Scroll to:
Clinical and diagnostic significance of apoptosis markers in myocardial infarction in the setting of chronic obstructive pulmonary disease
https://doi.org/10.21886/2219-8075-2021-12-4-46-53
Abstract
Objective: To study the levels of heat shock protein 70 (HSP70) and annexin V-dependent apoptosis of cells in myocardial infarction (MI) with a different course of the disease and in the setting of chronic obstructive pulmonary disease (COPD).
Materials and Methods: Sixty-five patients with MI were examined; 37 of them developed MI in the setting of COPD, and 28 patient had MI without COPD. The subjects were divided into subgroups depending on the presence of ST segment elevation. The control group included 30 somatically healthy individuals.
Results: In all examined patients with MI and in patients with MI with COPD, the number of annexin V-associated apoptotic cells and the level of HSP70 was statistically significantly higher than in healthy individuals. The value of the studied biomarkers was statistically significantly higher than in patients without COPD. In patients with MI with ST segment elevation, the values of the studied biomarkers were statistically significantly higher in patients without ST segment elevation in all the examined patients.
Conclusions: Higher values of the studied biomarkers in the group of comorbid patients compared with those with MI without COPD may be associated with intensified apoptosis in the setting of chronic systemic inflammation, which worsens the clinical course of both diseases. The greater significance of the levels of HSP70 and annexin V-dependent cell apoptosis in patients with MI with ST segment elevation compared with patients without ST segment elevation is due to the volume of myocardial lesion.
Keywords
For citations:
Naumov A.V., Prokofieva T.V., Polunina O.S., Saroyants L.V., Polunina E.A. Clinical and diagnostic significance of apoptosis markers in myocardial infarction in the setting of chronic obstructive pulmonary disease. Medical Herald of the South of Russia. 2021;12(4):46-53. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-46-53
Introduction
Currently, the occurrence rate of diseases associated with comorbid conditions, in particular Chronic Obstructive Pulmonary Disease (COPD), significantly increases. These conditions often lead to lethal outcomes [1]. According to some clinical and laboratory parameters, COPD aggravates the course of the majority of known diseases. In the group of diseases comorbid to COPD, cardiovascular diseases (CVD) are the most significant. In patients with COPD, comorbid CVDs are essentially associated with a higher risk of hospitalization within 30 days as well as an increase in the inpatient period [2]. Besides, CVDs increase the risk and duration of COPD exacerbations [3], and in turn, exacerbations increase the risk of further cardiovascular impairments [4].
The main cause of COPD is smoking that causes endothelial dysfunction. Patients with COPD have endothelial alterations in the pulmonary and systemic blood flow, which can promote pulmonary hypertension and/or cardiovascular events [5][6]. The issue of comorbid IHD and COPD pathology is complicated and remains understudied. The studied pathologies aggravate the course of чего? and accelerate the rate of mutually-affected development.
The understanding of pathogenetic aspects that associate COPD and various forms of CVD significantly expanded during the past decades [7][8] but the mechanisms that underlie them are still not clear. The key aspects include general risk factors, chronic systemic inflammation, lung hyperinflation, tissue hypoxia, pulmonary hypertension, oxidative stress, as well as some genetic factors, and COPD phenotype [9]. One of the general mechanisms that attract researchers’ interest is apoptosis. Apoptosis of endothelial cells is one of the factors that contribute to the development of pulmonary emphysema and is considered to be an additional criterion of endothelial and hemostasis impairment. In patients with MI, apoptosis plays a key role in the realization of the cardiac lesion.
Annexin V is a frequently used apoptosis marker. This is a calcium-dependent glycoprotein with a potent anticoagulant activity that exerts its effect in vitro. This feature is provided by the capacity of annexin V to compete with coagulative protein on phospholipid surface prolonging phospholipid-dependent coagulative reactions. Annexin V exerts a high affinity to apoptotic structures that produce a lot of phospholipids, in particular, phosphatidylserine (PS). This is confirmed by numerous studies both in vitro [10] and in vivo [11]. Annexin V is a protein with positive potential that exerts high affinity and specificity when binding with negatively charged PS on the surface of apoptotic cells. Phospholipids can be detected during fluorescent detection of annexin V with flow cytometry. There are data on the possibility of application of annexin V for the monitoring of apoptosis in real-time [12].
