Scroll to:
Vascular abnormalities visualized by multislice computed tomography of the abdomen: accidental findings or immediate causes of pain syndrome? (topic review)
https://doi.org/10.21886/2219-8075-2021-12-4-34-45
Abstract
Abnormalities of the abdominal aorta branches may cause chronic mesenteric ischemia, progressive pathological changes of the internal organs in this setting, and chronic pain syndrome. The causes of chronic mesenteric ischemia may be divided into atherosclerotic and non-atherosclerotic. Atherosclerosis of the unpaired branches of the abdominal aorta involves stenosis or occlusion. Other causes include fibromuscular dysplasia, vasculitis (Takayasu, segmental mediolytic arteriopathy), and median arcuate ligament syndrome. These syndromes, the pathogenesis of some of which remains controversial, lead to nonspecific complaints such as abdominal pain, weight loss, and others. Digital subtraction angiography or duplex ultrasound may provide hemodynamic information in cases of vascular disease in this area. However, multislice spiral computed tomography is in many cases the first choice because it allows for a comprehensive assessment of the state of blood vessels and associated morphological changes of internal organs. Structural changes accompanying these syndromes can also occur in patients who are undergoing a medical examination for other reasons. However, these syndromes should not be diagnosed solely on the basis of imaging; instead, the findings should be compared with the clinical presentation, which implies collaboration of radiologists and clinicians.
Keywords
For citations:
Arutiunova N.K., Araslanova L.V., Riabchenko V.A., Pisarenko E.A., Ter-Ananiants E.I. Vascular abnormalities visualized by multislice computed tomography of the abdomen: accidental findings or immediate causes of pain syndrome? (topic review). Medical Herald of the South of Russia. 2021;12(4):34-45. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-34-45
Introduction
Chronic mesenteric ischemia (CMI), which is characterized by such manifestations as non-specific abdominal pain after a meal and weight loss that affects patients’ nutrition, can develop into acute mesenteric ischemia, which is a dangerous and often lethal disease. Still, CMI remains an understudied, often non-diagnosed and non-treated disease. This primarily occurs because of the lack of respective knowledge in the medical society. It is suggested that an increase in life expectancy, rate of cardiovascular diseases among the senior population, and metabolic disorders will contribute to an increase in the occurrence rate of CMI. Modern methods of radio diagnostics and expansion of indications to these studies provided earlier and precise diagnostic of the disease and improved the disease outcome.
The review was aimed to evaluate the issue of CMI and modern possibilities of radio diagnostics, bolus contrast-enhanced multislice spiral computed tomography (CE-MSCT), or multislice spiral computed angiography (MSCT-A) to propose the interdisciplinary algorithm of monitoring for this group of patients as well as to compare the data on the most studied anomalies of abdominal vessels with their clinical picture, anatomy, and pathogenesis, to evaluate the main patterns that would help radiologists, physicians, gastroenterologists, vascular surgeons, general practitioners, and medical specialists that work with this group of patients.
Materials and Methods
A systematic review of the published studies was made according to standard recommendations. The authors searched publications made during the past decade in Russian and English language electronic databases PubMed, Google Scholar, Google books, Ciberleninka. The search was made for keywords that included “сhronic mesenteric ischemia, multislice spiral computed tomography angiography, atherosclerosis, fibromuscular dysplasia, vasculitis, and median arcuate ligament syndrome” in English language databases and “синдром хронической абдоминальной ишемии, мультиспиральная компьютерно-томографическая ангиография, атеросклероз, фиброзно-мышечная дисплазия, васкулиты, синдром внешней компрессии чревного ствола” in Russian language databases. The search for articles was performed in four directions that included atherosclerosis of the abdominal aorta and its unpaired visceral branches, fibromuscular dysplasia, vasculitis, and median arcuate ligament syndrome. All publications were checked for methodological validity before the inclusion in the article.
The peculiarities of CMI diagnostics are associated with non-specific symptoms such as abdominal pain, weight loss, altered defecation patterns, nausea, etc. [1].
It should be highlighted that apart from visualization of parenchymatous organs, a widely used ultrasonic investigation allows specialists to evaluate the blood flow in vessels. However, its application is limited in cases that are similar to ischemic vascular abdominal pathology because it cannot evaluate collateral blood flow, condition of parenchymatous organs in bolus-enhanced condition, and the status of intestinal walls [1][2].
CE-MSCT or MSCT-A are acknowledged methods of visualization of the first line due to its rate, availability, and capacity to diagnose alternative reasons for abdominal pain [3].
The technique of the study will vary depending on the chosen protocol.
Description of the method of study
The patient received orally 500-800 ml of contrast agent (water) for stomach and small intestinal contrast. A total of 100-120 ml of Iodine-containing contrasting agent was injected in the ulnar vein with an injector (18-20G) at the rate of 4-5 ml/s for adequate evaluation of the arterial and venous systems. The arterial phase imaging (25-30 seconds after the injection of the contrast agent) was obtained from the level above the celiac trunk to the level of common iliac arteries. The portal vein phase imaging (60-70 seconds after the introduction of the contrast agent) was obtained from the level above the diaphragm and below the pubic symphysis [4][5].
The collected and analyzed data was processed with a special working station and included 2D multiplanar images, maximum intensity projection (MIP), and volumetric rendering (VR) for 3D images of blood vessels [6][7].
MSCT-A images were checked for arterial stenosis or occlusion. For example, vascular occlusion is defined as a complete lack of lumen contrast, hemodynamically significant vascular stenosis was defined as a reduction of the lumen diameter to >50%, and hemodynamically insignificant stenosis is a reduction of lumen diameter to <50% [6].
