Scroll to:
Investigation of the infertility structure and outcomes of ART programs in patients of late reproductive age
https://doi.org/10.21886/2219-8075-2022-13-2-59-71
Abstract
Purpose: comparative study of the structure of infertility and outcomes of ART programs among patients of different age groups.
Materials and methods: the case histories of 180 patients with infertility were studied: Group I ≥ 35 years old; Group II <35 years old. Laboratory and immunohistochemical studies were carried out, and the outcomes of ART were assessed.
Results: in group I, a shortened menstrual cycle was determined in comparison with group II (27,15 ± 3,39 days vs 29,57 ± 2,38 days, p = 0,001). Also, in group I, the following were more often found: inflammatory diseases (p = 0,05); history of unilateral tubectomy (p = 0,019); increased FSH levels (9,73 ± 2,43 vs 8,74 ± 2,50); decrease in the concentration of LH, progesterone, AMH (p <0,05). In the cells of the aspirate of the uterine cavity in patients of group I, there was an increased content of progesterone receptors and a decreased concentration of estrogen receptors (p <0,05). Patients of group I received a smaller number of oocytes (8,34 ± 3,51 vs 10,78 ± 4,37) and quality embryos by the 5th day of cultivation (82,7% vs 87,97%; p <0,05). The number of pregnancies in groups I and II was 22,22% and 36,67%, respectively, and live births – 14,44% and 27,78% (p <0,05). Patients who gave birth had increased progesterone levels, greater endometrial thickness, more oocytes with transvaginal puncture, and high-quality embryos.
Conclusion: the factors that reduce fertility were: genital pathology, inhibition of ovarian function, depletion of the follicular reserve. Fewer live births are associated with defects in embryonic and implantation factors.
For citations:
Uryupina K.V., Kutsenko I.I., Kravtsovа E.I., Lukoshkina I.N., Tomina O.V., Kaushanskaya L.V. Investigation of the infertility structure and outcomes of ART programs in patients of late reproductive age. Medical Herald of the South of Russia. 2022;13(2):59-71. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-59-71
Introduction
In the past few decades, women have shown a tendency to a later decision in childbearing [1], which causes a number of problems related to progressive age-related fertility disorder. One of the reasons for this phenomenon may be the natural decrease in follicular reserve, accompanied by a decrease in the production of antimullerian hormone (AMH), inhibin B, and an increase in follicle-stimulating hormone (FSH) concentration during the folliculin phase [2][3]. Nevertheless, the prognostic value of these hormones in relation to infertility is not always confirmed. The association between markers of ovarian reserve (AMH, FSH, and inhibin B) and fertility in patients aged 30–44 years was studied, and no association between the decreased ovarian reserve and decreased fertility was found [4]. Another study confirmed a direct correlation between the number of antral follicles obtained by stimulation in the long protocol and the rate of clinical pregnancy: in women with 1–8 antral follicles, pregnancy occurred in 28.25% of cases, and when the number of antral follicles was >18, pregnancy was diagnosed in 40.13% of women [5].
Another cause of fertility disorders in older reproductive age is a severe gynecological history. Infectious processes and chronic endometritis are certainly risk factors for infertility. For example, 45% of infertile patients have a history of chronic endometritis. Other risk factors include high body mass index, stress, and smoking of both partners [6]. In patients with endometriosis, the probability of conception even using in vitro fertilization is significantly reduced [7]. The hypothalamic-pituitary-ovarian axis dysregulation observed in endometriosis and the inflammatory response induced by ectopic endometrial foci form an unfavorable follicular microenvironment, which ultimately reduces oocyte competence and endometrial receptivity [8]. Endometrial hyperplastic processes, intrauterine adhesions, submucosal and intramural myomatous nodes, and adenomyosis significantly complicate embryo implantation [9]. Oncological pathology and related therapy can lead to decreased fertility [10].
In a significant number of cases, assisted reproductive technologies (ART) and oocyte cryopreservation help to overcome the age-related fertility decline if pregnancy is deliberately postponed. However, even in this case, the probability of success is significantly reduced compared to patients in younger age groups [11]. The above facts emphasize the relevance of a comprehensive study of the causes of infertility in patients of older reproductive age in order to improve the effectiveness of ART programs in this population.
The study is aimed to perform a comparative study of the structure of infertility and the outcomes of ART programs among patients of different age groups.
Materials and Methods
The study was conducted based on the facilities of the Obstetrics and Gynecology Reproductive Clinic "Embryo" (Sochi). All participants (n=180) gave their informed consent to participation in the study and publication of the results. Inclusion criteria were age 25–48 years old, at least 3 years of infertility, fertile or subfertile sperm of the partner, normal karyotype in both partners, preserved menstrual cycle, and absence of severe somatic or oncological pathology.
The data of clinical, laboratory, and instrumental methods were studied, including sex hormone levels, AMH levels, markers of infectious agents, pelvic organs ultrasound, immunohistochemical examination of a uterine aspirate (pipelle biopsy), and determination of progesterone (PR) and estrogen (ER) receptors, plasma cells, natural killer (NK) cells, leukemia inhibitory factor (LIF), evaluation of oogenesis and embryogenesis and analysis of the outcomes of ART procedures.