Apoptosis is regulated by stress-induced heat shock proteins (HSP). One of the main representatives of such proteins is 70 kDa HSP (HSP70). HSP70 is a protein of self-protection that maintains cellular hemostasis in stress conditions. It acts as a molecular chaperone and plays an important role in the folding, assembly, transport, and degradation of proteins. It also helps to prevent the denaturation and aggregation of protein [13]. However, this protein exerts not only an anti-apoptotic effect. There is evidence that it can also affect the opposite pro-apoptotic effect. In other words, the mechanisms of association between apoptosis and expression of HSP remain unclear.
A better understanding of the conditions that cause apoptosis during myocardial infarction and after it as well as an understanding of cellular mechanisms that control apoptosis can lead to the development of new therapeutic strategies limiting myocardial tissue damage in patients with infarction.
The study aimed to evaluate the levels of HSP70 and annexin V-dependent apoptosis of cells in patients with myocardial infarction (MI) of a different course and in the setting of chronic obstructive pulmonary disease (COPD).
Materials and Methods
A total of 65 patients with myocardial infarction were examined, 37 of them developed MI in the setting of COPD, and 28 of them did not have COPD as comorbid pathology. The study was conducted in the Regional Vascular Center of Alexandro-Mariinsky Hospital in Astrakhan in 2018-2019. All patients signed informed consent before the inclusion in the study.
The age median of the studied patients was 52.8 [ 45.8; 60] years old. MI was verified based on clinical recommendations “Fourth Universal Definition of Myocardial Infarction” of the European Society of Cardiology (2018). The criteria of inclusion in the main groups were age <60 years old and MI type I. The study did not include patients with other types of MI, end-stage liver disease, renal failure (GFR < 30 ml/min), and oncological diseases.
In the group of patients with MI and COPD, diagnosis “Pulmonary pathology” and the severity of COPD were verified earlier. All patients with COPD had II-III degree of the disease in the non-acute period. The diagnosis COPD was verified according to the program “Global strategy of diagnostics, treatment, and prevention of chronic obstructive pulmonary disease” (revision 2019). The median of COPD duration was 17.5 [ 7.4; 24.6] years. All patients had smoking in the anamnesis. At the moment of hospitalization, there were 87.8% of smokers with COPD. The mean index of smoking was 34.6 packs-years. In 96% of cases, patients were long-term smokers by the moment of inclusion in the study.
To evaluate the associations between the studied parameters, patients with MI were divided into subgroups depending on the elevation of ST-segment on ECG (with elevation and without it). Among patients with MI without comorbid COPD, 14 patients were diagnosed with STEMI and 14 patients – non-STEMI. Among patients with MI and comorbid COPD, 19 patients had STEMI and 18 patients had non-STEMI.
The control group included 30 somatically healthy volunteers that underwent examination at various polyclinics in Astrakhan. Volunteers from the control group were comparable with the group of patients with MI by age and sex and were somatically healthy.
The study was approved by the Regional Independent Ethical Committee (dated 15.09.2016, Protocol №1). The study was conducted according to the international GCP standards.