There is also another point of view. Patients with abdominal ischemia symptoms and damage of one vessel of the celiac trunk or the upper mesenteric artery stenosis to ≥70% can be considered significant. In patients with manifested symptoms of vast lesion of the upper mesenteric artery and its branches, stenosis to ≥50% can be considered significant [8].
The most common secondary signs of ischemia on CT images included thickening of the intestinal wall, focal lack of intestinal wall contrasting, dilatation of the intestine, ascites, intestinal pneumatosis, free gas in the peritoneal cavity, and infarction of the parenchymal organs [8]. General contraindications to CE-MSCT were pregnancy, previous severe reactions to the contrast agent, claustrophobia, and lack of contact with a patient [7][9][10].
There were no publications on a direct comparison of CE-MSCT and MRI of mesenchymal vessels. For renal arteries, it was shown that MSCT-A was more informative than CE-MRI angiography [9]. The sensitivity of MSCT-A for the diagnostic of mesenchymal ischemia was 100%, the specificity was 95–100% [1].
The main causes of CMI
Chronic mesenteric ischemia includes stenosis-induced or occlusion-induced ischemia, vasculitis (Takayasu’s arteritis (TA), segmental arterial mediolysis (SMA)), and celiac axis compression syndrome [11].
The cause of pain syndrome is mesenteric ischemia induced by a direct ischemic effect with further pain mediator release as well as “steal syndrome” with respective pathogenetic mechanisms of pain syndrome development [12][13].
Atherosclerotic alterations in the mesenteric arteries
The most common causes of CMI include atherosclerotic occlusion or severe stenosis of mesenteric arteries. Stenosis to >50% is observed in 18% of patients older than 65 years old. However, only some of them have manifested symptoms [14].
Nearly in 50% of patients with atherosclerotic damage of peripheral or coronary vessels, a lesion of non-paired branches of the abdominal aorta is observed [15]. According to Krishnamurthy et al., angiography reveals clinically significant stenosis of two and more mesenteric arteries in 20.4% of patients with ischemic heart disease [16]. The occurrence rate of atherosclerosis of the abdominal aorta in patients with atherosclerosis of lower extremities was evaluated in some publications. According to some authors, it reached 60-100% and correlated with the expression of stenosis in both vascular pools [17][18].
The symptoms occur after a meal, which is associated with an enhancement in the blood flow, and are manifested as a classic clinical triad (postprandial abdominal pain, weight loss, and food refusal) that is observed in nearly half of patients with chronic mesenteric ischemia [19][20]. The main three non-paired arteries that arise from the abdominal aorta are the celiac artery, superior mesenteric artery (SMA), and inferior mesenteric artery (IMA). Since a collateral blood flow is present at several levels, patients might not experience symptoms even in the case of stenosis of several arteries simultaneously. When SMA is occluded, pancreaticoduodenal arteries feed the intestine via the hepatic and gastroduodenal arteries. When the celiac artery and SMA are occluded, the small intestine is fed from the IMA pool via the left colic branch. The symptoms usually arise from stenosis and/or occlusion of two and more vessels, although there are exceptions to this rule [21][22].
This pathology is usually diagnosed with CT angiography without issues. Atherosclerotic deposits are primarily localized in the entrance of the celiac artery and SMA [22].
A clinical example from the author’s archive data is presented in Fig. 1.
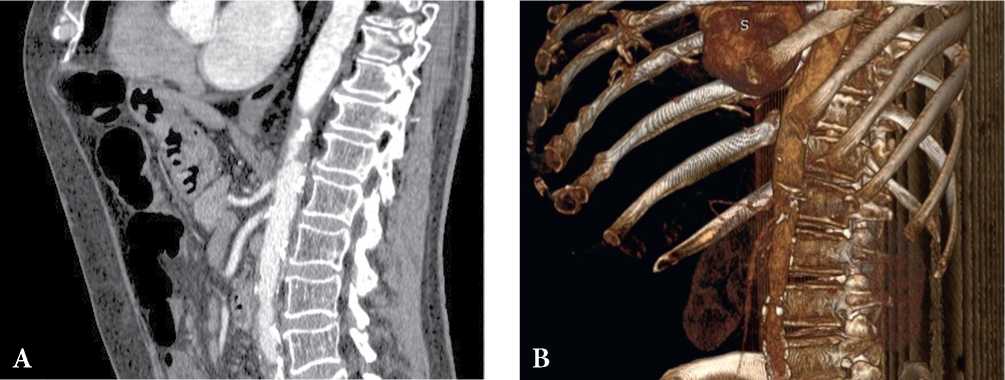
Рисунок 1. КТ-признаки атеросклеротического стеноза чревного ствола и верхней брыжеечной артерии. Пациент, 64 лет, боли в животе, неустойчивый стул. Стеноз чревного ствола до 60%, стеноз верхней брыжеечной артерии до 60% (A, B). Данные из архива авторов.
Figure 1. СТ findings are consistent with atherosclerotic stenosis of the celiac trunk and superior mesenteric artery. Patient, male, 64 years old, abdominal pain, unstable stool. Сeliac trunk stenosis up to 60%, superior mesenteric artery stenosis up to 60% (A, B). The authors' archive data.
Fibromuscular dysplasia
Non-atherosclerotic causes of structural changes in the vascular wall include fibromuscular dysplasia (FMD). It is a rare but well-known cause of chronic mesenteric ischemia [23].