The cohort of patients was divided into two equal age groups. Group I included 90 women ≥35 years of age (38.78±3.31 years), and Group II included 90 women younger than 35 years of age (29.5±2.60 years). Subsequently, depending on the applied ART protocol, the groups were divided into subgroups. A short regimen (using gonadotropin-releasing hormone antagonists, ant-GnRH) was given to 45 patients in Group I (IA) and 48 patients in Group II (IIA). A long regimen (gonadotropin-releasing hormone agonists, aGnRH) was given to 45 patients in Group I (IB) and 42 patients in Group II (IIB).
Statistical analysis of the data was performed using the SPSS program. Differences in the values of categorical variables were assessed by means of contingency tables using the Pearson Chi-square test. For continuous variables, the authors used Student's t-test for normal distribution and the Mann-Whitney U-test for nonparametric data. The threshold value of significance was p=0.05.
Results
The duration of infertility for the whole cohort was 6.77±3.92 years. In Group I, it was 9.02±4.51 years; in Group II, it was 4.51±1.74 years (p<0.05), which seems to be a natural consequence of age differences between the groups. Within each group, the duration of infertility by subgroup (depending on the stimulation protocol) did not differ significantly.
The age of menarche was not statistically different between the groups and was 13.58±1.6 years old in Group I and 13.43±1.64 years old in Group II. The duration of the menstrual cycle averaged 28.36±3.16 days. Significant differences in its duration were observed between the groups. The shorter duration of the menstrual cycle was observed among the patients in Group I (p=0.001), which may be related both to the pathology accumulated with age and to a decrease in PR production by the corpus luteum in the second phase of the menstrual cycle. No significant differences were observed within each group, confirming the homogeneity of the group.
Significant differences (p<0.05) in the rate of primary and secondary infertility were found between the two age groups. In Group I, secondary infertility was diagnosed in 51 patients out of 90, and in Group II – in 11 out of 90. The probable explanation for this distribution is that the rate of pathology leading to infertility increased with age and the patients in Group I may have been previously fertile. The tubal-peritoneal infertility factor was found more frequently in patients from Group I than in Group II (p=0.063).
This distribution can probably be explained by the age-related accumulation of inflammatory pathology that can impair fallopian tube patency. To clarify this aspect, the authors compared the rate of genital pathology in both groups. The incidence of hereditary clotting disorders preventing conception and pregnancy was significantly higher in Group I (36.6% vs 11.1%, p=0.001). Analysis of the rate of extragenital pathology revealed no statistically significant difference between the groups (p>0.05).
The total number of surgical interventions in Group I patients was significantly higher (p=0.009). Unilateral tubectomy was performed most frequently (p=0.019), probably indicating the presence of an initial pro-inflammatory background.
The average hormonal profile parameters of all patients were within the normal reference intervals. However, there was a statistically significant increase in the FSH level in Group I patients, which was 9.73±2.43 pg/ml versus 8.74±2.50 pg/ml in Group II (p=0.008). Elevated FSH levels may be a marker of a tendency toward a decreased follicular reserve, which is observed in patients of older age groups. Decreased luteinizing hormone (LH) values in Group I of 7.05±2.4 pg/ml versus 8.01±2.1 in Group II patients (p=0.011) may indicate insufficient ovarian stimulation by the pituitary gland. LH deficiency or an untimely peak in LH concentration may be the cause of ART failure. The chosen preconception regimen had no significant effect on LH and FSH levels. Elevated prolactin levels can delay oocyte maturation and lead to anovulation, but in the cohort, this index was mostly within normal values (86–650 IU/L). The concentration of prolactin did not practically differ between the groups and depending on the preconception preparation schemes. It was 193.57±60.46 pg/ml in Group I and 208.22±96.88 pg/ml in Group II, p>0.05. The level of estradiol in peripheral blood was not statistically significantly different between age groups and within groups, being 299.222±145.79 pg/ml in Group I and 287.64±142.75 pg/ml in Group II (p>0.05). The PR concentration in Group I was 29.03±17.95 pg/ml and was significantly lower (p<0.05) than in Group II (34.77±17.24), which indicates relative hyperestrogenemia in older women of childbearing age associated with preserved estradiol level.
Antimueller hormone (AMH), along with follicular reserve and age of patients, is a predictor of pregnancy. Overall, the mean AMH level for the cohort was 3.13±1.93 ng/ml, and there were significant differences revealed between the two groups. The decreased value of AMH in patients from Group I (2.78±1.92 ng/ml) versus 3.48±1.89 ng/ml (Group II) may indicate impaired follicle maturation, which in turn, decreases the likelihood of pregnancy.
