Scroll to:
Evaluation eff ects of IDPP4 therapy diff erent duration on the functional state of α- and β- cells in patients with type 2 diabetes mellitus
https://doi.org/10.21886/2219-8075-2022-13-1-88-97
Abstract
Purpose: evaluate the eff ects of DPP4i on fasting and postprandial insulin and glucagon secretion by examining basal secretion and response to food loading.
Materials and methods: patients (n = 54) were divided into treatment groups: long-term (more than a year) therapy with iDPP4 with Metformin, Metformin + sulfonylurea, fi rst-time therapy with iDPP4. Biochemical parameters, levels of insulin, glucagon, C-peptide before and aft er a standard breakfast were measured. Th e HOMA IR and HOMA β indices were calculated. Results: we obtained a signifi cant diff erence in fasting glucagon and insulin levels between the iDPP4 over a year and Metformin + SM groups. In addition, insulin levels before and aft er standard breakfast, C-peptide aft er standard breakfast, and fasting glucagon decreased aft er 6 months of fi rst-time DPP4 therapy.
Summary: the data obtained indicate the ability of iDPP4 to positively infl uence the two earliest and most signifi cant links in the pathogenesis of type 2 diabetes.
Keywords
For citations:
Tuchina T.P., Kolchanova I.A., Meltonyan A.R., Abramyan L.K., Babenko A.Yu., Galagudza M.M. Evaluation eff ects of IDPP4 therapy diff erent duration on the functional state of α- and β- cells in patients with type 2 diabetes mellitus. Medical Herald of the South of Russia. 2022;13(1):88-97. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-1-88-97
Introduction
Currently, the number of patients with diabetes mellitus type 2 (DM2) is constantly increasing worldwide. DM2 is one of the socially significant diseases that indirectly leads to the patient’s death because of complications. The main pathogenic factors of DM2 include insulin resistance and hyperinsulinemia [1].
According to the latest data, not only insulin resistance by HOMA-IR but also hyperinsulinemia (insulin level > 15 mU/L) are associated with an increased risk of cardiovascular diseases and episodes. Dipeptidyl peptidase 4 inhibitor (DPP4i) is one of the modern groups of drugs that exerts a complex effect on the mechanisms of DM2 development and progression. It has been shown that this group of drugs positively affects insulin secretion. However, apart from the experimentally and clinically proven positive effect on the secretory function of β-cells, researchers discussed a decrease in the activity of α-cells through the improvement of incretin secretion [2].
These effects were evaluated in several randomized clinical studies. In these studies, DPP4i were indicated as a monotherapy or in addition to other classes of antihyperglycemic drugs. Often, the evaluation included only insulin concentration and fasting glucagon. There were no such studies in real clinical practice, so the authors conducted a study to evaluate the effects of DPP4i on fasting and postprandial insulin and glucagon secretion by examining basal secretion and response to food loading. It is known that postprandial metabolic disorders affect the progression of endothelial dysfunction. Thus, the correction of postprandial changes has a high clinical significance [3][4]. Besides, the authors evaluated the effects of DPP4i therapy of various durations, including the patients in the study at the start of DPP4i therapy and during long-term therapy with this group of drugs.
The authors studied the secretory function of β-cells by the evaluation of the dynamics of insulin and C-peptide levels before and after food loading and by the HOMA-β index. The involvement of different methods is determined by the fact that the HOMA index provides a correct evaluation of insulin resistance and the reserve of β-cells in patients at prediabetic stages. In patients with DM2 who received various variants of therapy and had very variable glucose levels, the precision of these methods is low. This determined the necessity in the evaluation of the dynamics of insulin and C-peptide in the probe with a standard breakfast in this study. The levels of glucagon before and after a standard breakfast were used to evaluate the function of α-cells.
As was mentioned above, insulin resistance is one of the main pathogenetic factors of DM2 development and complications. Its evaluation was one of the study objectives. Insulin resistance was evaluated by the HOMA-IR index.
The study aimed to evaluate the effects of DPP4i on fasting and postprandial insulin and glucagon secretion by examining basal secretion and response to food loading.
