Scroll to:
Modern view of the problem of missed abortion
https://doi.org/10.21886/2219-8075-2021-12-3-6-11
Abstract
Non-developing pregnancy is an urgent obstetric problem, which is included in the structure of reproductive losses and. The authors performed a systematic analysis of the data presented in the modern scientifi c literature on the epidemiology, etiology, pathogenesis, and diagnosis of non-developing pregnancy. Th e review includes data from foreign and domestic articles on this topic found in PubMed over the past 7 years.
For citations:
Andreeva M.V., Shevtsova E.P., Zabolotneva K.O., Lyutaya E.D., Sivko T.S. Modern view of the problem of missed abortion. Medical Herald of the South of Russia. 2021;12(3):6-11. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-3-6-11
NDP is included in the structure of reproductive losses; it is an urgent obstetric problem. In Russia, the prevalence of spontaneous gestation termination is one-fourth of all registered pregnancies, while NDP accounts for 45 to 88.6% [1][2]. The most relevant period for studying NDP is the first trimester of gestation, since most of the cases of NDP (up to 80%) fall on it [1].
In the etiology of NDP, chromosomal aberrations play a special role [1][3]. Autosomal trisomy is responsible for more than half of pathological karyotypes. Monosomy is detected in 20–25% of cases [4]. Translocation (2–10%) can cause NDP in cases where repeated cases of NDP or spontaneous miscarriage are registered in a married couple [4].
In 10–25% of women with habitual miscarriage, congenital anomalies of the uterine structure are detected during the examination [1][5]. Acquired defects of the anatomical structure (isthmic-cervical insufficiency, fibroids with submucous nodes, intrauterine synechiae) play a more significant role in the NDP development [6][7].
Since 2006, based on the resolution of the World Congress of Obstetricians and Gynecologists FIGO, each NDP case should be considered as the one associated with chronic endometritis (CE). CE is a combination of morphological and functional changes in the endometrium of inflammatory origin, which are accompanied by changes in the physiological cyclic transformation and receptivity of tissues [8]. In women with miscarriage, the diagnosis of CE was verified by the results of a histological examination in 61.0–73.1% of cases [9–12]. In modern conditions, viral and bacterial pathogens are often verified in CE, more often as part of a viral-bacterial mixed infection. The chronization of the inflammatory process in the endometrium is also determined by the growth of resistance of the microflora to pharmacotherapy.
Numerous studies have proved that CE acts as a modifier of local immunity. Specific antigens in endometrial tissue induce differentiation of T-helper cells into two subpopulations: Th-1 and Th-2. Th-1 cells secrete interferon-γ, interleukin-2 (IL-2), and tumor necrosis factor-β, and Th-2 – IL-4, IL-5, and IL-10. Both subpopulations with the predominant influence of Th1 are responsible for the production of tumor necrosis factor-α [10][9][13]. The physiological course of pregnancy is provided by humoral immune reactions of the Th2-type. In turn, the cellular link of Th1-type immunity can have an abortive effect.
The pathological activation of natural killer (NK) cells and macrophages contributes to fetal loss. NK cells are directly involved in the dissolution of the trophoblast. The enhanced cytokine production and secretion caused by macrophage activation affect NK cells [9][12]. The proteins involved in the inflammatory response, proliferation, and apoptosis in the endometrium during the “implantation window” are determined by the expression of 25 genes. It was shown that the activity of genes directly encoding pro-inflammatory cytokines, growth factors, and apoptosis processes was significantly changed in patients with CE. Thus, the expression of IGFBR1, BCL2, and BAX increased, while the expression of IL-11, CC-14, IGF-1, and CASP8 decreased [3]. Modification of the activity of genes in the endometrium in the case of CE causes a decrease in its receptivity, which can become a probable cause of NDP.
The concept of endometrial receptivity is directly associated with the formation of pinopodia in it and an increase in the progesterone level, LIF, leukemia inhibiting factor receptor (LIFR), and integrin αVβ3 [13]. The suppression of the HOXA 10 genes leads to a sharp decrease in the number of pinopodia [13]. They also regulate the proliferation of endometrial stromal cells and the morphogenesis of epithelial cells. At the point of formation of pinopodia, the embryo and endometrium carry out a signaling interaction [11][13]. Interleukin-6, LIF, which are important components of blastocyst development and implantation, are expressed on pinopodia [3]. The realization of the LIF effects is achieved due to the receptors consisting of LIFR and gp130 (transmembrane proteins). LIF is responsible for the activation of the JAK/STAT, MAPK, and PIPK signaling pathways in various types of cells [3]. Thus, blastocyst implantation did not occur in the endometrium of mice homozygous for the defective LIF gene, which confirms the influence of LIF on implantation in general [3].