The endometrial thickness measured at ultrasound examination in the cohort averaged 7.78±2.45 mm: 7.50±2.21 mm in Group I and 8.06±2.66 mm (p=0.125) in Group II. The decrease in endometrial thickness in the older group confirms the suggestion of an insufficient proliferative phase, which is complemented by a decrease in PR levels in the older group as shown above. A decreased ovarian reserve (determined by the number of mature follicles on ultrasound) was observed in 30 (33%) patients ≥35 years old and only in 3 (3.3%) patients under 35 years old (p<0.05), since the follicular cell pool decreases with age.
Pipelle biopsy taken on days 19–21 of the menstrual cycle in Group I revealed significantly lower ER concentrations and higher PR concentrations in the glandular epithelium and endometrial stromal cells compared to the same parameters in Group II. Group I patients had slightly higher ER levels and significantly lower PR values in their blood compared to Group II patients' concentrations of these hormones. The increased concentration of PR receptors may be a compensatory response to the decreased PR concentration in patients ≥35 years of age (Table 1).
Table 1
Concentration (in points) of estrogen (ER) and progesterone (PR) receptors in the glandular epithelium and stromal cells of the endometrium
|
Receptors and their localization |
I group |
II group |
All cohort |
p |
|
ER in the cells of the glandular epithelium |
112,12± 40,96 |
124,92± 41,39 |
118,52± 41,56 |
0,038 |
|
ER in stromal cells |
121,72± 27,67 |
132,68± 30,30 |
127,2± 29,45 |
0,012 |
|
PR in the cells of the glandular epithelium |
174,23± 30,23 |
160± 30,05 |
167,52± 30,80 |
0,003 |
|
PR in stromal cells |
163,49± 30,57 |
138,96± 33,35 |
151,22± 34,19 |
0,001 |
The table below shows the content of the other remaining endometrial markers (Table 2).
Table 2
Infertility markers in the endometrium (in the number of cells in the visual field expressing this marker)
|
Markers and their localization |
I group |
II group |
p |
|
HLA-DR (MHC II) |
18,23± 8,81 |
19,91± 8,40 |
0,217 |
|
CD56 (NK-cells) |
12,96± 5,03 |
14,34± 5,26 |
0,073 |
|
CD138 (plasma cells) |
2,07± 1,39 |
2,02± 0,76 |
0,837 |
|
LIF in the glandular epithelium, % |
22± 7,59 |
26,1± 5,86 |
0,003 |
|
LIF in stromal cells, % |
21,3± 5,96 |
26,1± 3,00 |
0,003 |
There were no significant differences in most of the specified parameters. A significance level approaching the threshold (p=0.073) was determined for the level of CD56 (NK cells), the increased number of which indicates the tension of cellular immunity primarily in patients from Group II. An increased level of NK cells is associated with infertility [12]. Group I patients had a statistically significant decrease in LIF in both glandular epithelium and stromal cells, indicating a reduced ability of the endometrium to implant.
As a result of 180 cycles of ovulation stimulation, 1721 oocytes were obtained: 751 in Group I and 970 in Group II. In Group I, the number of obtained oocytes was significantly lower, being 8.34±3.51 versus 10.78±4.37 oocytes per induction in Group II (p<0.001). The median values were 8 and 10 oocytes per patient, respectively (Figure 1).
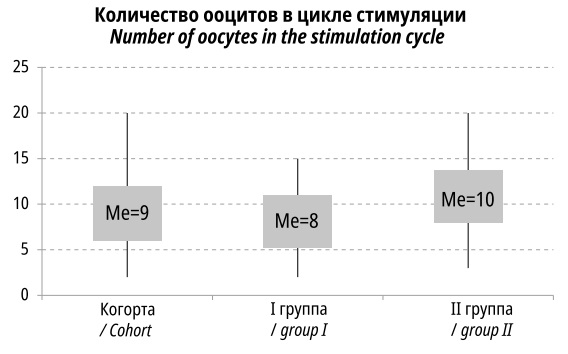
Figure 1. The number of oocytes obtained in the ovulation stimulation cycle (the rectangle covers 25–75% of the percentile, the upper and lower poles of the diagrams reflect the maximum and minimum values)
A probable explanation for the lower number of oocytes in the older age group is the decreased ovarian reserve, which is consistent with the decreased number of antral follicles, as mentioned earlier.
Of the 1721 oocytes obtained during ovulation induction, 1386 oocytes (80.5% of all obtained) were considered mature and of good quality, the rest were immature (12.5%) or degenerate (7%) (Figure 2). The number of mature oocytes in Group I was 533, and in Group II, it was 853.
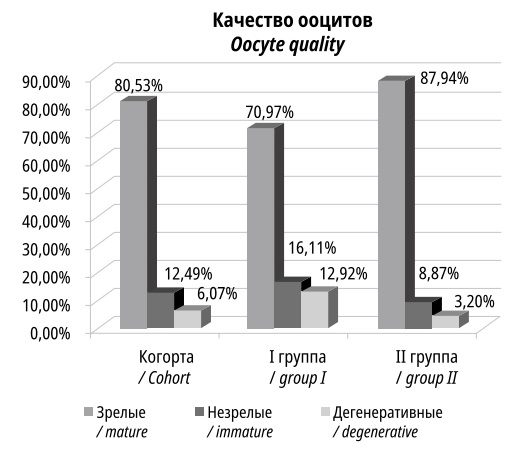
Figure 2. The number of mature, immature and degenerative oocytes
The decreased number of mature oocytes in Group I may be a consequence of insufficient stimulation by the pituitary gland and age-related decay of ovarian function. Good quality mature oocytes were used for ART procedures.