Materials and Methods
All the patients included in the study signed a form of informed consent. The study design was approved by the local ethical committee of the Almazov National Medical Research Center. From April 2019 to December 2020, 54 patients with DM2 that met the study entry criteria were included in a cross-sectional study. Patients from group I received DPP4i therapy for over a year. Patients from group II first started DPP4i therapy in addition to Metformin. The control group received a combination of Metformin and sulphonylurea (SU) for over a year. The clinical study design is presented in Fig. 2.
The evaluated biochemical parameters are presented in Table 1.
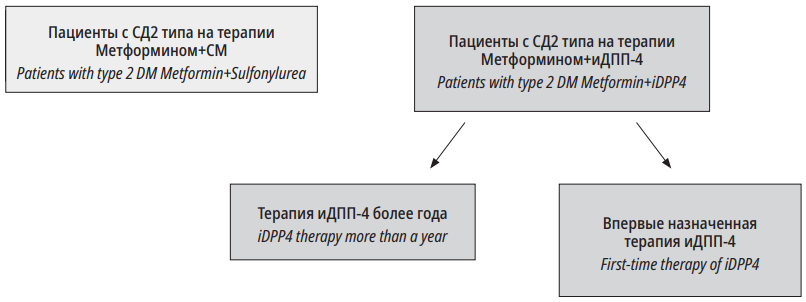
Рисунок 1. Дизайн исследования
Figure 1. Research design
Таблица / Table 1
Дизайн исследования
Research design
|
Исследования / Researches Группы / Groups |
Глюкоза крови, гликированный гемоглобин, инсулин, глюкагон, С-пептид натощак и после стандартного завтрака Blood glucose, glycated hemoglobin, insulin, glucagon, C-peptide on an empty stomach and after a standard breakfast |
||
|
Исходно 1st visit |
6 мес. 6 months |
12 мес. 12 months |
|
|
Метформин+СМ, n=18 Metformin+sulfonylurea, n=18 |
+ |
|
|
|
Метформин+иДПП4(более года), n=18 Metformin+IDPP4(more than a year), n=18 |
+ |
|
|
|
Метформин+иДПП4(впервые), n=18 Metformin+IDPP4(for the first time), n=18 |
+ |
+ |
|
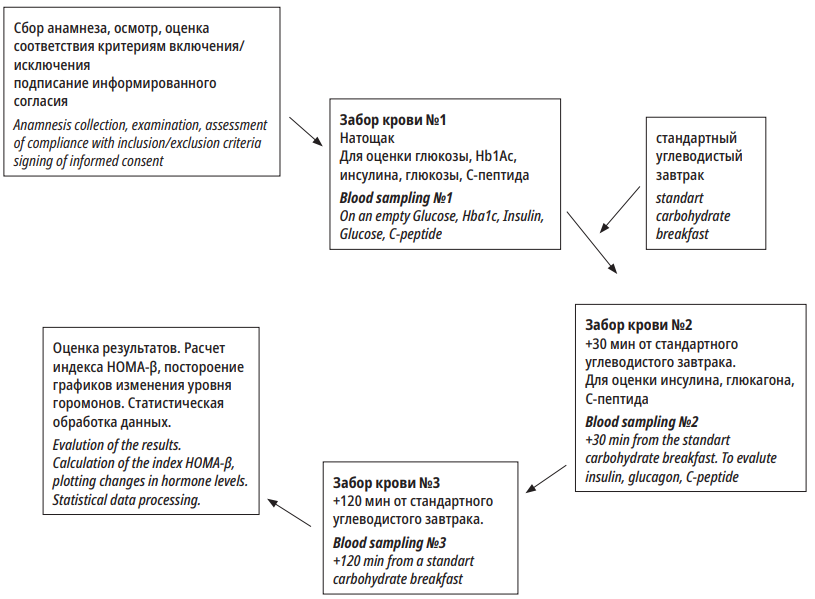
Рисунок 2. Схема визита
Figure 2. Visit plan
General characteristics of the examined participants and study design
Study entry criteria:
- Duration of DM2 > 3 years.
- Patients’ age 45–75 years old.