FMD is a heterogeneous group of vascular pathology that is characterized by idiopathic, non-inflammatory, and non-atherosclerotic angiopathy of small and medium arteries [23][24]. The prevalence of this pathology is unknown. Most commonly, it is diagnosed in young women aged 30 to 50 years old [25][26].
FMD can develop without the manifestation of symptoms. The developed symptoms can be associated with renal arteries pathology (hypertension or failure caused by the renal artery stenosis) caused by the lesion of the carotid and vertebral arteries (impulse noise, transitory ischemic attack, stroke), and coronary artery lesion (angina, infarction) [26][27][28]. The expressed clinical manifestations of mesenteric ischemia develop early rarely because of collateral blood supply [28].
Structural changes in the vascular walls are associated with fibrous or fibromuscular thickening. Any layer of the vascular wall can be affected (intima, media, or adventitia) without the signs of inflammation [25][26][29][30].
In 90-95% of cases, the media is affected. As a result, in most cases, stenosis develops with areas of expansion (small aneurysms). Less frequently, uniform stenosis of the vascular wall develops. FMD “weakens” the vascular wall, which contributes to the formation of the arterial wall dissection [26][31].
It can affect any medium-size artery and is primarily characterized by a bilateral localization (for example, in the case of the renal artery lesion) with the involvement of several arteries. The complications can include spontaneous dissection, distal embolization of the clot formed in the cases of an aneurysm, and hemorrhage. Digital subtraction angiography remains the gold standard of FMD diagnostic because it allows specialists to visualize minor or peripheral lesions. A specific feature of the most common medial type is an alternation of stenosis and dilatations that is called a string of beads sign. Less frequently, intimal and adventitial types are characterized by focal concentric stenosis of long segments or diverticular bulging. The advantages of MSCT and MRI are in the possibility to evaluate the ischemic lesion of the abdominal organs [28][31][32].
Typical angiographic signs include vascular loops, fusiform vascular ectasia, a string of beads, and stenoses. Fig. 2 shows an example of FMD from the authors’ archive.
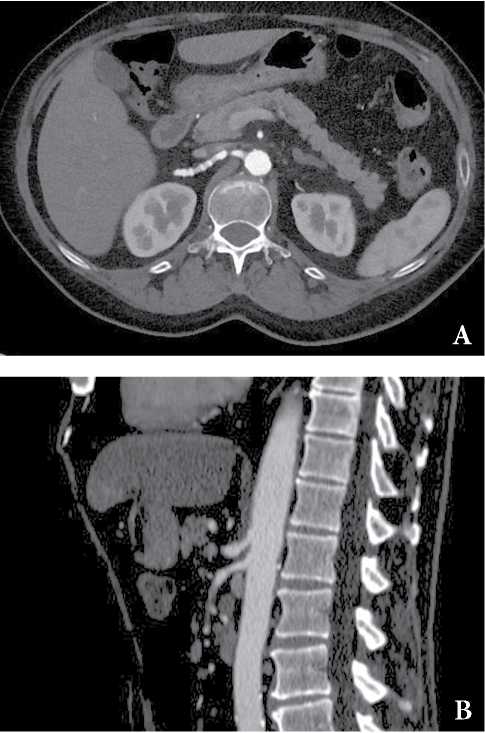
Рисунок 2. КТ-картина фиброзно-мышечной дисплазии (ФМД) с вовлечением почечных артерии, аневризмой чревного ствола (A) и стенозом верхней брыжеечной артерии (B) у пациента 65 лет с признаками абдоминальной ишемии. Данные из архива авторов.
Figure 2. СТ findings are consistent with fibromuscular dysplasia (FMD) involving renal arteries, celiac trunk aneurysm (A) and stenosis of the superior mesenteric artery (B) in a symptomatic 65-year-old male patient. The authors' archive data.
Differential diagnosis during the visualization includes atherosclerosis, usually in the beginning or in the proximal part of the artery, vasculitis, elevated ESR +/- fever, traumatic iatrogenic damage of the vessels (possible segmental arterial mediolysis) [26][28][31].
Takayasu arteritis
The most common condition among vasculitis and connective tissue diseases that causes chronic mesenteric arteritis is Takayasu arteritis [33]. Takayasu arteritis (АТ) is known as idiopathic arteriopathy. It is granulomatous vasculitis of large vessels that primarily affects the aorta and its main branches, and sometimes, pulmonary and coronary arteries [34][35]. The causes of the disease are unknown, however, it is considered that this pathology is similar to giant cell arteritis. This pathology prevails in women (<50 years old). The typical age of symptoms manifestation was 15-30 years old. Some researchers highlight the facts of geographical coincidence of the cases of tuberculosis and Takayasu arteritis, which suggests an association between these diseases. Probably tuberculosis causes an immune-mediated reaction of large vessels. Still, this hypothesis remains controversial [34].
Takayasu arteritis classification (Numano) [36]:
- Type I – only branches of the aortal arch are affected.
- Type Iiа – ascending part and/or aortal arches are affected. Braches of the aortic arches can also be affected. The rest part of the aorta is not affected.
- Type Iib – descending part of the thoracic aorta is affected with/without lesion of the ascending part and aorta arch or its branches. The abdominal aorta is not affected.
- Type III – descending part of the thoracic aorta, abdominal aorta, and/or renal arteries are affected. The ascending part and aortal arch with its branches are not affected.
- Type IV – only the abdominal aorta and/or renal arteries are affected.
- Type V – generalized type, a combination of features of other types.
The lesion of coronary and pulmonary arteries are marked as C(+) or P(+), respectively.
