Scroll to:
Changes in body composition depending on the rs1801282 PPARG polymorphism in patients with different variants of treatment of early carbohydrate metabolism disorders
https://doi.org/10.21886/2219-8075-2021-12-4-27-33
Abstract
Objective: To study the effect of the rs1801282 PPARG polymorphism on changes in the body composition of patients with early carbohydrate metabolism disorders in groups with different variants of treatment.
Materials and Methods: The study involved 64 patients (8 men and 56 women) with early carbohydrate metabolism disorders. At baseline, all patients underwent genotyping for the rs1801282 PPARG polymorphism and body composition determination with bioelectrical impedance analysis (BIA). Then, the patients were divided into two groups depending on the type of therapy. The patients from Group 1 (40 subjects, mean age 45.2±15.4 years) kept a generally accepted diet with the exclusion of simple carbohydrates and limitation of complex carbohydrates and fats. The patients from Group 2 (24 subjects, mean age 51.2±14.5 years) took metformin in addition to the diet therapy. The effects of different types of treatment on body composition changes were assessed with follow-up BIA 3 months after the start of treatment.
Results: Carriers of the mutant G allele of rs1801282 PPARG in the metformin and diet therapy group showed a significant increase in the content of body cell mass (1.28±0.51% vs 0.36±0.37%; P = 0.021) compared with CC homozygotes in the absence of differences in body weight changes (P > 0.05).
Conclusions: The presence of the mutant allele G of rs1801282 PPARG promotes the increase in body cell mass in case of adding metformin to the diet therapy in patients with early carbohydrate metabolism disorders.
Keywords
For citations:
Valeeva F.V., Medvedeva M.S., Khasanova K.B., Turtseva T.S., Yilmaz T.S. Changes in body composition depending on the rs1801282 PPARG polymorphism in patients with different variants of treatment of early carbohydrate metabolism disorders. Medical Herald of the South of Russia. 2021;12(4):27-33. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-27-33
Introduction
One of the leading trends in modern medicine is the individualization of therapy for various pathologies, taking into account the genetic characteristics of the patient. It is known that depending on the polymorphism of the genes responsible for metabolism, patients are able to respond differently to the treatment.
Therapy of early carbohydrate metabolism disorder (CMD) in order to prevent the development of type 2 diabetes mellitus is one of the key problems of endocrinology. Previously, while detecting these disorders, patients were exclusively trained in the principles of balanced nutrition without prescribing concomitant medicine therapy, but in recent years there have been more and more recommendations from leading endocrinological communities in favor of prescribing metformin [1][2]. In addition to the hypoglycemic effect, metformin has the ability to reduce body weight mainly due to loss of visceral fat [3], however, the expected effect is observed only in some patients [4].
Peroxisomes are intracellular organelles that play an important role in many cellular metabolic processes, including alpha- and beta-oxidation of fatty acids, synthesis of plasmalogens and neutralization in liver cells of glyoxylate involved in the conversion of fatty acids into carbohydrates [5]. Nuclear receptors activated by peroxisome proliferators (peroxisome proliferator-activated receptors, PPAR) are an important regulator of the process of differentiation of adipocytes and a modulator of intracellular insulin-dependent signaling cascades [6]. One of the key PPAR isotypes involved in the regulation of adipogenesis is the PPARγ receptor encoded by the PPARG gene [7].
PPARG is located at the 25th locus of the short arm of the 3rd pair of chromosomes [8]. Activation of the PPARγ receptor regulates the expression of genes involved in glucose transport [9], and also triggers apoptosis in mature adipocytes, resulting in stimulation of adipogenesis and the formation of young adipocytes more sensitive to the action of insulin [10]. The rs1801282 PPARG polymorphism occurs when proline is substituted for alanine (C > G; Pro12Ala) in the codon of the 12th exon B and is one of the most-studied single nucleotide polymorphisms of this gene. It is known that the presence of the G allele is associated with a decrease in the activity of PPARγ [11]. Thus, the C allele of the rs1801282 polymorphism, on the contrary, is associated with an increase in the transcriptional activity of PPARγ [12].
The PPARG gene, in accordance with its role in lipid metabolism, is described as a genetic marker of obesity and various obesity-related diseases [13], however, data on the effect of gene mutation on the development of overweight are contradictory. Thus, the G allele of the Pro12Ala polymorphism is associated with obesity in the populations of Brazil and Italy [14][15], but in the Chinese population, the mutation mediated a protective effect [16]. Thus, the single nucleotide rs1801282 PPARG polymorphism is a potential candidate capable of modulating the response of patients to diet therapy and pharmacotherapy in early CMD from the patient's body composition indicators.