To measure the levels of apoptotic cells, the authors used a kit ANNEXIN V-FITC/7 AAD (Beckman Coulter, USA). The reaction of reagents is based on a high affinity of annexin V and PS and specific binding of 7-aminoactinomycin (7AAD) with nucleotide pairs guanine-cytosine in a DNA. The isolation of mononuclears was made by Ficoll-Hypac density gradient centrifuging of venous blood (Pharmacia Fine Chemicals, Sweden). A population of lymphocytes was used to detect apoptotic and/or necrotic cells with 2D histograms. Apoptosis was analyzed during flow cytometry using “Navios” (Beckman Coulter, USA). Control cells included in the setting of compensation and quadrant were represented only by cells stained with annexin A FITC and cells stained only with 7ADD. The cells were defined as intact cells (viable cells (AV-/7AAD-)), cells at the early stage of apoptosis (AV+/7AAD-), cells at the late stage of apoptosis (AV+/7AAD+), and dead cells (AV-/7AAD+). Each subpopulation was expressed as a percent of the total mononuclear number. The concentration of HSP70 (ng/ml) was measured by the method of enzyme immunoassay using a kit (“Enzo Life Science”, USA), microplate photometer “Invitrologic” (Russia), microplate washer “Stat Fax 2600” (USA), and shaker “ST-3 SkyLine” (Latvia).
Statistical processing of the data was made using the software STATISTICA 10.0. Descriptive characteristics are represented by a median and 25 and 75 percentiles. To compare two independent groups, the authors used a rank variance analysis with the application of the non-parametric Wilcoxon-Mann-Whitney test. For three and more groups, the Kruskal-Wallis test was used. The differences were considered statistically significant at р <0.05.
Results
The analysis of the levels of circulating annexin V-mononuclears in the peripheral blood in patients with MI and somatically healthy volunteers showed that the highest count of apoptotic cells was observed in patients with MI and COPD (Fig. 1).
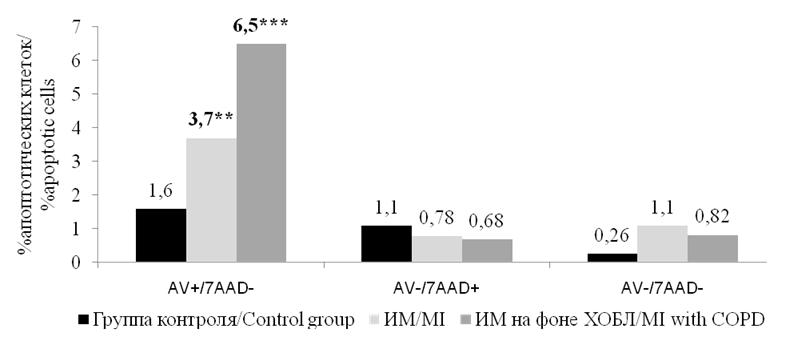
Рисунок 1. Медианы количества циркулирующих аннексин V-мононуклеаров (%) в периферической крови у больных ИМ и соматически здоровых лиц. Примечание: ** — р <0,01, *** — р<0,001; статистически значимые различия с группой контроля.
Figure 1. Medians of the number of circulating annexin V-mononuclears (%) in peripheral blood in patients with MI and somatically healthy individuals. Note: ** — p<0.01, *** — p<0.001, statistically significant differences with the control group.
In this group of patients, the content of apoptotic mononuclears was 6.5 [ 3.4–12.3] %, which was significantly higher than in the control group, wherein the content of these cells was 1.6 [ 0.8–2.4] % (р <0.001). It was also higher than in patients with MI without COPD (3.7 [ 2.1–4.9] %) (р<0.01). Patients with MI and without COPD also had statistically significant differences in comparison with the control group (р<0.01). There were no statistically significant differences in the content of other circulating mononuclears, viable cells (intact cells), cells at the late stage of apoptosis, and dead cells.
The study of the levels of HSP70 showed that regardless of the presence of comorbid bronchopulmonary pathology in patients with MI, this parameter was significantly higher in comparison with somatically healthy volunteers. The highest level of HSP70 was observed in the group of patients with MI and COPD (Fig. 2).