The clinical picture includes various ischemic symptoms caused by stenosis or clot formation. First symptoms are usually manifested as fatigue, fever, night sweats, weight loss, and arthralgia. Frequently, anemia with elevated levels of inflammatory markers is observed. This phase resolves with the beginning of the chronic phase that is characterized by inflammatory and obliterating alterations in the aorta and its branches. Peripheral pulse often decreases or is absent. For this reason, this disease is also called a “pulseless disease”.
CT-picture presents a uniform and expended thickening of the intestinal wall. In the active phase, enhanced contrast of the intestinal wall, stenosis, occlusion of the main aortic branches, aneurysmal dilatation of the aorta or its branches, and formation of pseudoaneurysm can be observed [37][38][39].
Fig. 3 presents an example of a CT image of Takayasu arteritis.
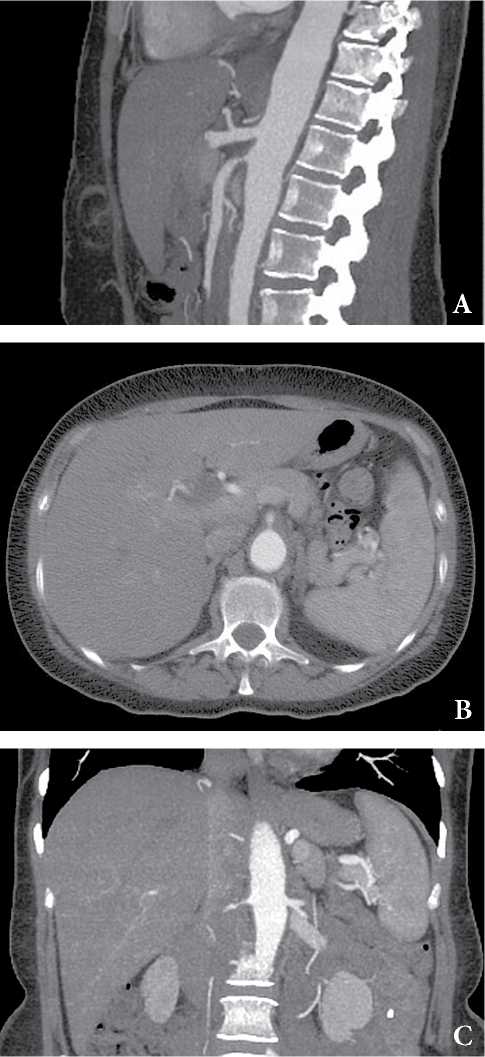
Рисунок 3. КТ-признаки артериита Такаясу. Пациентка, 37лет, перемежающаяся боль в животе. Признаки утолщения стенки грудной и брюшной аорты (A), стеноз до 60% ВБА (A, B) и правой почечной артерии (C). Случай любезно предоставлен доктором Мэтью Лукисом, Radiopaedia.org, rID: 52141.
Figure 3. СТ-findings are consistent with Takayasu arteritis. Patient, female, 50 years old. Intermittent abdominal pain. Wall thickening of the thoracic and abdominal aorta (A), up to 60% stenosis of the superior mesenteric artery (A, B) and right renal artery (C). Case courtesy of Dr Matthew Lukies, Radiopaedia.org, rID: 52141.
Segmental arterial mediolysis
A group of rare vasculitides, that lead to chronic mesenteric ischemia, also includes segmental arterial mediolysis (SAM), which is considered to be a rare arteriopathy [9]. The main histological process of SAM is the lysis of smooth muscles of the arterial wall, which leads to the intramural hemorrhage, sacculated or dissecting aneurysm, thrombosis, or hemorrhage [40][41].
SAM primarily affects medium branches of the superior mesenteric artery. The etiology of the disease is unknown but there is an association observed with episodes of internal vessel narrowing (for example, shock, hypoxia, recent vast surgery, vasopressor infusion) [40][42][43]. There is some histologic similarity with FMD, which is considered to be a differential diagnosis, but clinical signs and distribution of lesions are usually typical.
Lately, the occurrence rate of the disease increased due to a wider implementation of CT angiography and awareness-raising in the radiological society. The morbidity rate can reach up to 1 case per 100,000 patients per year [40][43].
Abdominal pain, distention, shock in severe cases, and hematocrit fall are typical symptoms. In middle-aged patients with non-traumatic spontaneous mesenteric hemorrhage, segmental arterial mediolysis is the most probable primal cause. In acute cases, the lethality rate is up to 50% [43].
CT picture is characterized by fusiform aneurysms, stenosis, dissections, and occlusions of arteries. A sequence of aneurysms and stenosis (string-of-beads sign) is observed. Their distribution tends to avoid bifurcations [42].
An example is presented in Fig. 4.