A total of 1,386 oocytes were fertilized, of which only 977 (70.5%) embryos of good and satisfactory quality were obtained.
The number of oocytes suitable for fertilization was lower (4.11±1.43 vs. 6.74±2.92) in patients 35 years and older than in Group II (p<0.001). The median values were also significantly different (Figure 3).
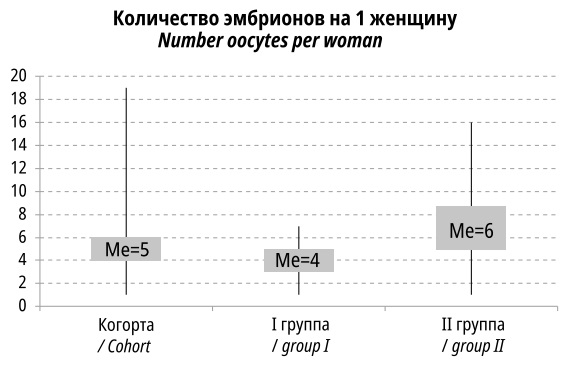
Figure 3. Number of fertilized eggs per woman
Differences in the preconception preparation protocols did not lead to significant differences in the number of the obtained embryos (Table 3).
Table 3
Number of received embryos in subgroups
|
Indicators |
IA |
IБ |
IIA |
IIБ |
|
Total amount |
186 |
184 |
333 |
274 |
|
Average±SD |
4,13±1,42 |
4,09±1,46 |
6,94±3,08 |
6,52±2,75 |
|
Median |
4 |
4 |
6 |
6 |
|
25%-persentile |
3 |
3 |
5 |
4,25 |
|
75%-persentile |
5 |
5 |
9 |
8 |
|
Min |
1 |
1 |
1 |
1 |
|
Max |
6 |
7 |
16 |
12 |
|
Significance |
P=0,607 |
P=0,636 |
||
Thus, there were no significant differences, associated with the stimulation protocol, detected up to the time of fertilization of the oocytes. The main differences were observed only between the different age groups.
On the 3rd and 5th days after fertilization, embryo quality was checked (performed by an embryologist). The final Gardner assessment was performed on day 5 (Table 4). Poor quality embryos were discarded.
Table 4
Assessment of the quality of embryos according to Gardner
|
Number of embryos |
All cohort |
I group |
II group |
|
|
Total amount |
840 |
306 |
534 |
|
|
Average±SD |
4,51±1,05 |
4,32±0,98 |
4,69±1,09 |
|
|
Median |
5 |
4 |
5 |
|
|
25%-persentile |
4 |
4 |
4 |
|
|
75%-persentile |
5 |
5 |
6 |
|
|
Min |
3 |
3 |
3 |
|
|
Max |
6 |
6 |
6 |
|
|
Significance |
|
p=0,018 |
||
Note: significant differences are marked in bold (p <0,05)
Statistical analysis of the data revealed a significant decrease in embryo quality (p=0.018) in Group I patients in the older reproductive age. Group II showed higher embryo quality.
On day 5, there were 306 embryos suitable for embryo transfer in Group I, and 534 embryos in Group II (p<0.001), which approximately corresponded to the distribution of the obtained oocytes. Relative to the number of quality embryos obtained immediately after fertilization, the content of quality embryos by day 5 of cultivation was 85.98% (82.70% in Group I, and 87.97% in Group II (Figure 4). Aneuploidy, which occurrence increases with the age of the patient, could have contributed to the loss of quality embryos.
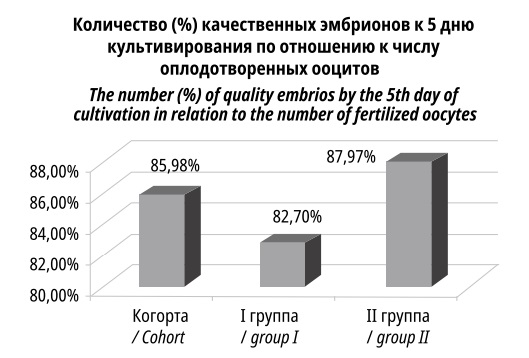
Figure 4. The ratio of the number of good quality embryos to the number of fertilized oocytes
The choice of the stimulation protocol had no effect on embryo quality (p=0.940). Thus, the quality of the obtained embryos depended mainly on age rather than on the stimulation protocol.
Of the total cohort, 53 (29.40%) patients were verified to be pregnant based on the results of ultrasound and laboratory tests. In Group I, pregnancy occurred in 20 (22.22%) cases, and in Group II, in 33 (36.67%) cases (p=0.034). There were no significant differences depending on the stimulation protocol (Table 5).