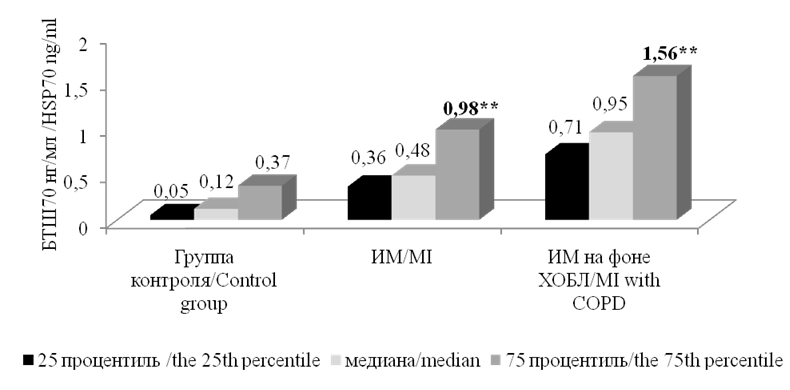
Рисунок 2. Уровни БТШ70у больных ИМ и здоровых лиц. Примечание: ** — статистически значимые различия с группой контроля, р <0,01.
Figure 2. The levels of BTSH70U of MI patients and healthy individuals. Note: ** — statistically significant differences with the control group, p <0.01.
In patients from this group, the level of HSP70 was 0.95 [ 0.71–1.56] ng/ml, which was significantly higher in comparison with the group of patients with MI and without COPD (0.48 [ 0.6–0.98] ng/ml) (р <0.05) and the control group (0.12 [ 0.05–0.37] ng/ml) (р<0.01). In the group of patients with MI and without COPD, the level of HSP70 was significantly higher (p<0.01) in comparison with the control group.
The study of the associations between the levels of annexin-dependent apoptosis in patients with MI and ST-segment elevation showed that patients with MI and ST-segment elevation had increased levels of apoptotic cells regardless of the comorbid bronchopulmonary pathology (Table 1).
Таблица / Table 1
Содержание циркулирующих аннексин V-мононуклеаров (%) в зависимости от подъёма сегмента ST при ИМ
The content of circulating annexin V-mononuclears (%) depending on ST segment elevation in MI
|
Показатели / Indicators |
ИМ / MI |
ИМ на фоне ХОБЛ / MI withCOPD |
||
|
ИМпST / STEMI, n=14 |
ИМбпST / non-STEMI, n=14 |
ИМпST / STEMI, n=19 |
ИМбпST / non-STEMI, n=18 |
|
|
Интактные клетки (аннексинV-7AAD-клетки) / Intact cells (AnnexinV-7AADcells) |
94,5 [ 92,8–96,5] |
96,3 [ 95,3–97,2] |
90,2 [ 84,4–94,3] |
92,4 [ 82,7–95,5] |
|
Ранняя стадия апоптоза (аннексинV+7AAD- клетки) / Early stage of apoptosis (annexinV+7AAD cells) |
3,9 [ 2,3–4,9] р1<0,05 |
2,4 [ 1,8–3,1] |
7,6 [ 4,4–12,3] р1 <0,01 р2 <0,05 р3=0,16 |
5,9 [ 3,1–9,7] р1 <0,05 |
|
Поздняя стадия апоптоза (аннексин V+7AAD+ клетки) / Late stage of apoptosis (annexin V+7AAD+ cells) |
0,95 [ 0,76–1,34] |
0,73 [ 0,59–0,87] |
1,2 [ 0,77–1,53] |
1,01 [ 0,89–1,13] |
|
Погибшие клетки (аннексин V-7AAD+ клетки) / Dead cells (annexin V-7AAD+ cells) |
0,65 [ 0,44–0,96] |
0,58 [ 0,45–0,71] |
0,97 [ 0,55–1,79] р1 <0,05 р2 <0,05 р3 <0,05 |
0,67 [ 0,47–0,75] |
Note: p1 — statistically significant differences with the group of non-STEMI; p2 — statistically significant differences with the group of STEMI; p3 — statistically significant differences with the group of non-STEMI with COPD.
In the group of STEMI, the content of cells at the early stage of apoptosis was 3.9 [ 2.3–4.9] %, which was significantly higher (p<0.05) than in the group of non-STEMI, wherein the level of these cells was 2.4 [ 1.8–3.1] %. The most significant increase in the level of apoptotic cells at this stage of apoptosis was observed in patients with MI and ST-segment elevation in the group of comorbid patients (7.6 [ 4.4–12.3] %), which significantly exceeded this parameter both in the group of non-STEMI (P<0.01) and a group of STEMI (р <0.05).