The study aimed to study the effect of the rs1801282 polymorphism of the PPARG gene on the body composition of patients with early disorders of carbohydrate metabolism in groups with different therapy options.
Methods and materials
The study involved 64 people (8 men and 56 women). The criterion for inclusion in the study was the presence of early CMD, namely impaired fasting glycemia (the level of glycemia in capillary blood on an empty stomach is more than 5.6, but less than 6.1 mmol/l at a glycemic level 2 hours after a standard carbohydrate load of less than 7.8 mmol/l), impaired glucose tolerance (the level of glycemia in capillary blood 2 hours after a standard carbohydrate load of more than 7.8 mmol/l, but less than 11.1 mmol/l) or type 2 diabetes mellitus detected for the first time (the level of glycemia in capillary blood on an empty stomach is more than 6.1 mmol/l and/or 2 hours after the standard carbohydrate load is more than 11.1 mmol/l). The exclusion criteria from the study were age less than 18 years, pregnancy, lactation, decreased kidney function (glomerular filtration rate (GFR) <45 ml / min / 1.73 m2), liver (increased levels of liver enzymes, namely alanine aminotransferase (ALT) and aspartate aminotransferase (AST), more than three times), the presence of cancer in the anamnesis, taking drugs that affect fat and carbohydrate metabolism.
At the beginning of the study, all the patients underwent genotyping with the determination of rs1801282 PPARG polymorphism gene by polymerase chain reaction (PCR) in real time using a rotary amplifier Rotor Gene Q; the substrate for the reaction was DNA obtained from whole blood lymphocytes by the sorbent method (“Ampli-Prime DNA-sorb-B”, Moscow). The body composition was also evaluated by bioimpedance measurement using an impedance analyzer (Diamant-STORK, St. Petersburg). Further, the patients were divided into two therapeutic groups by randomization. The first group was on a diet generally accepted for disorders of carbohydrate metabolism with the exception of simple and restriction of complex carbohydrates and fats. This group included 40 people (7 men and 33 women, average age – 45.2 ± 15.4 years). The second group received metformin therapy in addition to the diet, this group included 24 people (1 man and 23 women, the average age was 51.2 ± 14.5 years). Then, for three months, once every two weeks, patients were monitored by checking food diaries, conducting intermediate measurements. Three months later, bioimpedance was repeated for all the patients.
Statistical processing of the results was carried out using the R environment for statistical data processing. While analyzing the data, the ANCOVA method was used with two independent categorical variables and their interaction. In order to assess the compliance of the genotype frequency distribution of the studied sample with the Hardy-Weinberg law, the criterion χ2 was applied. The arithmetic mean (M) and the standard error of the mean (SEM) were used to describe the quantitative data; the standard deviation (SD) was used to characterize the age of the studied groups. The dynamics of changes in bioimpedance parameters was calculated as a percentage of the measurement at the starting point.
Results
The study of the rs1801282 PPARG polymorphism in patients revealed a distribution of the frequency of alleles and genotypes of the studied polymorphism corresponding to the Hardy–Weinberg distribution (χ2 = 1.22; p >0.05). 67.6% of patients were carriers of the C allele in the homozygous variant, 26.8% of patients were heterozygous CG and 5.6% of patients were carriers of the mutant G allele in the homozygous variant; the prevalence of the C allele in the study sample of patients was 81%, The G allele is 19% (Fig.1). The data obtained correlate with the database of the 1000 Genomes project, according to which the prevalence of the C allele in the European population is 88%, the G allele is 12%; CC homozygotes account for 76.9% of the population, CG – 22.1%, GG – 1%.
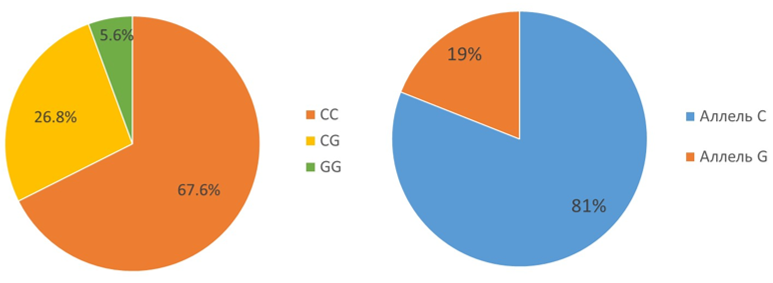
Figure 1. Frequency of occurrence of genotypes, alleles of rs1801282 PPARG in the study sample.