- Stable hypotensive, hypolipidemic therapy for 3 months before the inclusion in the study.
For the group Metformin+DPP4i (>1 year) – patients who received therapy with biguanides in combination with DPP4i drugs for over a year.
For the group Metformin+DPP4i (first prescription) – patients on Metformin monotherapy who were first prescribed DPP4i.
For the group Metformin+SU – patients with DM2 that received therapy with biguanides in combination with SU for over a year and who never received DPP4i.
Criteria for study exclusion
- Serious decompensation of comorbid diseases.
- Absolute insulinopenia (C-peptide < 0.25 ng/ml).
- Insulin therapy, long-term therapy with other groups of antidiabetic drugs (for over 3 months and within < 6 months before the inclusion in the study).
- Uncorrectable dyslipidemia.
- Alcohol abuse.
- BMI ≥40 kg/m².
- Oncological diseases in medical history without sustained remission for 2 years.
- Systemic autoimmune diseases, immune depressant therapy, immune modulation therapy, biological drugs therapy, etc.
- Patient’s low adherence to the therapy.
- Pregnancy, lactation.
- Intolerance to Metformin/SU/DPP4i components.
Criteria of preterm withdrawal from a prospective study:
- Major surgical interventions, vast injuries, severe infections, acute conditions.
- The indication/change of antihypertensive, hypolipidemic therapy during the study.
The patients’ examination included medical history, complaints, medical records evaluation, analysis of constant pharmacotherapy, and stoking status. Self-control of glycemia by the patients and compensation of DM2 were also analyzed.
Patients with comorbid diseases, including cardio-vascular pathology, continued their earlier indicated therapy.
Blood pressure was measured twice with a tonometer Healthcare CS-105 (Russia) after a 5-minute rest with a 10-minute interval. The analysis included the mean values. The patient's height was measured with a height meter RM-1 Diacoms. Body mass was measured with medical electronic weights VEM-150-Massa-K once with a precision of up to 100g.
Laboratory methods
Laboratory tests were performed in a laboratory LRK-1/KPK of Almazov National Medical Research Centre(Roche Diagnostics, USA) with commercial kits Cobas Integra, Roche (Germany). Blood probes for insulin, glucagon, and C-peptide were taken with K3EDTA vacutainers and aprotinin. The transportation of blood probes from the unit for medical procedures to the lab was organized in a cool accumulator container (Severok 400, Russia). Serum was obtained by centrifuging for 15 minutes at 3000 rpm, 4 °C. The obtained serum was stored at -70 °C. After defrosting, insulin, glucagon, and C-peptide were detected in the serum (“Peninsula”, Elisa method). The norm of glycated hemoglobin was up to 6%. The fasting insulin level in norm was 22–123 pmol/L.
There are no norms for the postprandial insulin level. However, it is believed that a two-fold increase in the level of insulin in comparison with the basal level is a sign of a saved reserve of β-cells. Normal values for C-peptide are 0.78–1.89 ng/ml. Reference values of glucagon were 75–140 pg/ml.
To calculate the HOMA IR index, the following formula was used: fasting insulin*fasting glucose/22.5. To evaluate the reserve of the function of β-cells, the authors used HOMA β = 20 × fasting insulin / (glucose fasting – 3.5). It is believed that the norm for HOMA β in a healthy person older than 35 years old is 100%, and the norm for HOMA IR in the same conditions is -1.0. Insulin resistance is detected at HOMA IR > 2.5.
Statistical analysis
The obtained data were analyzed in the software package IBM SPSS STATISTICA for Windows. The data were compared with the Mann-Whitney test. The results were presented as medians and quartiles (Me, 25% quartile – 75% quartile). The results were statistically significant at Bonferroni adjusted p<0.05. A model of linear regression was used for multi-variant analysis.
Results
The authors compared the level of secretion of hormones that characterize the function of the pancreas, the level of glycated hemoglobin, and the HOMA β and HOMA IR indices in patients on long-term therapy with DPP4i+Metformin and in patients in the control group who received Metformin+SU. Besides, the authors studied the dynamics of the changes in the secretion of hormones, levels of glycated hemoglobin, and the HOMA β and HOMA IR indices in the group of patients who started DPP4i therapy for the first time. Table 2 contains the data by the groups and time of parameters measurement.