In the group of patients with comorbid COPD and non-STEMI, this parameter was 5.9 [ 3.1–9.7] %, which was significantly higher (р <0.05) than this parameter in the group of non-STEMI. A comparison of the content of dead cells showed that in the comorbid group with ST-segment elevation, their levels were significantly higher (p<0.05) than in other groups of patients. There were no statistically significant differences in the levels of other cells.
The analysis of the dependence between the level of HSP70 and ST-segment elevation (Table 2) showed that STEMI patients with comorbid COPD had the highest levels of HSP70 (1.22 [ 0.95–1.56] ng/ml.
Таблица / Table 2
Значение уровня БТШ70 (нг/мл) в зависимости от подъёма сегмента ST при ИМ
HSP70 levels (ng/ml) depending on ST segment elevation in MI
|
ИМ/MI |
ИМ на фоне ХОБЛ/MI with COPD |
||
|
ИМпST / STEMI, n=14 |
ИМбпST / non-STEMI, n=14 |
ИМпST / STEMI, n=19 |
ИМбпST / non-STEMI, n=18 |
|
0,57 [ 0,46–0,98] р1 <0,05 р3=0,16 |
0,41 [ 0,36–0,69] |
1,22 [ 0,95–1,56] р1 <0,01 р2 <0,05 р3 <0,05 |
0,81 [ 0,71–1,2] р2 <0,05 р3=0,16 |
Note: p1 — statistically significant differences with the group of non-STEMI; p2 — statistically significant differences with the group of STEMI; p3 — statistically significant differences with the group of non-STEMI with COPD.
This parameter was significantly higher than in STEMI group (0.57 [ 0.46–0.98] ng/ml) (р <0.05) and in non-STEMI group (0.41 [ 0.36–0.69] ng/ml) (р <0.01). Besides, statistically significant differences were observed in the group of non-STEMI and comorbid COPD (0.81 [ 0.71–1.2] ng/ml) (р <0.05).
In the group of patients with MI, there was a statistically significant increase in the level of HSP70 in patients with ST-segment elevation in comparison with a group of non-STEMI (0.41 [ 0.36–0.69] ng/ml) (р <0.05). In all the groups, the levels of HSP70 were significantly higher in patients with ST-segment elevation in comparison with a group of non-STEMI.
Discussion
More than 70% of patients older than 65 years old have comorbid diseases. Primarily, at least one of the disease have a cardiovascular character [14]. A typical patient with COPD has a similar risk to die from cardiovascular or respiratory pathology [15].
Earlier, it was shown that patients with COPD had an increased count of apoptotic cells, which is associated with a high level of pro-apoptotic factors that activate the process of apoptosis [16]. In patients with MI, an enhancement of endothelium apoptosis and an increase in the pro-coagulant activity of the blood are observed [17].
STEMI is the most severe outcome of coronary disease. It is registered in 12.8% of all lethal cases worldwide [18]. In the cases of ischemic lesion, along with necrotic death of cardiomyocytes, the process of apoptosis gets activated [19]. Apoptosis gets initiated within several minutes after an ischemic episode and precedes necrosis. Apoptosis is characterized by shrinkage of cells, destruction of chromatin, and systematic DNA degradation. The apoptotic process can equally involve cardiomyocytes, interstitial, and endothelial cells. Some authors suggest that post-necrotic apoptosis remains for a long time. It is responsible for ventricular remodeling and progression of heart failure [20]. The revealed elevation of annexin V-positive mononiclears in the blood in patients with MI occurs as a result of endothelial damage associated with the degradation of atherosclerotic plaque. Annexin V binds with PS on the surface of apoptotic cells and increases protective reaction that reduces the risk of thrombosis with the activation of pro-coagulant reactions [21].