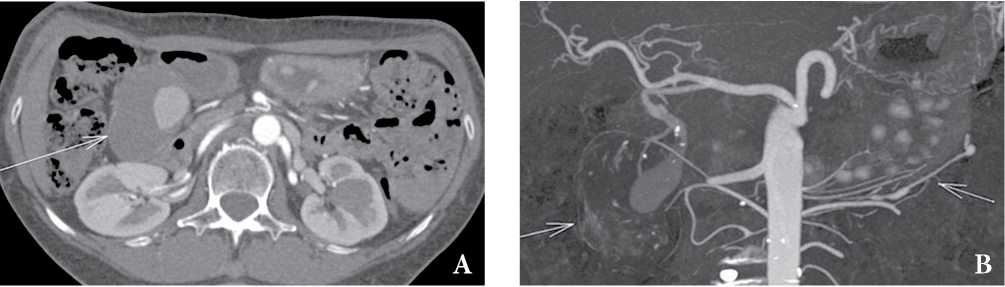
Рисунок 4. Сегментарный артериальный медиолиз, женщина 64 лет. КТ-ангиограмма показывает большую аневризму гастродуоденальной артерии (A, B, длинная стрелка) и симптом “нити бус” (B, короткая стрелка). Случай любезно предоставлен доктором Томасом Сноу, Radiopaedia.org, rID: 30333
Figure 4. Segmental arterial mediolysis. CT angiogram shows a large gastroduodenal artery aneurysm (A, B, long arrows) and the string-of-beads sign of the left gastroepiploic artery (B, short arrow). Case courtesy of Dr Thomas Snow, Radiopaedia.org, rID: 30333
Median arcuate ligament syndrome
The causes of external impact on abdominal vessels that lead to the development of mesenteric ischemia include median arcuate ligament syndrome (Dunbar syndrome or Harjola-Marable syndrome), which is characterized by external compression of the celiac trunk by the diaphragmatic crurae [44]. There is no consensus on what should be considered a pathology and what is variant anatomy because this anomaly is met in symptomless patients [45][46].
In 10–24% of patients, the ligament can go in front of the artery. In some of them, the ligament compresses the celiac artery, which impairs the blood flow and provokes symptoms [47]. The expressed compression develops nearly in 1% of patients and persists during inhaling [48]. Its occurrence rate is higher in women (mean age is 30-50 years old) and slim patients [46]. Clinical symptoms include position-dependent chronic pain in the abdomen, especially after a meal, which intensifies in the supine position at the expiratory height, nausea/vomit, and weight loss [46].
It is suggested that the etiology of abdominal pain is ischemic. It is caused by blood flow failure caused by compression. Alternatively, it was suggested that there was a neuropathic component associated with an impact on the celiac plexus [46].
CT picture of the celiac trunk compression includes a focal narrowing of the upper proximal celiac trunk section, which has a hooked or J-like appearance, signs of collaterals, and lack of associated atherosclerosis. CT-angiography is used additionally to evaluate post-stenotic dilatation, branches of the celiac trunk (gastroduodenal and common hepatic artery), and thickening of the median arcuate ligament. The thickness of the median arcuate ligament of more than 4 mm is considered to be abnormal [46].
Often, the breathing phase significantly affects the degree of the celiac trunk narrowing. Primarily, stenosis is more expressed at the end of exhaling and less expressed at the end of inhaling. Thus, it is recommended to visualize during the final inhale to reduce the possibility of detection of clinically insignificant narrowing (false positive) [49][50]. Examples of celiac trunk compression stenosis and combined vascular pathology from the authors’ archive are presented in Figs. 5 and 6.
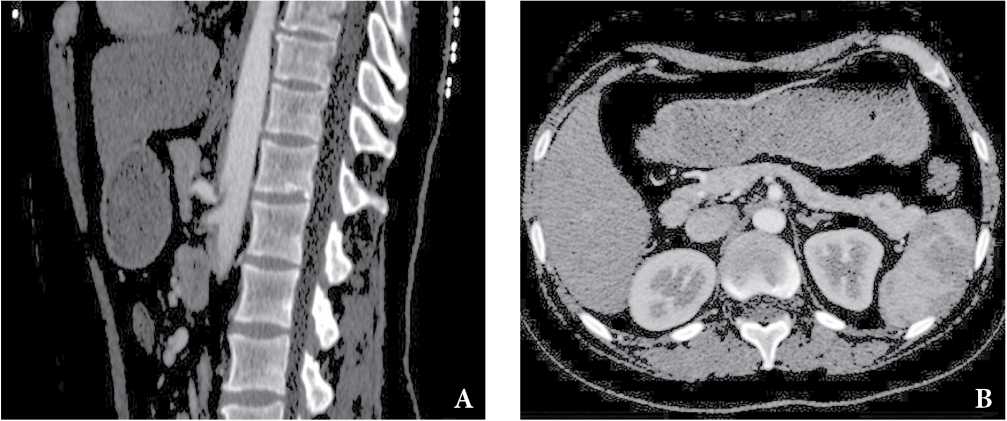
Рисунок 5. КТ-признаки компресcии чревного ствола дугообразной связкой. Пациентка, 38 лет, хронические абдоминальные боли до 8–9 баллов, согласно визуальной аналоговой шкале (ВАШ). Стеноз чревного ствола до 75% в устье, характерный изгиб чревного ствола в виде «крючка», постстенотическая дилатация (A), срединная дугообразная связка, сдавливающая основание чревного ствола (B).
Figure 5. СТ findings are consistent with median arcuate ligament syndrome. Patient, female, 38 years old; with chronic abdominal pain up to 8-9 VAS points. Stenosis of the celiac trunk up to 75% at the orifice, characteristic hooked appearance of the celiac trunk, post-stenotic dilatation (A), median arcuate ligament, compressing the base of the celiac trunk (B).