Таблица / Table 2
Полученные результаты измерения уровней инсулина, глюкагона, С-пептида, гликированного гемоглобина, индексов HOMA β и HOMA IR по группам, до и через 30 и 120 мин. после стандартного завтрака
The obtained results of measuring the levels of insulin, glucagon, c-peptide, glycated hemoglobin, HOMA β and HOMA IR indices by groups, before and after 30 and 120 minutes after a standard breakfast
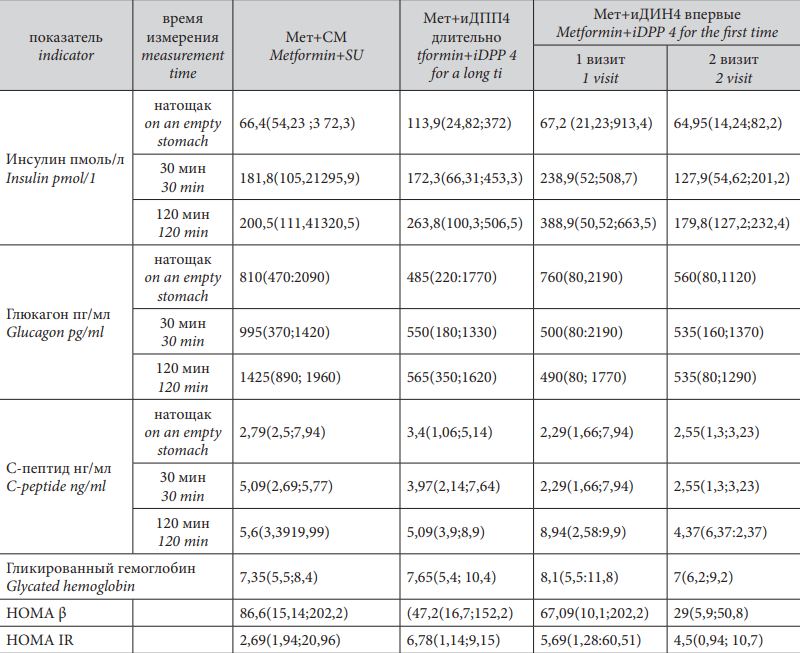
The levels of glycated hemoglobin did not differ in the group of patients who received Metformin+SU (7.35(5.5;8.4)) and Metformin+DPP4i (7.65(5.4;10.4)), p>0.05.
In the group of patients who received DPP4i for the first time in addition to Metformin, the level of glycated hemoglobin during the first visit was 8.1(5.5;11.8), and by the second visit, its level significantly decreased to 7(6.2;9.2), p<0.05.
Evaluation of insulin secretion
The authors revealed significant differences in the levels of fasting insulin in the groups of patients who received Metformin and DPP4i for a long time (113.9(24.82;372 pmol/L) and Metformin+SU (66.4(54.23;372.3 pmol/L) (p<0.05). However, it should be mentioned that in both groups, this parameter was within the normal values.
There were no significant differences revealed in the postprandial level of insulin.
At the same time, the comparison of the dynamics of fasting and postprandial insulin levels before the introduction of DPP4i into the therapy and 6 months after the beginning of the therapy revealed statistically significant differences both in the fasting insulin levels and postprandial dynamics. Fasting insulin levels significantly decreased (1st visit – 67.2 (21.23;913.4) pmol/L, 2nd visit – 64.95(14.24;82.2) pmol/L, p=0.0425. However, both parameters were within the reference values for insulin.
Thirty minutes after food loading, the insulin levels were significantly higher (238.9 (52;508.7)) during the 1st visit than during the 2nd visit (127.9 (54.62;201.2)), p <0.05.
During the 1st visit, 120 minutes after the food loading, the level of insulin was again significantly higher (388.9 (50.52;663.5)) than during the 2nd visit (179.8 (127.2;232.4)), p <0.05.
It should be noted that these patients (before DPP4i prescription) had a significantly higher level of glycemia (HbA1c 8.1).