Thus, the sensitivity of the endometrium to progesterone decreases within CE, which is a possible mechanism for the violation of its generative function [14]. The degradation of estrogens and the activation of growth factors (EGF, TGFa, β, VEGF) during a long-term inflammatory process causes a local increase in the concentration of estrogens, leading to excessive endometrial proliferation [14][15]. The latter is not capable of adequate secretory transformation due to a decrease in the number of progesterone receptors in the cells of the endometrial glands, stroma, as well as on regulatory Th-lymphocytes [14][15].
A number of literature sources have provided convincing data on the significant role of endometrial proteins in implantation processes, the most significant of which is α2-microglobulin of fertility (AMGF). AMGF acts as an indicator of the activity of the uterine glands, and placental α1-microglobulin (PAMG) acts as an indicator of the endometrium decidualization [1][2]. Within CE, the production of endometrial proteins with an immunosuppressive effect (AMGF, PAMG) is reduced [16].
Ultrasound examination for NDP
Currently, ultrasound is the most informative method of diagnosing an undeveloped uterine pregnancy. When performing a sonographic study, it is necessary to differentiate a frozen gestation from a progressive one and exclude an ectopic pregnancy. In the early stages, preference is given to transvaginal ultrasound; if it is impossible, sonography is performed transabdominally.
From the ultrasound viewpoint, there are two variants of NDP: anembryonia and early death of the embryo (fetus). Therefore, Figure 1 shows the state of anembryonia.

Figure 1. Transvaginal ultrasound examination. Pregnancy 7 weeks and 5 days. The diameter of the ovum is 25 mm. Anembryony.
While performing an ultrasound examination, it is necessary to differentiate the frozen gestation from the progressive one and exclude ectopic localization. Figure 2 shows an example of an undeveloped pregnancy of 6 weeks and 4 days.
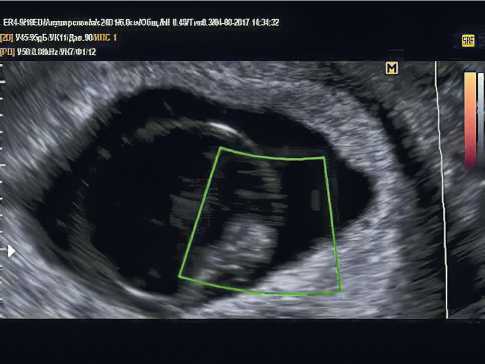
Figure 2. Transvaginal ultrasound examination. Pregnancy 6 weeks and 4 days. There is no heartbeat. Non-developing pregnancy.
In the early stages, preference is given to transvaginal ultrasound; if it is impossible, sonography is performed transabdominally.
The ultrasound data make it possible to differentiate between two types of anembryonia [1]. The anembryonia of type I is characterized by the absence of an embryo, the ovum is up to 20–25 mm, the uterus corresponds to 5–7 weeks of pregnancy. At the same time, the parameters of the uterus and ovum do not correspond to the gestational period, and dynamic observation does not change the characteristics. The growth rate of the ovum in anembryonia of type II is normal, but the embryo is absent or its remains may be fixed in the form of a thin hyperechoic line (“vertebral fold”). At the period of 10–11 weeks of pregnancy, the ovum reaches 45–55 mm in diameter, but starting from the 8th week, it is not possible to verify the laying of the villous chorion. Thus, Figure 3 shows the early embryo death.

Figure 3. Transvaginal ultrasound examination. Pregnancy 8 weeks and 2 days. Non-developing pregnancy (early embryo death).
The following ultrasound diagnostic criteria for NDP were determined as well: 1) the fetus heartbeat with its coccygeal-parietal size of 7 mm or more is not recorded; 2) the embryo with an average diameter of the ovum of 25 mm or more is absent [17][18]. The diameter of the ovum is necessarily measured in three projections, and then its average value is calculated. In the presence of at least one of the above criteria, a diagnosis of NDP is made [18], but it is should be confirmed by the second specialist of ultrasound diagnostics1. The working group of the National Institute for Health and Clinical Excellence (NICE) of the UK also considers the possibility of re-examination of the patient after 7–14 days for a final conclusion [18].
The US criteria of NDP for repeated studies are detected as follows: 1) the absence of an embryo with a heartbeat 2 weeks or more after the detection of an ovum without a vitelline sac; 2) the absence of an embryo with a heartbeat 11 days or more after the detection of an ovum with a vitelline sac [17][18][19].
There are also prognostic ultrasound criteria for NDP: 1) the absence of a fetal heartbeat with a computed tomography (CT) scan of less than 7 mm; 2) the absence of an embryo with an average diameter of the fetal sac of 16–24 mm; 3) the absence of an embryo with a heartbeat 7–13 days after the detection of an ovum without a vitelline sac; 4) the absence of an embryo with a heartbeat 7–10 days after the detection of an ovum with a vitelline sac; 5) the absence of an embryo after 6 weeks from the beginning of the last menstruation; 6) the size of the vitelline sac is more than 7 mm; 7) the discrepancy between the size of the fetal sac and the size of the embryo (the difference between the average diameter of the fetal sac and the CT of the fetus is less than 5 mm) [1][17][19].