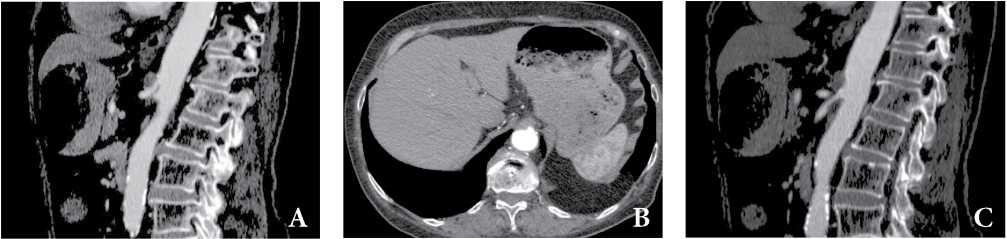
Рисунок 6. КТ-признаки сочетанной патологии компресcии чревного ствола дугообразной связкой и атеросклероз ветвей брюшной аорты. Пациентка, 68 лет, хронические абдоминальные боли, в последнее время отмечает усиление боли после еды, похудание, отсутствие аппетита. Стеноз чревного ствола до 50% в устье, характерный изгиб чревного ствола в виде «крючка» с наличием кпереди от изгиба утолщенной дугообразной связки (A, B), срединная дугообразная связка, сдавливающая основание чревного ствола (B), стеноз ВБА в проксимальном сегменте за счет мягкой циркулярной атеросклеротической бляшки (С).
Figure 6. СТ findings are consistent with combined pathology of compression of the celiac trunk by the arcuate ligament and atherosclerosis of the branches of the abdominal aorta. Patient, female, 68 years old; with chronic abdominal pain; recently noted an increase in pain after eating, weight loss, and a lack of appetite. Celiac trunk stenosis up to 50% at the orifice, characteristic hooked appearance of the celiac trunk with the presence of a thickened arcuate ligament anterior to the bend (A, B), median arcuate ligament, compressing the base of the celiac trunk (B), SMA stenosis in the proximal segment behind account of a soft circular atherosclerotic plaque (C).
Conclusion
CE-MSCT or MSCT-A is a preferable method for the diagnostic of structural changes in patients with pain syndrome and suspected chronic mesenteric ischemia. It allows specialists to evaluate the condition of vessels as well as post-ischemic alterations in the visceral organs.
In patients with non-specific gastrointestinal complaints (chronic pain syndrome after a meal, weight loss, etc.) and lack of evident cause and effect relation with visceral pathology, chronic mesenteric ischemia should also be excluded.
Clinical specialists should discuss diagnostic tactics with radiologists when selecting the optimal method of diagnostics.
Frequently, an occlusive form of mesenteric ischemia in patients older than 65 years old is caused by atherosclerotic changes in unpaired aortic branches. Primarily, the symptoms appear when two or more branches are affected.
When patients have complaints typical for ischemic pain syndrome and verified atherosclerotic damage of coronary and lower extremity arteries, there is a high risk of occlusive lesion of the abdominal aorta branches.
Rare non-atherosclerotic causes of vascular lesion that lead to mesenteric ischemia include vasculitides (Takayasu’s disease, segmental arterial mediolysis) and FMD.
When patients have complaints typical for mesenteric ischemic syndrome in combination with a typical lesion of renal arteries and unpaired abdominal aorta branches, FMD should be excluded.
When a patient has signs of chronic mesenteric ischemia after vast surgical interventions in the anamnesis that were associated with shock, hypoxia, and injection of vasopressors, segmental arterial mediolysis should be excluded.
Young patients with suspected mesenteric ischemia and position-dependent abdominal pain with a typical narrowing of the celiac trunk proximal segment and hypertrophy of the diaphragm crurae might have median arcuate ligament syndrome.
References
1. Terlouw LG, Moelker A, Abrahamsen J, Acosta S, Bakker OJ, et al. European guidelines on chronic mesenteric ischaemia — joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia. United European Gastroenterol J. 2020; 8(4):371-395. DOI: 10.1177/2050640620916681.
2. Yaghmai V, Brandwein W. Abdominal Computed Tomography Angiography. 2014. In: Richard M. Gore, Marc S. Levine. Textbook of Gastrointestinal Radiology. 2021. ISBN: 9780323640824
3. Schieda N, Fasih N, Shabana W. Triphasic CT in the diagnosis of acute mesenteric ischaemia. Eur Radiol. 2013; 23(7):1891- 900. DOI: 10.1007/s00330-013-2797-y.
4. Kirkpatrick ID, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology. 2003; 229(1):91-8. DOI: 10.1148/radiol.2291020991.
5. Laghi A, Ferrari R, Mangiapane F, Trenna S, Marin D, Passariello R. CT Angiography of Splanchnic Vessels. In: Chapman A.H. (eds) Radiology and Imaging of the Colon. Medical Radiology (Diagnostic Imaging). Springer, Berlin, Heidelberg; 2004. DOI: 10.1007/978-3-642-18834-3_22.
6. Cademartiri F, Raaijmakers RH, Kuiper JW, van Dijk LC, Pattynama PM, Krestin GP. Multi-detector row CT angiography in patients with abdominal angina. Radiographics. 2004; 24(4):969- 84. DOI: 10.1148/rg.244035166.
7. Baert A, Passariello R. Multidetector-Row CT Angiography. Springer Science & Business Media; 2006. ISBN:3540269843.
8. Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, et al. CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol. 2007; 189(1):W13-9. DOI: 10.2214/AJR.04.1374.
9. Shih MC, Hagspiel KD. CTA and MRA in mesenteric ischemia: part 1, Role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007; 188(2):452-61. DOI: 10.2214/AJR.05.1167.
10. Kurochkin S.V., Zidikhanov D.I. Computed tomography angiography in the diagnosis of coarctation of the aorta. Complex Issues of Cardiovascular Diseases. 2017; 6(4):169-175. (In Russ.). DOI: 10.17802/2306-1278-2017-6-4-169-175.
11. Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009; 192(2):408-16. DOI: 10.2214/AJR.08.1138. PMID: 19155403.
12. Patel R, Waheed A, Costanza M. Chronic Mesenteric Ischemia. 2021 Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. PMID: 28613499.
13. Johnston KW, Lindsay TF, Walker PM, Kalman PG. Mesenteric arterial bypass grafts: early and late results and suggested surgical approach for chronic and acute mesenteric ischemia. Surgery. 1995; 118(1):1-7. DOI: 10.1016/s0039-6060(05)80002-9.
14. Mensink PB, Moons LM, Kuipers EJ. Chronic gastrointestinal ischaemia: shifting paradigms. Gut. 2011; 60(5):722-37. DOI: 10.1136/gut.2009.199695.
15. Fitzpatrick LA, Rivers-Bowerman MD, Thipphavong S, Clarke SE, Rowe JA, Costa AF. Pearls, Pitfalls, and Conditions that Mimic Mesenteric Ischemia at CT. Radiographics. 2020; 40(2):545- 561. DOI: 10.1148/rg.2020190122.
16. Krishnamurthy G, Menon A, Kannan K, Prakash S, Rajendran A, Philips D. Coronary artery disease and mesenteric artery stenosis — Two sides of the same coin? — Long term prospective analysis. Intractable Rare Dis Res. 2019; 8(4):245-251. DOI: 10.5582/irdr.2019.01087.
17. Cai H, Fu F, Wang Y, Li J, Cao J, et al. [Correlation between the stenosis degree of aorto-iliac artery and superior mesenteric artery in patients with lower extremity atherosclerotic occlusive disease by CT angiography]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018; 30(7):635-639. (In Chinese). DOI: 10.3760/cma.j.issn.2095-4352.2018.07.004.
18. Dolgushina A.I., Shaposhnik I.I., Seljanina A.A., Kuznecova A. S., Genkel V.V. Chronic mesenteric ischemia: current state of the problem. Experimental and Clinical Gastroenterology. 2020;(6):166-172. (In Russ.). DOI: 10.31146/1682-8658-ecg178-6-166-172.
19. Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg. 2003; 17(3):323-8. DOI: 10.1007/s10016-001-0249-7.
20. Hagspiel KD, Angle JF, Spinosa DJ, Matsumoto AH. Mesenteric ischemia: angiography and endovascular interventions. In: Longo W, Peterson GJ, Jacobs DL, eds. Intestinal ischemia disorders: pathophysiology and management. St. Louis, MO: Quality Medical Publishing; 1999.
21. Ruehm SG, Weishaupt D, Debatin JF. Contrast-enhanced MR angiography in patients with aortic occlusion (Leriche syndrome). J Magn Reson Imaging. 2000; 11(4):401-10. DOI: 10.1002/(sici)1522-2586(200004)11:4<401::aid-jmri8>3.0.co;2-h.
22. Bhatti AA, Chugtai A, Haslam P, Talbot D, Rix DA, Soomro NA. Prospective study comparing three-dimensional computed tomography and magnetic resonance imaging for evaluating the renal vascular anatomy in potential living renal donors. BJU Int. 2005; 96(7):1105-8. DOI: 10.1111/j.1464-410X.2005.05809.x.
23. Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet J Rare Dis. 2007; 2:28. DOI: 10.1186/1750-1172-2-28.
24. Willoteaux S, Faivre-Pierret M, Moranne O, Lions C, Bruzzi J, et al. Fibromuscular dysplasia of the main renal arteries: comparison of contrast-enhanced MR angiography with digital subtraction angiography. Radiology. 2006; 241(3):922-9. DOI: 10.1148/radiol.2413050149.
25. Furie DM, Tien RD. Fibromuscular dysplasia of arteries of the head and neck: imaging findings. AJR Am J Roentgenol. 1994; 162(5):1205-9. DOI: 10.2214/ajr.162.5.8166011.
26. Varennes L, Tahon F, Kastler A, Grand S, Thony F, et al. Fibromuscular dysplasia: what the radiologist should know: a pictorial review. Insights Imaging. 2015; 6(3):295-307. DOI: 10.1007/s13244-015-0382-4.
27. Michelis KC, Olin JW, Kadian-Dodov D, d’Escamard V, Kovacic JC. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol. 2014; 64(10):1033-46. DOI: 10.1016/j.jacc.2014.07.014.
28. Olin JW, Sealove BA. Diagnosis, management, and future developments of fibromuscular dysplasia. J Vasc Surg. 2011; 53(3):826-36.e1. DOI: 10.1016/j.jvs.2010.10.066.
29. Willoteaux S, Faivre-Pierret M, Moranne O, Lions C, Bruzzi J, et al. Fibromuscular dysplasia of the main renal arteries: comparison of contrast-enhanced MR angiography with digital subtraction angiography. Radiology. 2006; 241(3):922-9. DOI: 10.1148/radiol.2413050149.
30. Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet J Rare Dis. 2007; 2:28. DOI: 10.1186/1750-1172-2-28.
31. Rubin GD, Rofsky NM. CT And MR Angiography Comprehensive Vascular Assessment. USA: Lippincott Williams & Wilkins; 2008. ISBN:078174525X
32. Jahnlova D, Veselka J. Fibromuscular Dysplasia of Renal and Carotid Arteries. Int J Angiol. 2015; 24(3):241-3. DOI: 10.1055/s0034-1396931.
33. Mehta R, Deepak, John A, Shine, Raj, Balakrishnan. Takayasu arteritis presenting as chronic mesenteric ischemia. Indian J Gastroenterol. 2004; 23(2):73-4. PMID: 15176543.