Evaluation of C-peptide secretion
The comparison of the baseline levels of C-peptide in the groups of patients who received Metformin+SU and Metformin+DPP4i did not reveal any significant differences either fasting or after food loading. The comparison of the dynamics of fasting and postprandial C-protein levels before the introduction of DPP4i and 6 months after the start of the therapy revealed statistically significant differences in the level of C-peptide 120 minutes after. The levels of C-peptide were significantly lower (1st visit – 8.94 (2.58;9.9) ng/ml), 2nd – 4.37 (6.37;2.37) ng/ml, p <0.05.
Evaluation of glucagon secretion
The evaluation of the secretory function of α-cells showed that in all the studied groups, there was manifested fasting hyperglucagonemia. In the group of patients who received Metformin+SU (810 (470;2090)), it was significantly higher than in the group of patients who received Metformin+DPP4i (485 (220;1770)), p<0.05 despite a comparable control of glycemia. There were no significant differences revealed in the level of glucagon after a standard breakfast. The comparison of the dynamics in the glucagon levels before the introduction of DPP4i and 6 months after the start of the therapy revealed statistically significant differences in its fasting levels (1st visit – 760 (80,2190), 2nd visit – 560 (80,1120) p<0.05). Its levels significantly decreased during DPP4i therapy. There were no statistically significant differences in the levels of glucagon 30 and 120 minutes after a standard breakfast. Thus, expressed hyperglucagonemia was observed in treatment groups. However, PDD4i therapy was associated with its significantly weaker expression at the early stage of therapy (6 months) and during long-term treatment.
Evaluation of HOMA β and HOMA IR indices
HOMA β index did not differ significantly in the groups of Metformin+SU 86.6 (15.14;202.2) and Metformin+DPP4i (47.2 (16.7;152.2)), p>0.05, as well as HOMA-IR index, p>0.05.
Despite a significant dynamics of the level of insulin in the group of patients who received DPP4i for the first time in addition to Metformin, there were no significant changes revealed in HOMA β (1st visit – 67.09 (10.1;202.2), 2nd visit – 29 (5.9;50.8), p>0.05, and HOMA IR, p >0.05, indices.
Multivariant analysis
The authors evaluated the effect of the duration of DM, age, sex, phenotype, BMI, duration of therapy, HOMA β and HOMA IR indices, glycated hemoglobin, and fasting glucose level on the parameters and dynamics of the changes in the levels of insulin, glucagon, and C-peptide.
A model of linear regression was designed (Table 3).
Таблица / Table 3
Результаты многофакторного анализа (оценивалось влияние различных факторов на показатели инсулина до (А) и после(В) стандартного завтрака)
Results of multivariate analysis (the influence of various factors on insulin parameters before and after a standard breakfast was evaluated)
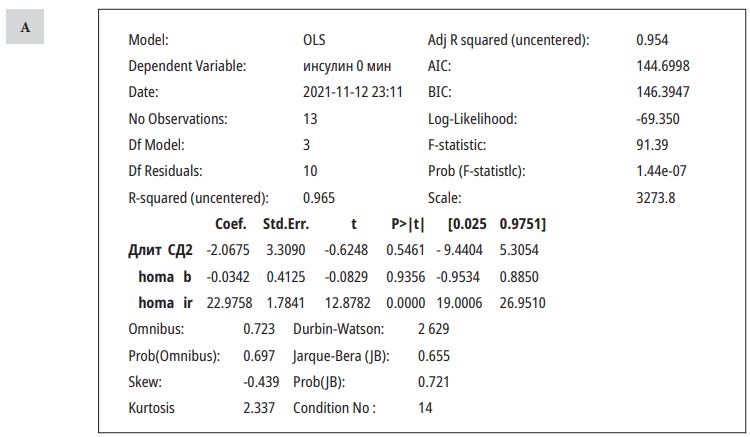
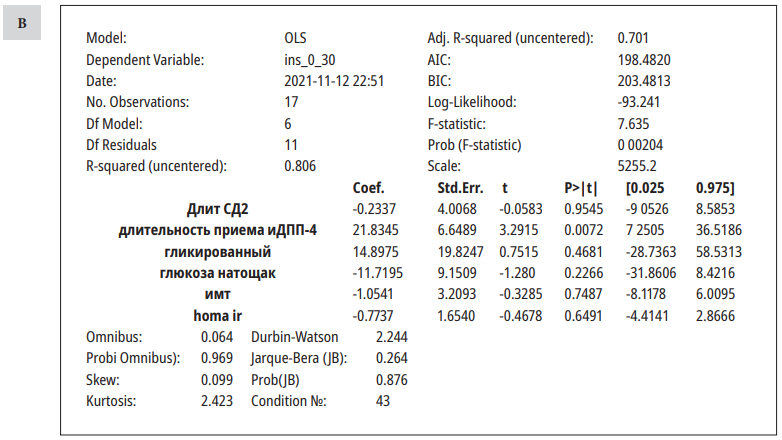
Fasting insulin levels were significantly affected by the HOMA IR index, and postprandial insulin levels were affected by the duration of DPP4i therapy. Other parameters did not influence the insulin levels.