The main factor that causes the expression of HSP70 is ischemia. In such conditions, the production of HSP70 can be the mechanism of adaptation of the myocardium to its damage. Wei et al demonstrated that an increase in the expression of HSP70 in cardiomyocytes frequently develops because of ischemic or dilated cardiomyopathy [22]. Elevated levels of HSP70, revealed in the present study, could be associated with the anti-apoptotic properties of HSP70. It was shown that HSP70 can protect cells from apoptosis induced by TNF-α after the activation of effector caspases and retain the process of cellular death caused by Cytochrome C, which is also true for cardiomyocytes [23].
Conclusion
The study results revealed an elevation of annexin V-associated apoptotic cells and the levels of HSP70 that contribute to the resistance of hypoxia and optimization of reperfusion. It is suggested that this is a manifestation of a protective reaction to reduce a thrombogenic potential of the blood in patients with MI. The authors suggest that higher levels of annexin V-positive mononuclears and HSP70 in the blood of patients with STEMI are provided by the volume of the myocardial lesion. It is known that patients with non-STEMI have non-occlusive thrombosis of the coronary artery, while the development of STEMI occurs because of a complete thrombotic occlusion of the coronary artery. Higher values of the studied biomarkers in the group of comorbid patients with MI and comorbid COPD in comparison with patients that had MI without comorbid COPD can be associated with the intensification of apoptosis caused by chronic systemic inflammation that worsens the clinical course of both diseases.
References
1. Pogosova N.V., Sokolova O.Yu., Yufereva Yu.M., Osipova I.V.,Ryamzina I.N. First results of analysis of the Russian part of the European register on cardiac rehabilitation Eurocared (European cardiac rehabilitation database). Kardiologiia. 2015; 55(2):49-56. (In Russ.). eLIBRARY ID: 23169147
2. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. 2018; 12:1753465817750524. DOI: 10.1177/1753465817750524.
3. Goedemans L, Bax JJ, Delgado V. COPD and acute myocardial infarction. Eur Respir Rev. 2020; 29(156):190139. DOI: 10.1183/16000617.0139-2019.
4. Kunisaki KM, Dransfield MT, Anderson JA, Brook RD, Calverley PMA, et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am J Respir Crit Care Med. 2018; 198(1):51-57. DOI: 10.1164/rccm.201711-2239OC.
5. Pizarro S, García-Lucio J, Peinado VI, Tura-Ceide O, Díez M, et al. Circulating progenitor cells and vascular dysfunction in chronic obstructive pulmonary disease. PLoS One. 2014; 9(8):e106163. DOI: 10.1371/journal.pone.0106163. Erratum in: PLoS One. 2014; 9(12):e115566.
6. García-Lucio J, Peinado VI, de Jover L, Del Pozo R, Blanco I, et al. Imbalance between endothelial damage and repair capacity in chronic obstructive pulmonary disease. PLoS One. 2018; 13(4):e0195724. DOI: 10.1371/journal.pone.0195724
7. Trinkmann F, Saur J, Borggrefe M, Akin I. Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)-Current Considerations for Clinical Practice. J Clin Med. 2019; 8(1):69. DOI: 10.3390/jcm8010069.
8. Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agustí A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. Am J Respir Crit Care Med. 2016; 194(11):1319-1336. DOI: 10.1164/rccm.201604-0690SO.
9. Lee H, Shin SH, Gu S, Zhao D, Kang D, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med. 2018; 16(1):178. DOI: 10.1186/s12916-018-1159-7.
10. Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000; 2(7):399-406. DOI: 10.1038/35017029.
11. Hofstra L, Liem IH, Dumont EA, Boersma HH, van Heerde WL, et al. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet. 2000; 356(9225):209-12. DOI: 10.1016/s0140-6736(00)02482-x.
12. Kupcho K, Shultz J, Hurst R, Hartnett J, Zhou W, et al. A realtime, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis. 2019; 24(1-2):184-197. DOI: 10.1007/s10495-018-1502-7.
13. Mathangasinghe Y, Fauvet B, Jane SM, Goloubinoff P, Nillegoda NB. The Hsp70 chaperone system: distinct roles in erythrocyte formation and maintenance. Haematologica. 2021; 106(6):1519- 1534. DOI: 10.3324/haematol.2019.233056.
14. Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013; 187(7):728-35. DOI: 10.1164/rccm.201209-1665OC.
15. Kuzmichev B.Yu., Voronina L.P., Tarasochkina D. S., Polunina O. S., Prokofieva T.V., et al. Hyperhomocysteinemia as a risk factor for a complicated course of myocardial infarction against the background of the chronic obstructive pulmonary disease. Astrakhan medical journal. 2019; 14(3):79-87. (in Russ.) eLIBRARY ID: 41550888
16. Vasina L.V., Lugovaya Ä.V., Petrischev N.N., Serebryanaya N.B. Pathogenic significance of relative alteration in V-binding mononuclears and CD 59+-lymphocites of peripheral blood in patients with acute coronary syndrome. Medicо-Biological and Socio-Psychological Problems of Safety in Emergency Situations. 2088;(1):74-80. (in Russ.). eLIBRARY ID: 21139575
17. Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011; 2(12):e244. DOI: 10.1038/cddis.2011.130.
18. Masoudi FA, Ponirakis A, Yeh RW, Maddox TM, Beachy J, et al. Cardiovascular care facts: a report from the national cardiovascular data registry: 2011. J Am Coll Cardiol. 2013; 62(21):1931- 1947. DOI: 10.1016/j.jacc.2013.05.099.
19. Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005; 115(3):565-71. DOI: 10.1172/JCI24569.
20. Shekhar A, Heeger P, Reutelingsperger C, Arbustini E, Narula N, et al. Targeted Imaging for Cell Death in Cardiovascular Disorders. JACC Cardiovasc Imaging. 2018; 11(3):476-493. DOI: 10.1016/j.jcmg.2017.11.018.
21. Osende Olea JI. Anexina V en jóvenes con infarto de miocardio: nuevas respuestas traen nueva preguntas [Annexin V levels in young patients with acute myocardial infarction: new answers bring new questions]. Rev Esp Cardiol. 2002; 55(12):1223-5. (in Spanish). DOI: 10.1016/s0300-8932(02)76792-4.
22. Wei YJ, Huang YX, Shen Y, Cui CJ, Zhang XL, et al. Proteomic analysis reveals significant elevation of heat shock protein 70 in patients with chronic heart failure due to arrhythmogenic right ventricular cardiomyopathy. Mol Cell Biochem. 2009; 332(1- 2):103-11. DOI: 10.1007/s11010-009-0179-1.
23. Patterson C, Cyr D. Welcome to the machine: a cardiologist’s introduction to protein folding and degradation. Circulation. 2002; 106(21):2741-6. DOI: 10.1161/01.cir.0000041145.30519.6b.
About the Authors
A. V. NaumovAndrey V. Naumov, Post-graduate student of the Department of Internal Diseases of Pediatric Faculty
Astrakhan
T, V. Prokofieva
Tatyana V. Prokofieva, Cand. Sci. (Med.), Associate Professor of the Department of Internal Diseases of Pediatric faculty
Astrakhan
O. S. Polunina
Olga S. Polunina, Dr. Sci. (Med.), Professor, Head of the Department of Internal Diseases of Pediatric Faculty
Astrakhan
L. V. Saroyants
Lyudmila V. Saroyants, Dr. Sci. (Med.), head of laboratory and experimental Department
Astrakhan
E. A. Polunina
Russian Federation
Ekaterina A. Polunina, Dr. Sci. (Med.), Associate Professor of the Department of Internal Diseases of Pediatric Faculty
Astrakhan
Review
For citations:
Naumov A.V., Prokofieva T.V., Polunina O.S., Saroyants L.V., Polunina E.A. Clinical and diagnostic significance of apoptosis markers in myocardial infarction in the setting of chronic obstructive pulmonary disease. Medical Herald of the South of Russia. 2021;12(4):46-53. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-46-53







