34. Mehta R, Deepak, John A, Shine, Raj, Balakrishnan. Takayasu arteritis presenting as chronic34. Mehta R, Deepak, John A, Shine, Raj, Balakrishnan. Takayasu arteritis presenting as chronic mesenteric ischemia. Indian J Gastroenterol. 2004; 23(2):73-4. PMID: 15176543.
35. Yang J, Peng M, Shi J, Zheng W, Yu X. Pulmonary artery involvement in Takayasu’s arteritis: diagnosis before pulmonary hypertension. BMC Pulm Med. 2019; 198(1):225. DOI: 10.1186/s12890-019-0983-7.
36. Nastri MV, Baptista LP, Baroni RH, Blasbalg R, de Avila LF, et al. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics. 2004; 24(3):773-86. DOI: 10.1148/rg.243035096.
37. Broncano J, Vargas D, Bhalla S, Cummings KW, Raptis CA, Luna A. CT and MR Imaging of Cardiothoracic Vasculitis. Radiographics. 2018; 38(4):997-1021. DOI: 10.1148/rg.2018170136.
38. Sueyoshi E, Sakamoto I, Uetani M. MRI of Takayasu’s arteritis: typical appearances and complications. AJR Am J Roentgenol. 2006; 187(6):W569-75. DOI: 10.2214/AJR.05.1093.
39. Gotway MB, Araoz PA, Macedo TA, Stanson AW, Higgins CB, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005; 184(6):1945-50. DOI: 10.2214/ajr.184.6.01841945.
40. Chao CP. Segmental arterial mediolysis. Semin Intervent Radiol. 2009; 26(3):224-32. DOI: 10.1055/s-0029-1225666.
41. Michael M, Widmer U, Wildermuth S, Barghorn A, Duewell S, Pfammatter T. Segmental arterial mediolysis: CTA findings at presentation and follow-up. AJR Am J Roentgenol. 2006; 187(6):1463-9. DOI: 10.2214/AJR.05.0281.
42. Hur JH, Chun EJ, Kwag HJ, Yoo JY, Kim HY, et al. CT Features of Vasculitides Based on the 2012 International Chapel Hill Consensus Conference Revised Classification. Korean J Radiol. 2017; 18(5):786-798. DOI: 10.3348/kjr.2017.18.5.786.
43. Slavin RE. Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation. Cardiovasc Pathol. 2009; 18(6):352-60. DOI: 10.1016/j.carpath.2008.09.001.
44. Hagspiel KD, Leung DA, Angle JF, Spinosa DJ, Pao DG, et al. MR angiography of the mesenteric vasculature. Radiol Clin North Am. 2002; 40(4):867-86. DOI: 10.1016/s0033-8389(02)00027-1.
45. Kopecky KK, Stine SB, Dalsing MC, Gottlieb K. Median arcuate ligament syndrome with multivessel involvement: diagnosis with spiral CT angiography. Abdom Imaging. 1997; 22(3):318- 20. DOI: 10.1007/s002619900199.
46. Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg. 2016; 151(5):471-7. DOI: 10.1001/jamasurg.2016.0002.
47. Lindner HH, Kemprud E. A clinicoanatomical study of the arcuate ligament of the diaphragm. Arch Surg. 1971; 103(5):600-5. DOI: 10.1001/archsurg.1971.01350110102016.
48. Cornell SH. Severe stenosis of the celiac artery. Analysis of patients with and without symptoms. Radiology. 1971; 99(2):311– 316. DOI: 10.1148/99.2.311.
49. Fong JK, Poh AC, Tan AG, Taneja R. Imaging findings and clinical features of abdominal vascular compression syndromes. AJR Am J Roentgenol. 2014; 203(1):29-36. DOI: 10.2214/AJR.13.11598.
50. Sardar P, White CJ. Chronic mesenteric ischemia: Diagnosis and management. Prog Cardiovasc Dis. 2021; 65:71-75. DOI: 10.1016/j.pcad.2021.03.002.
About the Authors
N. K. ArutiunovaRussian Federation
Nina K. Arutiunova, doctor of the Consultative Department
Rostov-on-Don
L. V. Araslanova
Russian Federation
Larisa V. Araslanova, Cand. Sci. (Med.), Head of the Department of Diagnostic Radiology, Regional Consultative and Diagnostic Center; Associate Professor of the Department of Personalized and Translational Medicine, Rostov State Medical University
Rostov-on-Don
V. A. Riabchenko
Russian Federation
Victoria A. Riabchenko, Cand. Sci. (Med.), doctor of the Department of Diagnostic Radiology, Regional Consultative and Diagnostic Center; Assistant of the Department of Personalized and Translational Medicine, Rostov State Medical University
Rostov-on-Don
E. A. Pisarenko
Russian Federation
Elena A. Pisarenko, doctor of the Department of Diagnostic Radiology
Rostov-on-Don
E. I. Ter-Ananiants
Russian Federation
Elena I. Ter-Ananiantc, doctor of the Department of Radiation Diagnostics
Rostov-on-Don
Review
For citations:
Arutiunova N.K., Araslanova L.V., Riabchenko V.A., Pisarenko E.A., Ter-Ananiants E.I. Vascular abnormalities visualized by multislice computed tomography of the abdomen: accidental findings or immediate causes of pain syndrome? (topic review). Medical Herald of the South of Russia. 2021;12(4):34-45. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-34-45







