Table 5
Difference in pregnancy rate depending on age (group) and stimulation protocol (subgroup)
|
|
Cohort, % (n) |
|||
|
Frequency |
29,43% (53) |
|||
|
Groups |
I (≥35 years old) |
II (<35 years old) |
||
|
Frequency |
22,22% (20) |
36,67% (33) |
||
|
P (Chi-square) |
p=0,034 |
|||
|
Subgroups |
IA |
IБ |
IIA |
IIБ |
|
Frequency |
10% (9) |
12,22% (11) |
22,22% (20) |
14,44% (13) |
|
P (Chi-square) |
p=0,612 |
p=0,293 |
||
The results are consistent with published data on a decrease in fertility in patients with age, especially in the 31–38 year range [13][14]. Potential predictors of pregnancy for the entire cohort of patients were also analyzed (Table 6).
Table 6
Distribution of a number of parameters in the onset and absence of pregnancy for the whole cohort
|
Indicators |
Options |
(+) pregnancy |
(-) pregnancy |
P |
|
FSH |
Средн.±SD |
9,41±1,91 |
9,16±2,72 |
0,626 |
|
Prolactin |
Средн.±SD |
194,1±72,67 |
203,72±84,15 |
0,468 |
|
Progesterone |
Средн.±SD |
40,44±17,50 |
28,33±16,71 |
<0,001 |
|
Е2 |
Средн.±SD |
301,08±151,09 |
289,25±141,56 |
0,617 |
|
LH |
Средн.±SD |
7,89±1,91 |
7,37±2,48 |
0,132 |
|
AMG |
Средн.±SD |
2,84±1,98 |
3,25±1,91 |
0,189 |
|
Endometrial thickness |
Средн.±SD |
9,18±2,25 |
7,19±2,30 |
<0,001 |
|
Oocyte count |
Средн.±SD |
11,21±4,33 |
8,87±3,86 |
0,001 |
|
Number of embryos by day 5 |
Средн.±SD |
5,60±3,27 |
4,28±2,24 |
0,023 |
As can be seen from the data presented in the table, the increased occurrence rate of pregnancy coincides with higher PR levels, greater endometrial thickness, and an increased number of oocytes and embryos by day 5 of cultivation. These parameters indicate better endometrial readiness for implantation and higher quality of embryos in patients with pregnancy (Tables 6, 7).
Table 7
The content of hormones in the blood of patients
|
Groups |
I |
II |
||
|
Pregnancy |
(+) pregnancy |
(-) pregnancy |
(+) pregnancy |
(-) pregnancy |
|
N |
20 |
70 |
33 |
57 |
|
FSH, M±SD |
10,09±1,74 |
9,63±2,59 |
8,99±1,92 |
8,60±2,79 |
|
p |
0,455 |
0,471 |
||
|
0,008 |
||||
|
Prolactin, M±SD |
206,5±53,14 |
189,87±62,24 |
186,59±82,16 |
220,74±103,07 |
|
p |
0,280 |
0,107 |
||
|
0,225 |
||||
|
Progesterone, M±SD |
36,88±21,93 |
26,79±16,13 |
42,61±14,12 |
30,23±17,35 |
|
p |
0,067 |
<0,001 |
||
|
0,030 |
||||
|
Е2, M±SD |
335,55±169,42 |
288,84±137,92 |
280,19±137,34 |
289,75±147,15 |
|
p |
0,208 |
0,762 |
||
|
0,547 |
||||
Although the FSH level (before the stimulation protocol) was elevated in the pregnant patients, this difference was not significant between pregnant and nonpregnant patients. Still, there were previously identified elevated FSH levels in Group I patients. Differences in PR levels between pregnant and nonpregnant patients were more pronounced in Group II (p<0.001 and p=0.067, respectively) (Table 8).
Table 8
Parameters of patients with different ART outcomes
|
Groups |
I |
II |
||
|
Pregnancy |
(+) pregnancy |
(-) pregnancy |
(+) pregnancy |
(-) pregnancy |
|
N |
20 |
70 |
33 |
57 |
|
LH, M±SD |
7,83±2,25 |
6,82±2,45 |
7,92±1,72 |
8,05±2,37 |
|
p |
0,085 |
0,882 |
||
|
0,011 |
||||
|
AMG, M±SD |
1,74±1,32 |
3,07±1,97 |
3,51±2,02 |
3,46±1,82 |
|
p |
0,007 |
0,956 |
||
|
0,012 |
||||
|
Endometrial thickness, M±SD |
8,95±1,73 |
7,08±2,16 |
9,33±2,53 |
7,32±2,46 |
|
p |
0,001 |
<0,001 |
||
|
0,129 |
||||
|
Oocyte count, M±SD |
9,20±3,53 |
8,10±3,49 |
12,42±4,37 |
9,82±4,12 |
|
p |
0,225 |
0,004 |
||
|
<0,001 |
||||
|
Number of embryos by day 5, M±SD |
3,55±1,36 |
3,36±1,60 |
6,85±3,47 |
5,40±2,40 |
|
p |
0,308 |
0,055 |
||
|
<0,001 |
||||
AMH levels were lower in patients ≥35 years of age with established pregnancy than in non-pregnant women. This may be due to the peculiarities of the examined population, as well as due to the fact that the AMH test was performed in the cycles preceding ART. In Group II, there were no significant differences in the AMH level.