As a result of statistical processing of the obtained data, the relationship of the polymorphism of the studied gene with changes in body weight, waist circumference and hips was not observed in the study groups (p >0.05). Also, while analyzing changes in bioimpedance parameters, no relationship was found between the polymorphism rs1801282 PPARG and the dynamics of fat mass and total body water (p >0.05). However, among patients receiving metformin on the background of diet therapy, carriers of the mutant G allele were characterized by a more pronounced set of active cell mass (1.28 ± 0.51% vs. 0.36 ± 0.37%, p = 0.021; Table. 1, Fig. 2). In the diet therapy group, the G allele did not affect changes in active cell mass among patients (p >0.05; Table 2).
Table 1
Changes in body cell mass in patients with early carbohydrate metabolism disorders treated with metformin + diet therapy depending on the rs1801282 PPARG polymorphism
|
|
Body cell mass, % |
||
|
Baseline |
3 months |
Δ |
|
|
CC |
42.28±0.72 (SD = 2.68) |
42.64±0.63 (SD = 2.35) |
0.36±0.37 (SD = 1.37) |
|
CG/GG |
40.74±1.38 (SD = 4.37) |
42.02±1.49 (SD = 4.73) |
1.28±0.51 (SD = 1.59) |
Table 2
Changes in body cell mass in patients with early carbohydrate metabolism disorders treated with diet therapy on;y depending on the rs1801282 PPARG polymorphism
|
Генотип |
Body cell mass, % |
||
|
Baseline |
3 months |
Δ |
|
|
CC |
42.64±0.58 (SD = 3.1) |
43.56±0.62 (SD = 3.36) |
0.92±0.27 (SD = 1.44) |
|
CG/GG |
43.14±1.19 (SD = 3.96) |
43.19±1.26 (SD = 4.18) |
0.05±0.48 (SD = 1.61) |
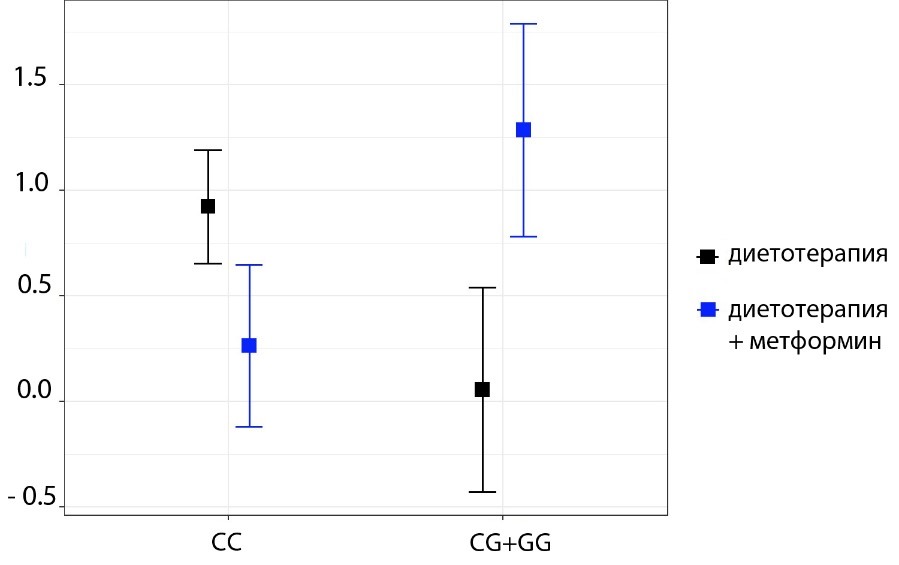
Figure 2. Changes of body cell mass (%) in patients with early carbohydrate metabolism disorders treated with various types of therapy depending on the rs1801282 PPARG polymorphism.
Discussion
The definition of PPARG polymorphism has acquired great importance in the field of nutrition genetics, while data on the effect of the mutant G allele on the outcomes of diet therapy are ambiguous. According to studies conducted in Brazilian and Central European populations, the decrease in body fat mass in response to the generally accepted diet therapy for overweight, lasting for three months, was more pronounced in carriers of the G allele than in CC homozygotes [17][18]. The opposite results were shown by a study by Matsuo et al.: the G allele was more common among patients resistant to diet therapy [19]. However, the authors of this study did not find the effect of the mutant G allele on changes in the fat component and body weight in general 3 months after the start of observation of patients who are exclusively on diet therapy. This may be explained by the fact that the G allele does not affect the short-term results of diet therapy, but contributes to a change in the compositional composition of the body with longer-term use of diet therapy.