These signs only allow suspecting NDP; dynamic ultrasound is necessary to confirm or exclude it. Some researchers point out that it is impractical to use the calculation of the difference between the diameter of the ovum and the CTD as a prognostic criterion [20], and the phenomenon of the “yolk stalk sign” has additional value [21][22]. The yolk stalk is a tubular structure connecting the vitelline sac and the body of the embryo. In the early stages of gestation, they are located close to each other, so it is not possible to visualize the yolk stalk normally. If an embryo without a heartbeat begins to separate from the vitelline sac before reaching the values of 5 mm or more, then in this situation they talk about the phenomenon of the “yolk stalk”. The results of the studies confirm the correlation of this sign with a frozen pregnancy in the dynamic observation of patients [21][22].
Therapeutic tactics for NDP
The traditional tactic for NDP is surgical extraction of the deceased ovum, while the process of endometrial repair takes longer than after an artificial abortion [2]. Therefore, an important direction of studying the problem of NDP is the search and development of new approaches to the management of patients after emptying the uterine cavity in order to reduce the frequency of infectious complications, prevent the chronization of the inflammatory process. In this regard, attention is drawn to the possibility of using quantum therapy (QT) in the postoperative period in patients with NDP [23][24].
The QT method based on magnetic-infrared laser therapy contributes to the normalization of all parts of the regulation of the adrenal glands, sexual, immune, and other systems [23][24]. Under the influence of low-intensity pulsed laser radiation, a total reaction of organs and tissues is generated, which causes an analgesic and anti-inflammatory effect. The functional of the microcirculatory bed is improved; the repair processes are accelerated. The activity of specific and non-specific links of immunity is stimulated. The excitability of the vegetative centers decreases. The trophism of damaged tissues improves against the background of an increase in the overall level of adaptation of the body [23][24]. At the level of individual organs and tissues, including the uterus, receptor sensitivity increases, the duration of the phase of inflammation and interstitial edema of tissues decreases, etc. [23][24]
The above-mentioned effects of QT, the positive experience of using it for the treatment of wound infection and chronic inflammatory processes [23, 24] of other localization determine the scientific and practical interest in knowing the possibilities of using QT in patients after the interruption of NDP in order to prevent the chronization of the inflammatory process and its recurrence.
1. Letter of the Ministry of Health of the Russian Federation dated June 7, 2016 No. 15-4/10/2-3482 “Miscarriage in early pregnancy: diagnosis and management tactics”.
References
1. Radzinsky V.E. et al. Non-developing pregnancy: Methodological recommendations of MARS (Interdisciplinary Association of Reproductive Medicine Specialists). Moscow: StatusPraesens magazine editorial offi ce; 2015. (In Russ.).
2. Radzinskii V.E., Dimitrova V.I., Maiskova I.Yu. Nerazvivayushchayasya beremennost´. M.: GEOTAR-Media; 2009. (In Russ.).
3. Di Pietro C, Cicinelli E, Guglielmino MR, Ragusa M, Farina M, et al. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2013;69(5):509-517. https://doi.org/10.1111/aji.12076
4. Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27(8):2297-2302. https://doi.org/10.1093/humrep/des179
5. Tabolova V.K., Korneeva I.E. Infl uence of chronic endometritis on the outcomes of programs of assisted reproductive technologies: morpho-functional and molecular-genetic characteristics. Obstetrics and gynecology. 2013;10:17-22. (In Russ.). eLIBRARY ID: 20841398
6. Ailamazyan E.K., Kulakova V.I., Radzinskii V.E., Savel´eva G.M., eds. Akusherstvo. Natsional´noe rukovodstvo. M.: GEOTAR-Media; 2009. (In Russ.).
7. Puscheck E.E., Scott Lucidi R. FACOG Early Pregnancy Loss Workup / Updated: Jun 08, 2018. – URL: https://reference.medscape.com/article/266317-workup
8. Kogan E.A., Demura T.A. Molecular and morphological aspects of endometrial receptivity disorders in chronic endometritis. Archives of pathology. 2012;3:15-17. (In Russ.). eLIBRARY ID: 22288730
9. Andreeva M.V., Neklyudova A.V. Ways to overcome infectious complications in obstetrics. Bulletin of Volgograd State Medical University. 2019;4(72):21-25. (In Russ.). DOI: 10.19163/1994-9480-2019-4(72)-21-25
10. Radzinskii V.E., Orazmuradova A.A., eds. Beremennost´ rannih srokov. Ot pregravidarnoi podgotovki do zdorovoi gestacii. Media Bureau "Status Presence"; 2018. (In Russ.).