The evaluation of the impact of the above-mentioned parameters on the levels of glucagon and C-peptide did not reveal significant associations.
Discussion
Recent studies have shown that there are five stages in the development of DM2. The earliest changes that form before the development of carbohydrate metabolism disorders are hyperinsulinemia/ insulin resistance and hyperglucagonemia [5]. These changes primarily determine all further pathological changes that lead to both the development of hyperglycemia and DM2 and cardiovascular complications associated with DM2. The data obtained in the present study show a positive effect of DPP4i therapy on the secretion of insulin and glucagon.
The addition of DPP4i to Metformin therapy showed a positive effect on the levels of insulin, C-peptide, and glucagon. This was associated with a decrease in the expression of hyperinsulinemia and hyperglucagonemia, which can indicate a tendency to the normalization of α- and β-cells functioning, which was observed in earlier experimental studies [6]. The level of fasting insulin in patients who received DPP4i was higher than in patients who received SU for a long time, and the delta between fasting and postprandial insulin levels was smaller. This can indicate the beginning of β-reserve exhaustion during SU therapy and preservation of the cellular function of β-cells during DPP4i therapy. At the same time, insulin levels were within the normal values in both groups. Such effects of DPP4i are verified by experimental and clinical studies.
According to the published data, DPP4i can enhance the replication of β-cells and inhibit their apoptosis [7][8]. Some researchers suggested that drugs from this group could not only improve the function of the remaining β-cells but also stimulate the formation of new β-cells in patients with DM2 [9]. It was revealed that via glucagon-like peptide 1 (GLP-1), DPP4i also contributed to the cellular differentiation of immature progenitor islet cells into β-cells or re-differentiation of them from other types of exocrine cells [10]. According to the published data, GLP-1 stimulates the transcription of D1 cyclin, which is required for the G1 and S phases of the cell cycle [11][12]. The present study showed that the proliferative and anti-apoptotic effects of GLP-1 could be achieved by pharmacological but not physiological concentrations of incretins [13]. Besides, the preservation of the mass of β-cells can be observed due to a direct resistance to apoptosis in cells that express its receptors [14]. One of the studies showed that GLP-1 delayed morphological changes that occurred in human islets in patients with DM2. This could occur because of the enhanced expression of anti-apoptotic protein bcl-2 and decreased secretion of the caspase-3 active form [8].
A decrease in the pathological secretion of glucagon could be explained by the mechanism of paracrine interaction between α and β-cells. Some studies confirmed that α- and β-cells of the pancreas were involved in paracrine interactions and mutually regulated their count and functions. It was shown that the normalization of the function of β-cells could inhibit the activity of α-cells [15]. It is known that during uncontrolled DM2, the level of glucagon increases [16], which can reflect the general dysfunction of pancreatic islets. However, the authors did not reveal any significant associations between the degree of DM2 control and glucagon levels, while there was a significant association with the type of therapy. Besides, increased secretion of glucagon can be a potential indicator of the degree of transdifferentiation of α- and β-cells. Transdifferentiation of β-cells is determined by the loss of their phenotype and transformation into a completely new islet endocrine cell, which can secrete not only insulin but also glucagon [17]. Thus, these changes in patients who started DPP4i therapy for the first time can be associated with the transdifferentiation of glucagon-producing cells into insulin-producing cells.