Special attention of scientists is drawn to the study of the effect of diet therapy in combination with hypoglycemic drugs, depending on the variant rs1801282 PPARG. Despite the fact that a significant part of the studies studied the association of this polymorphism with the results of thiazolidinedione therapy [20], there are a number of studies that studied the effect of rs1801282 PPARG on the effectiveness of metformin treatment. In the DPP study, among patients receiving metformin therapy and following the generally accepted overweight diet, carriers of the G allele had a more pronounced decrease in body weight than CC homozygotes; also, in the presence of the G allele, a decrease in the amount of body fat was noted, in carriers of CC this effect of combined therapy was not manifested [21]. The data obtained with DPP are confirmed by a study previously conducted by Masud S, Ye S., according to which among people following a diet and receiving metformin therapy, bodyweight loss was more pronounced in carriers of the G-allele [22]. The authors of this study found no differences in changes in fat mass and body weight in general between patients with different variants of rs1801282 PPARG when metformin was added to diet therapy, however, we found the effect of the G allele on changes in the amount of active cell mass when taking metformin, which had not previously been evaluated in other studies. It is known that the amount of active cell mass reflects the number of metabolically active tissues (cells of the nervous system, internal organs, muscle tissue) and characterizes the sufficiency of the protein component of nutrition. Thus, an increase in the content of active cell mass when metformin is added to carriers of the mutant allele G rs1801282 PPARG is a sign of increased anabolic processes in the body, improving the physical performance of patients already in the early stages of drug administration.
This study is limited in duration and reflects short-term changes in the body composition of patients with various therapy options. Therefore, further observation of patients and an increase in the sample will help to clarify the effect of the mutant G allele on changes in the compositional composition of the body of patients with different variants of CMD therapy at different periods of therapy.
Conclusions
- The change in the amount of adipose tissue, as well as total water in the body when taking metformin on the background of diet therapy three months after the start of treatment is not associated with the carrier of the G allele of rs1801282 PPARG among patients with early disorders of carbohydrate metabolism.
- A set of active cell mass is noted among carriers of the G allele of rs1801282 PPARG when metformin is added to the conventional diet three months after the start of therapy for early carbohydrate metabolism disorders.
References
1. Dedov I.I., Shestakova M.V., Mayorov A.Yu., Vikulova O.K., Galstyan G.R., et al. Standards of specialized diabetes care. Edited by Dedov I.I., Shestakova M.V., Mayorov A.Yu. 9th edition. Diabetes mellitus. 2019; 22(1S1):1-144. (In Russ.). DOI: 10.14341/DM221S1
2. Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm — 2020 executive summary. Endocr Pract. 2020; 26(1):107-139. DOI: 10.4158/CS-2019-0472.
3. Zhou J, Massey S, Story D, Li L. Metformin: An Old Drug with New Applications. Int J Mol Sci. 2018; 19(10):2863. DOI: 10.3390/ijms19102863.
4. Bankura B, Das M, Pattanayak A, Adhikary B, Bhattacharjee R, et al. Inter-patient Variability in Clinical Efficacy of Metformin in Type 2 Diabetes Mellitus Patients in West Bengal, India. Journal of Metabolic Syndrome. 2016;(5):2. DOI: 10.4172/2167-0943.1000198.
5. Waterham HR, Ferdinandusse S, Wanders RJ. Human disorders of peroxisome metabolism and biogenesis. Biochim Biophys Acta. 2016; 1863(5):922-33. DOI: 10.1016/j.bbamcr.2015.11.015.
6. Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 2021; 114:154338. DOI: 10.1016/j.metabol.2020.154338.
7. Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications — a review. Nutr J. 2014; 13:17. DOI: 10.1186/1475-2891-13-17.
8. Yahaya TO, Salisu TF. A Review of Type 2 Diabetes Mellitus Predisposing Genes. Curr Diabetes Rev. 2019; 16(1):52-61. DOI: 10.2174/1573399815666181204145806.
9. Kononenko I.V., Mayorov A.Yu., Koksharova E.O., Shestakova M.V. Pharmacogenetics of hypoglycemic agents. Diabetes mellitus. 2015; 18(4):28-34. (In Russ.). DOI: 10.14341/DM7681
10. Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998; 101(6):1354-61. DOI: 10.1172/JCI1235.