11. Plyasunova M.P., Khlybova S.V. Chronic endometritis as one of the urgent problems in modern gynecology. Vyatka medical bulletin. 2013;1:44-53. (In Russ.). eLIBRARY ID: 19114243
12. Sukhikh G.T., Shurshalina A.V. Hronicheskii endometrit. Rukovodstvo. M.: GEOTAR-Media; 2013. (In Russ.).
13. Dimitriadis E, Nie G, Hannan NJ, Paiva P, Salamonsen LA. Local regulation of implantation at the human fetal-maternal interface. Int J Dev Biol. 2010;54(2-3):313-22. DOI: 10.1387/ijdb.082772ed
14. Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofl uorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84(8):2963-71. DOI: 10.1210/jcem.84.8.5928
15. Sivko T.S., Andreeva M.V., Gadzhieva A.Kh. Non-developing pregnancy as a cause of reproductive losses. Almanac-2019: collection of articles. 2019:228-230. (In Russ.).
16. Kitaya K., Yasuo T. Immunohistochemistrical and clinicopathological characterization of chronic endometritis. Am J Reprod Immunol. 2011;66,5:410–415. DOI: 10.1111/j.1600-0897.2011.01051.x
17. Murugan VA, Murphy BO, Dupuis C, Goldstein A, Kim YH. Role of ultrasound in the evaluation of fi rsttrimester pregnancies in the acute setting. Ultrasonography. 2020;39(2):178-189. DOI: 10.14366/usg.19043
18. NICE guideline [NG126]. Ectopic pregnancy and miscarriage: diagnosis and initial management. Published date: 17 April 2019. Accessed at: https://www.nice.org.uk/guidance/ng126.
19. Preisler J, Kopeika J, Ismail L, Vathanan V, Farren J, et al. Defi ning safe criteria to diagnose miscarriage: prospective observational multicentre study. BMJ. 2015;351:h4579. DOI: 10.1136/bmj.h4579.
20. Kapfh amer JD, Palaniappan S, Summers K, Kassel K, Mancuso AC, et al. Diff erence between mean gestational sac diameter and crown-rump length as a marker of fi rsttrimester pregnancy loss aft er in vitro fertilization. Fertil Steril. 2018;109(1):130-136. DOI: 10.1016/j.fertnstert.2017.09.031
21. Filly MR, Callen PW, Yegul NT, Filly RA. Th e yolk stalk sign: evidence of death in small embryos without heartbeats. J Ultrasound Med. 2010 Feb;29(2):237-41. DOI: 10.7863/jum.2010.29.2.237
22. Acuña J, Rukh S, Adhikari S. Point-of-care ultrasound identifi cation of yolk stalk sign in a case of failed fi rst trimester pregnancy. World J Emerg Med. 2018;9(2):149-151. DOI: 10.5847/wjem.j.1920-8642.2018.02.012
23. Melkozerova O.A., Bashmakova N.V., Pogorelko D.V., Chistyakov M.A. Energy of low-frequency ultrasound in the restoration of the receptor fi eld of the endometrium aft er non-developing pregnancy. Obstetrics and gynecology. 2014;7:61-67. (In Russ.). eLIBRARY ID: 21801006
24. Zubarev PN, Risman BV. [Ultrasonic cavitation and ozonization in treatment of patients with pyo-necrotic complications of diabetic foot syndrome]. Vestn Khir Im I I Grek. 2011;170(1):48-53. (In Russ.). PMID: 21506355.
About the Authors
M. V. AndreevaРоссия
Margarita V. Andreeva, Dr. Sci. (Med.) Professor, Department of Obstetrics and Gynecology
Volgograd
E. P. Shevtsova
Россия
Elena P. Shevtsova, Cand. Sci. (Med.), Associate Professor, Department of Obstetrics and Gynecology
Volgograd
K. O. Zabolotneva
Россия
Kseniya O. Zabolotneva, Cand. Sci. (Med.), Associate Professor, Department of Obstetrics and Gynecology
Volgograd
E. D. Lyutaya
Россия
Elena D. Lyutaya, Dr. Sci. (Med.), Professor, Head of the Department of Radiation, Functional and Laboratory Diagnostics
Volgograd
T. S. Sivko
Россия
Tatyana S. Sivko, post-graduate student, Department of Obstetrics and Gynecology
Volgograd
Review
For citations:
Andreeva M.V., Shevtsova E.P., Zabolotneva K.O., Lyutaya E.D., Sivko T.S. Modern view of the problem of missed abortion. Medical Herald of the South of Russia. 2021;12(3):6-11. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-3-6-11
JATS XML






