In both groups, the endometrial thickness was significantly higher in patients with an incipient pregnancy, which reflects the better readiness of the uterine mucosa for embryo implantation. In Group II, the number of oocytes obtained (p=0.004) and quality embryos by day 5 (p=0.055) correlated better with the onset of pregnancy than in Group I.
In Group II, the number of oocytes and quality embryos by day 5 of cleavage was higher for pregnancy onset. It is possible that the structure of infertility differs by age, and at the age of 35 years old and older, the main causes of the disturbance are the endometrial condition and the quality of the embryos (the probability of aneuploidy increases), in the younger age groups the outcome of ART depends more on the efficiency of gametogenesis and the quality of the embryo itself. It is possible that in the older age group, the rate of infertility was more evidently associated with endometrial receptivity than embryo quality.
Of the 180 examined patients, 38 (21.11%) were recorded to have live births (13 (14.44%) in Group I, 25 (27.78%) (p=0.028) in Group II). Thus, there was an almost twofold excess of live births in Group II. Within both groups, there were no significant differences depending on the stimulation protocol (Group I, p=0.76; Group II, p=0.208).
Further, the authors evaluated possible predictors of live birth rate (Table 9).
Table 9
Distribution of parameters in women who gave birth and in the rest of the cohort
|
Indicators |
Options |
(+) childbirth |
(-) childbirth |
P |
|
FSH |
Average±SD |
9,34±1,67 |
9,21±2,69 |
0,707 |
|
Prolactin |
Average±SD |
187,67±72,19 |
204,43±82,90 |
0,258 |
|
Progesterone |
Average±SD |
40,39±17,27 |
29,63±17,28 |
0,001 |
|
Е2 |
Average±SD |
293,35±154,45 |
292,57±141,79 |
0,976 |
|
LH |
Average±SD |
8,20±1,77 |
7,35±2,44 |
0,035 |
|
AMG |
Average±SD |
2,80±2,03 |
3,22±1,90 |
0,211 |
|
Endometrial thickness |
Average±SD |
10,21±1,44 |
7,13±2,25 |
<0,001 |
|
Oocyte count |
Average±SD |
12,61±3,50 |
8,75±3,92 |
<0,001 |
|
Number of embryos by day 5 |
Average±SD |
6,32±3,13 |
4,22±2,31 |
<0,001 |
The results generally repeat the trends found in diagnosed pregnancy. Patients who gave birth had elevated levels of PR, LH, a thicker endometrium, and a higher number of oocytes and embryos by day 5 of culture (Table 10).
Table 10
Values of a number of parameters in patients
|
Groups |
I |
II |
||
|
Pregnancy |
(+) роды |
(-) роды |
(+) роды |
(-) роды |
|
N |
13 |
77 |
25 |
65 |
|
FSH, M±SD |
9,91±2,00 |
9,70±2,50 |
9,04±1,42 |
8,62±2,81 |
|
p |
0,769 |
0,482 |
||
|
0,008 |
||||
|
Prolactin, M±SD |
207,46±52,24 |
191,22±61,73 |
177,38±79,67 |
220,08±100,78 |
|
p |
0,373 |
0,061 |
||
|
0,258 |
||||
|
Progesterone, M±SD |
32,92±20,92 |
28,37±17,47 |
44,28±13,96 |
31,11±17,06 |
|
p |
0,401 |
0,001 |
||
|
0,001 |
||||
|
Е2, M±SD |
341,46±172,42 |
292,09±140,86 |
268,34±141,45 |
293,14±143,98 |
|
p |
0,261 |
0,464 |
||
|
0,976 |
||||
|
LH, M±SD |
8,28±1,79 |
6,84±2,47 |
8,16±1,80 |
7,95±2,28 |
|
p |
0,039 |
0,586 |
||
|
0,035 |
||||
|
AMG, M±SD |
1,69±1,36 |
2,96±1,95 |
3,37±2,10 |
3,52±1,82 |
|
p |
0,032 |
0,669 |
||
|
0,211 |
||||
|
Endometrial thickness, M±SD |
9,85±1,28 |
7,10±2,09 |
10,39±1,50 |
7,16±2,45 |
|
p |
<0,001 |
<0,001 |
||
|
<0,001 |
||||
|
Oocyte count, M±SD |
10,85±3,34 |
7,92±3,37 |
13,52±3,28 |
9,72±4,30 |
|
p |
0,006 |
<0,001 |
||
|
<0,001 |
||||
|
Number of embryos by day 5, M±SD |
4,08±1,32 |
3,29±1,55 |
7,52±3,16 |
5,32±2,57 |
|
p |
0,056 |
0,004 |
||
|
<0,001 |
||||
As in the analysis of pregnancy onset, the rate of live births obviously coincides with the increased levels of LH, PR, greater endometrial thickness, a larger number of obtained oocytes and embryos by day 5 of cultivation. For prolactin, FSH, and estradiol values, there were no significant correlations with the outcome of ART.