Conclusion
The authors did not reveal statistically significant dynamics in the functioning of β-cells by the HOMA-β index, which again highlights a lower value of this indicator in patients with DM2 who receive pharmacotherapy.
In general, the obtained data indicate the capability of DPP4i to affect the earliest and most significant parts of DM2 pathogenesis that also enhance the risk of the development of cardiovascular diseases in patients with DM2.
References
1. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31 Suppl 2:S262-8. DOI: 10.2337/dc08-s264
2. Herman GA, Stein PP, Th ornberry NA, Wagner JA. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Th er. 2007;81(5):761-7. DOI: 10.1038/sj.clpt.6100167
3. Sorkin JD, Muller DC, Fleg JL, Andres R. Th e relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care. 2005;28(11):2626-32. DOI: 10.2337/diacare.28.11.2626
4. Woerle HJ, Neumann C, Zschau S, Tenner S, Irsigler A, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77(2):280-5. DOI: 10.1016/j.diabres.2006.11.011
5. Rydén L., J. Grant P., D. Anker S., Berne C., Cosentino F., et al. Рекомендации по диабету, предиабету и сердечно-сосудистым заболеваниям. EASD/ESC. Российский кардиологический журнал. 2014;(3):7-61. DOI: 10.15829/1560-4071-2014-3-7-61
6. Taisiia Pavlovna T, Kseniia Petrovna S, Anna Anatolievna V, Ivan Sergeyevich U, Olga Vladimirovna R, et al. Dynamics of total volume of pancreatic α- and β -cells under the infl uence sulfonylureas and their combination with dipeptidyl peptidase-4 inhibitors. Endocrinol Diabetes Metab. 2021;4(3):e00238. DOI: 10.1002/edm2.238
7. Xu G, Stoff ers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270-6. DOI: 10.2337/diabetes.48.12.2270
8. Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144(12):5149-58. DOI: 10.1210/en.2003-0323
9. Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141(12):4600-5. DOI: 10.1210/endo.141.12.7806
10. Vasu S, Moff ett RC, Th orens B, Flatt PR. Role of endogenous GLP-1 and GIP in beta cell compensatory responses to insulin resistance and cellular stress. PLoS One. 2014;9(6):e101005. DOI: 10.1371/journal.pone.0101005
11. Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, et al. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188(3):481-92. DOI: 10.1677/joe.1.06160
12. Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52(1):12432. DOI: 10.2337/diabetes.52.1.124
13. Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96(26):14843-7. DOI: 10.1073/pnas.96.26.14843
14. Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5’-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144(4):1444-55. DOI: 10.1210/en.2002-220897
15. Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. δ-cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J Physiol. 2018;596(2):197-215. DOI: 10.1113/JP274581
16. Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84-116. DOI: 10.1210/er.2006-0007
17. Stefan Y, Orci L, Malaisse-Lagae F, Perrelet A, Patel Y, Unger RH. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31(8 Pt 1):694-700. DOI: 10.2337/diab.31.8.694
About the Authors
T. P. TuchinaRussian Federation
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов
I. A. Kolchanova
Russian Federation
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов
A. R. Meltonyan
Russian Federation
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов
L. K. Abramyan
Russian Federation
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов
A. Yu. Babenko
Russian Federation
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов
M. M. Galagudza
Russian Federation
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов
Review
For citations:
Tuchina T.P., Kolchanova I.A., Meltonyan A.R., Abramyan L.K., Babenko A.Yu., Galagudza M.M. Evaluation eff ects of IDPP4 therapy diff erent duration on the functional state of α- and β- cells in patients with type 2 diabetes mellitus. Medical Herald of the South of Russia. 2022;13(1):88-97. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-1-88-97
JATS XML







