11. Sarhangi N, Sharifi F, Hashemian L, Hassani Doabsari M, Heshmatzad K, et al. PPARG (Pro12Ala) genetic variant and risk of T2DM: a systematic review and meta-analysis. Sci Rep. 2020; 10(1):12764. DOI: 10.1038/s41598-020-69363-7.
12. Groop L, Pociot F. Genetics of diabetes — are we missing the genes or the disease? Mol Cell Endocrinol. 2014; 382(1):726-739. DOI: 10.1016/j.mce.2013.04.002.
13. Leońska-Duniec A, Ahmetov II, Zmijewski P. Genetic variants influencing effectiveness of exercise training programmes in obesity — an overview of human studies. Biol Sport. 2016; 33(3):207-14. DOI: 10.5604/20831862.1201052.
14. Castro GV, Latorre AFS, Korndorfer FP, de Carlos Back LK, Lofgren SE. The Impact of Variants in Four Genes: MC4R, FTO, PPARG and PPARGC1A in Overweight and Obesity in a Large Sample of the Brazilian Population. Biochem Genet. 2021; 59(6):1666-1679. DOI: 10.1007/s10528-021-10079-2.
15. Bordoni L, Marchegiani F, Piangerelli M, Napolioni V, Gabbianelli R. Obesity-related genetic polymorphisms and adiposity indices in a young Italian population. IUBMB Life. 2017; 69(2):108-115. DOI: 10.1002/iub.1596.
16. Wang X, Liu J, Ouyang Y, Fang M, Gao H, Liu L. The association between the Pro12Ala variant in the PPARγ2 gene and type 2 diabetes mellitus and obesity in a Chinese population. PLoS One. 2013; 8(8):e71985. DOI: 10.1371/journal.pone.0071985.
17. Rodrigues APS, Rosa LPS, Silveira EA. PPARG2 Pro12Ala polymorphism influences body composition changes in severely obese patients consuming extra virgin olive oil: a randomized clinical trial. Nutr Metab (Lond). 2018; 15:52. DOI: 10.1186/s12986-018-0289-4.
18. Chmurzynska A, Muzsik A, Krzyżanowska-Jankowska P, Mądry E, Walkowiak J, Bajerska J. PPARG and FTO polymorphism can modulate the outcomes of a central European diet and a Mediterranean diet in centrally obese postmenopausal women. Nutr Res. 2019; 69:94-100. DOI: 10.1016/j.nutres.2019.08.005.
19. Matsuo T, Nakata Y, Katayama Y, Iemitsu M, Maeda S, et al. PPARG genotype accounts for part of individual variation in body weight reduction in response to calorie restriction. Obesity (Silver Spring). 2009; 17(10):1924-31. DOI: 10.1038/oby.2009.199.
20. Pearson ER. Diabetes: Is There a Future for Pharmacogenomics Guided Treatment? Clin Pharmacol Ther. 2019; 106(2):329-337. DOI: 10.1002/cpt.1484.
21. Franks PW, Jablonski KA, Delahanty L, Hanson RL, Kahn SE, et al. The Pro12Ala variant at the peroxisome proliferator-activated receptor gamma gene and change in obesity-related traits in the Diabetes Prevention Program. Diabetologia. 2007; 50(12):2451-60. DOI: 10.1007/s00125-007-0826-6.
22. Masud S, Ye S; SAS Group. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med Genet. 2003; 40(10):773-80. DOI: 10.1136/jmg.40.10.773.
About the Authors
F. V. ValeevaRussian Federation
Farida V. Valeeva, Dr. Sci. (Med.), Professor, Head of Endocrinology Department
Kazan
M. S. Medvedeva
Russian Federation
Maria S. Medvedeva, PhD-fellow of Endocrinology Department
Kazan
K. B. Khasanova
Russian Federation
Kamilya B. Khasanova, PhD-fellow of Endocrinology Department
Kazan
T. S. Turtseva
Russian Federation
Tatyana S. Turtseva, Resident of Endocrinology Department
Kazan
T. S. Yilmaz
Russian Federation
Tatyana S. Yilmaz, PhD, Associate Professor of Endocrinology Department
Kazan
Review
For citations:
Valeeva F.V., Medvedeva M.S., Khasanova K.B., Turtseva T.S., Yilmaz T.S. Changes in body composition depending on the rs1801282 PPARG polymorphism in patients with different variants of treatment of early carbohydrate metabolism disorders. Medical Herald of the South of Russia. 2021;12(4):27-33. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-27-33







