Discussion
The study of a cohort of 180 patients with a history of infertility revealed several factors consistent with impaired fertility in patients ≥35 years old. As compared to Group II, they were found to have a longer duration of infertility (9.02±4.51 vs 4.51±1.74 years) and a higher incidence of secondary infertility (28.3% vs 6.1%). Patients older than 34 years old were more frequently diagnosed with inflammatory and oncological diseases (polyps, fibroids, salpingoophoritis, endometritis) accompanied by a high rate of the adhesive process, tubal-peritoneal infertility factor, and unilateral tubectomies in the anamnesis (p<0.05). The duration of the menstrual cycle was 27.15±3.39 days in Group I and 29.57±2.38 days in Group II (p=0.001), and this could reflect an age-related decrease in PR production by the corpus luteum.
The LH level in Group I (senior) was significantly decreased, while the FSH concentration, on the contrary, was increased compared to similar parameters in Group II. Low LH production may lead to insufficient ovarian stimulation by the pituitary gland, while the elevated FSH levels rather reflect a decrease in ovarian reserve that progresses with age. Low PR levels in patients ≥35 years old (29.03±17.95 vs 34.77±17.24) and a decreased AMH concentration (2.78±1.92 vs 3.48±1.89) compared to those in Group II were likely markers of depression of ovarian function. Endometrial thickness was not significantly reduced in Group I, being 7.50±2.21 mm, and 8.06±2.66 mm in Group II (p=0.125). A decreased ovarian reserve (according to ultrasound findings) was detected in 33% of patients in Group I and only 3.3% in Group II. A significant increase in the concentration of PR receptors and a decrease in the concentration of ER receptors and LIF levels were detected in uterine mucosal aspirates in Group I. Thus, the laboratory and instrumental data suggest an accumulated genital pathology, decreased ovarian reserve, and insufficient endometrial readiness for implantation in older patients.
A decrease in oocytes and embryos of good quality (p<0.05), which were obtained at different stages of cultivation, is consistent with the data on a decrease in ovarian reserve and impaired hormonal regulation in Group I. Moreover, the proportion of good-quality embryos decreased more intensively in Group I by day 5, which may indicate latent defects that cannot be detected only by morphological signs.
In Group I, 22.22% of the patients became pregnant, the number of live births was 14.44%; in Group II, the rates were 36.67% and 27.78%, respectively. Moreover, the stimulation protocol had no effect on the outcome of ART. This confirms the progressive decline in fertility described in the publications in patients aged 31–38 years [9][10]. The most reliable predictors of a favorable outcome of ART were an increased PR level, greater endometrial thickness, a large number of oocytes obtained by transvaginal puncture, and the number of quality embryos by day 5 of cultivation. The increased thickness of the functional layer of the endometrium appears to be associated with a high PR concentration in the second phase of the menstrual cycle and corresponds to better endometrial receptivity. The ultimate success of ART procedures is determined by a large set of factors, but with age, the role of endocrine, inflammatory, oncological pathologies that can affect female fertility increases. Besides, defects of the embryonic factor and implantation factor of the endometrium are observed.
Conclusion
The study on the infertility patterns in patients ≥35 and younger than 35 years of age led to the following conclusions:
1) Pregnancy in patients ≥35 years old as a result of the ART procedures was registered in 22.22%, and the live birth rate was 14.44%, which was significantly lower than in patients <35 years old, where pregnancy and live birth rates were 36.67% and 27.78%, respectively (p<0.05).
2) Patients ≥35 years of age had lower PR levels accompanied by a reactive increase in PR receptors in the endometrium.
3) There was a significant decrease revealed in endometrial implantation factor provided by LIF in patients ≥35 years of age.
4) The AMH concentration in the blood of patients in the older age group was significantly lower than in the younger group and was 2.78±1.92 vs 3.48±1.89 (p=0.012), indicating a decrease in ovarian reserve.
5) Patients in the ≥35-year-old group had a significantly lower number of mature oocytes and quality embryos than patients younger than 35 (p<0.05), consistent with age-related depletion of follicular reserve and decreased oocyte quality.
6) Favorable outcomes of ART were more often observed in patients with a larger functional endometrial layer, a high PR concentration, and a larger number of mature oocytes and quality embryos (p<0.05).
References
1. Solov'eva T.V., Karaseva A.S. Faktory determinacii pozdnego detorozhdeniya u zhenshchin fertil'nogo vozrasta v respublike Mordoviya. Kazanskij social'no-gumanitarnyj vestnik. 2018; №4: 32-35. DOI: 10.24153/2079-5912-2018-9-4-32-35. (In Russ.).
