MA, Nugent R, Krohn MA, Hillier S, Andrews ww. Bacterial colonization of the vagina during pregnancy in four ethnic groups. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1996;174(5):1618- 21. Doi: 10.1016/s0002-9378(96)70617-8.
2. Bebneva T.N., Letunovskaya A.B. Lactobacillus and estriol in maintenance of vaginal biocenosis. Farmateka. 2010;(9):24–28. (In Russ.). eLIBRARY ID: 15192731
3. Klimanov A. Yu. Features of organ-preserving surgical treatment of uterine fibroids by laparoscopic access in women of reproductive age: Abstract of the thesis. dis. … Candidate of Medical Sciences. Samara. 2012. - 24 p. (In Russ.)
4. Gajer P, Brotman RM, Bai G, Sakamoto j, Schütte UM, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. DOI: 10.1126/scitranslmed.3003605.
5. Hickey Rj, Zhou x, Pierson jD, Ravel j, Forney Lj. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res. 2012;160(4):267-82. DOI: 10.1016/j.trsl.2012.02.008.
6. Dobrokhotova Yu.E., Ibragimova D.M., Saprykina L.V. Uterine fibroids. Moscow: GEOTAR-Media; 2018. (In Russ.)
7. Dobrokhotova Yu.E., Ilyina I.Yu., Ibragimova D.M., Narimanova M.R. Uterine fibroids: alternative treatments. Reproduction problems. 2018;24;2:83-87. (In Russ.) DOI: 10.17116/repro201824283-87
8. Yu O, Scholes D, Schulze-Rath R, Grafton j, Hansen K, Reed SD. A US population-based study of uterine fibroid diagnosis incidence, trends, and prevalence: 2005 through 2014. Am J Obstet Gynecol. 2018;219(6):591.e1-591.e8. DOI: 10.1016/j.ajog.2018.09.039.
9. 9 Altukhova O.B., Radzinsky V.E., Polyakova I.S., Churnosov M.I. The role of growth factor genes in the development of uterine fibroids in combination with endometrial hyperplasia. Obstetrics and gynecology. 2021; 4:104 - 110. (In Russ.) DOI: 10.18565/aig.2021.4.104-110
10. Mäkinen N, Heinonen HR, Moore S, Tomlinson IP, van der Spuy ZM, Aaltonen LA. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011;2(12):966-9. DOI: 10.18632/oncotarget.370.
11. Samsonov A.E., Rymashevsky A.N., Volkov A.E., Terekhina L.A. Features of the effect of uterine fibroids on the course of pregnancy. Tauride Medical and Biological Bulletin. 2013; 16:2(1):205-207. (In Russ.) eLIBRARY ID: 22807263
12. Radzinsky V.E., Totchiev G.F. Uterine fibroids: a course on organ preservation. News bulletin. Moscow: Editorial staff of Status Praesens; 2014. (In Russ.)
13. Biglia N, Carinelli S, Maiorana A, D'Alonzo M, Lo Monte G, Marci R. Ulipristal acetate: a novel pharmacological approach for the treatment of uterine fibroids. Drug Des Devel Ther. 2014;8:285-92. DOI: 10.2147/DDDT.S54565.
14. Kiselev V.I., Radzinsky V.E., Shalaev O.N., Eseneeva F.M., Poloznikov A.A., et al. Characteristics of DNA-methylation in uterine fibroids. Molecular medicine. 2017;15(3):45-50. (In Russ.) eLIBRARY ID:29328837
15. Mas A, Tarazona M, Dasí Carrasco j, Estaca G, Cristóbal I, Monleón j. Updated approaches for management of uterine fibroids. Int J Womens Health. 2017;9:607-617. DOI: 10.2147/IjwH.S138982.
16. Boricheva D.A., Matsarskaya M.D., Shepovalova N.S. Uterine fibroids as a consequence of hormonal imbalance. Synergy of Sciences. 2018;20:650-663. (In Russ.) eLIBRARY ID:32484393
17. Voskresenskiy S., Grudnitskaya E., Tesakova M., Nebyshinec L., Shoroch I., Kunickaya O. Clinical and hormonal changes in uterine fibroid in a reproductive age. Reproductive health Eastern Europe. 2018;8(2):155-162. (In Russ.) eLIBRARY ID:32792719
18. Altukhova O.B., Radzinsky V.E., Polyakova I.S., Churnosov M.I. Involvement of estrogen and progesterone receptor gene polymorphisms in the development of uterine fibroids. Obstetrics and gynecology. 2020;3:127-132. (In Russ.) DOI: 10.18565/aig.2020.3.127-132
19. armolinskaya M.I., Polenov N.I., Kunitsa V.V. Uterine fibroids: the role of signaling pathways in the pathogenesis. A literature review. Journal of obstetrics and women’s diseases. 2020;69(5):113-124. (In Russ.). DOI: 10.17816/jOwD695113-124
20. Reis FM, Bloise E, Ortiga-Carvalho TM. Hormones and pathogenesis of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:13-24. DOI: 10.1016/j.bpobgyn.2015.11.015.
21. Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153(1):11-9. DOI: 10.1093/aje/153.1.11.
22. Sośnik H, jeleń M, Kosiński M, Sośnik K. Research on genesis of adipocytic metaplasia in uterine fibroids. Pol J Pathol. 2015;66(4):403-9. DOI: 10.5114/pjp.2015.57254.
23. Tikhomirov A.L., Grishin G.P., Lubnin D.M., Zinin D.S., Kocharyan A.A. Modern organ-preserving treatment of uterine fibroids. Consilium medicum. 2013;10(6):19–23. (In Russ.) eLIBRARY ID: 20267593
24. Tabarina E.P., Potaturkina-Nesterova N.I. The association of hysteromyoma with ureoplasm infection. Kazan Medical Journal. 2009; 90(3):408-411. (In Russ.). eLIBRARY ID: 12906435
25. Menshikov V.V. Methods of clinical laboratory research: Reference manual. T. 3. Moscow: Labora; 2009. (In Russ.)
26. Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42-50. DOI: 10.1016/j.maturitas.2016.05.015.
27. Kroon Sj, Ravel j, Huston wM. Cervicovaginal microbiota, women's health, and reproductive outcomes. Fertil Steril. 2018;110(3):327-336. DOI: 10.1016/j.fertnstert.2018.06.036.
28. Peelen Mj, Luef BM, Lamont RF, de Milliano I, jensen jS, et al. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta. 2019;79:30-39. DOI: 10.1016/j.placenta.2019.03.011.
29. Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. Human vaginal pH and microbiota: an update. Gynecol Endocrinol. 2018;34(6):451-455. DOI: 10.1080/09513590.2017.1407753.
30. Peric A, weiss j, Vulliemoz N, Baud D, Stojanov M. Bacterial Colonization of the Female Upper Genital Tract. Int J Mol Sci. 2019;20(14):3405. DOI: 10.3390/ijms20143405.
31. Kalinkina O.B., Tezikov Y.V., Lipatov I.S., Aravina O.R., Morozova O.N. Study of urogenital tract and gut microbiota in women with stage III and IV ovarian endometriosis. Perm Medical Journal. 2020;37(1):14-21. (In Russ.). DOI: 10.17816/pmj37114-21
About the Authors
E. S. NikitinaRussian Federation
Ekaterina S. Nikitina, Cand. Sci. (Med.), Assistant of the Department of Obstetrics and Gynecology No. 1
Rostov-on-Don
A. N. Rymashevsky
Russian Federation
Alexander N. Rymashevsky, Dr. Sci. (Med.), professor, Head of the Department of Obstetrics and Gynecology No. 1
Rostov-on-Don
Y. L. Naboka
Russian Federation
Yulia L. Naboka, Dr. Sci. (Med.), Professor, head of Department of microbiology and virology №1
Rostov-on-Don
M. A. Rymashevsky
Russian Federation
Mikhail A. Rymashevsky, Cand. Sci. (Med.), Assistant of the Department of Obstetrics and Gynecology No. 1
Rostov-on-Don
I. A. Gudima
Russian Federation
Irina A. Gudima, Dr. Sci. (Med.), associate professor, professor of the Department of microbiology and virology №1
Rostov-on-Don
E. G. Svirava
Russian Federation
Eteri G. Svirava, Cand. Sci. (Med.), Assistant Professor of Microbiology and Virology Department No. 1
Rostov-on-Don
Review
For citations:
Nikitina E.S., Rymashevsky A.N., Naboka Y.L., Rymashevsky M.A., Gudima I.A., Svirava E.G. Microbiota of the vagina and myoma nodes in uterine myoma. Medical Herald of the South of Russia. 2022;13(2):50-58. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-50-58
JATS XML







