2. Broer S.L., van Disseldorp J., Broeze K.A., Dolleman M., Opmeer B.C., Bossuyt P., Eijkemans M.J.C., Mol B.W.J., Broekmans F.J.M. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum. Reprod. Update. 2013; 19(1): 26-36. DOI: 10.1093/humupd/dms041.
3. Santoro N., Isaac B., Neal-Perry G., Adel T., Weingart L., Nussbaum A., Thakur S., Jinnai H., Khosla N., Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women. J. Clin. Endocrinol. Metab. 2003; 88(11): 5502-9. DOI: 10.1210/jc.2002-021839.
4. Steiner A.Z., Pritchard D., Stanczyk F.Z., Kesner J.S., Meadows J.W., Herring A.H., Baird D.D. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA. 2017; 318(14): 1367-1376. DOI: 10.1001/jama.2017.14588.
5. Liao S., Xiong J., Tu H., Hu C., Pan W., Geng Y., Pan W., Lu T., Jin L. Prediction of in vitro fertilization outcome at different antral follicle count thresholds combined with female age, female cause of infertility, and ovarian response in a prospective cohort of 8269 women. Medicine (Baltimore). 2019; 98(41): P.e17470. DOI: 10.1097/MD.0000000000017470.
6. Bashiri A., Halper K.I., Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018; 6(1): 121. DOI: 10.1186/s12958-018-0414-2.
7. Tanbo T., Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017; 96(6): 659-667. DOI: 10.1111/aogs.13082.
8. Broi M.G.D., Ferriani R.A., Navarro P.A. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist. Reprod. 2019; 23(3): 273-280. DOI: 10.5935/1518-0557.20190029.
9. Vlahos N.F., Theodoridis T.D., Partsinevelos G.A. Myomas and Adenomyosis: Impact on Reproductive Outcome. Biomed Res Int. 2017; 2017: 5926470. Published online 2017 Nov 6. DOI: 10.1155/2017/5926470.
10. Vuković P., Kasum M., Raguž J., Lonjak N., BilićKnežević S., Orešković I., BeketićOrešković L., Čehić E. Fertility preservation in young women with early-stage breast cancer. ActaClin Croat. 2019; 58(1): 147-156. DOI: 10.20471/acc.2019.58.01.19.
11. Fritz R., Jindal S.J. Reproductive aging and elective fertility preservation. // Ovarian Res. 2018; 11(1): 66. DOI: 10.1186/s13048-018-0438-4.
12. Azargoon A., Mirrasouli Y., ShokrollahiBarough M., Barati M., Kokhaei P.The State of Peripheral Blood Natural Killer Cells and Cytotoxicity in Women with Recurrent Pregnancy Loss and Unexplained Infertility. Int. J. Fertil. Steril. 2019; 13(1): 12-17. DOI: 10.22074/ijfs.2019.5503.
13. Krutova V.A., Kovalenko YA.A. Sovremennye predstavleniya o matochnoj forme besplodiya. Elektronnyj zhurnal. Sovremennye problem nauki i obrazovaniya. 2018; №3. URL: http://www.science-education.ru/ru/article/view?id=27568 (access: 19.04.2020). (In Russ.).
14. Steiner A.Z., Jukic A.M. Impact of female age and nulligravidity on fecundity in an older reproductive age cohort. Fertil Steril. 2016; 105(6): 1584-1588.e1. DOI: 10.1016/j.fertnstert.2016.02.028.
About the Authors
K. V. UryupinaRussian Federation
Kristina V. Uryupina, junior Researcher, Department of Obstetrics, Gynecology and Perinatology
Krasnodar
I. I. Kutsenko
Russian Federation
Irina I. Kutsenko, Dr. Sci. (Med.), Professor, head of the Department of obstetrics, gynecology and Perinatology
Krasnodar
E. I. Kravtsovа
Russian Federation
Elena I. Kravtsova, Cand. Sci. (Med.), associate Professor of obstetrics, gynecology and Perinatology
Krasnodar
I. N. Lukoshkina
Russian Federation
Irina N. Lukoshkina, Cand. Sci. (Med.), associate Professor of obstetrics,gynecology and Perinatology
Krasnodar
O. V. Tomina
Russian Federation
Oksana V. Tomina, Cand. Sci. (Med.), associate Professor of obstetrics, gynecology and Perinatology
Krasnodar
L. V. Kaushanskaya
Russian Federation
Lyudmila V. Kaushanskaya, Dr. Sci. (Med.), Professor, head of the Simulation Center
Rostov-on-Don
Review
For citations:
Uryupina K.V., Kutsenko I.I., Kravtsovа E.I., Lukoshkina I.N., Tomina O.V., Kaushanskaya L.V. Investigation of the infertility structure and outcomes of ART programs in patients of late reproductive age. Medical Herald of the South of Russia. 2022;13(2):59-71. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-59-71







































