##article.goto##
Daily periodicity of labor in pregnant women in physiological and complicated pregnancy depending on the sex of the fetus
https://doi.org/10.21886/2219-8075-2021-12-1-46-53
##article.abstract##
Objective: the study aimed to reveal the daily periodicity of labor, the nature of melatonin metabolism, and the outcome of childbirth in women with a physiological and complicated pregnancy, depending on the sex of the fetus.
Materials and methods: to study the chronophysiological characteristics of birth outcomes depending on fetal sex, 1 980 birth histories and stories of newborns were analyzed. The neonates were born between January 1 and December 31, 2016, in a maternity ward of the Federal State Budgetary Educational Institution of Higher Education “RostGMU” of the Ministry of Health of Russia. Melatonin production was identified by the level of urinary excretion of 6-sulfatoxymelatonin (6-SM) (its main metabolite) examining the morning portion of the urine of women by the ELISA method (at 8 am 3 ml of urine were collected in Eppendorf tube).
Results: it was revealed that fetal sex modulated the activity of the central regulatory mechanisms responsible for the daily period functional processes in the female body and the initiation of labor. The largest number of spontaneous births by male fetuses occurred in the early evening before midnight when daily illumination was decreased, while the birth of girls was observed more often in the period from midnight to early morning. At the same time, mothers of boys had lower production of melatonin compared to that of girls’ mothers.
Conclusions. The peculiarities of labor and birth complications associated with the sex of the fetus were identified.
##article.subject##
##article.forCitation##
Botasheva T.L., Andreeva V.O., Lebedenko E.Yu., Fabricant A.D., Khloponina A.V., Zheleznyakova E.V., Zavodnov O.P. Daily periodicity of labor in pregnant women in physiological and complicated pregnancy depending on the sex of the fetus. Medical Herald of the South of Russia. 2021;12(1):46-53. https://doi.org/10.21886/2219-8075-2021-12-1-46-53
Introduction
In the study of the obstetrical pathology mechanisms, a detailed study of integration mechanisms in the “mother – placenta – fetus” functional system (MPFFS) has great importance. Center and peripheral interactions in the MPFFS play a significant role in placental dysfunction pathogenesis [1][2][3][4][5][6].
According to the scant literature [7][8][9][10][11], one of the significant factors modulating the nature of fetalmaternal relationships in the MPFFS is the fetus gender. The evaluation category of these interactions is associated with a clear understanding that between the mother and the fetus organisms, mutual biochemical and hormonal signaling is carried out throughout the entire gestational period through the uteroplacental complex, which is a communication channel, and this signaling determines the formation of not just the MPFFS but also the “mother – placenta – female fetus” functional system” and “mother – placenta – male fetus functional system” with the characteristic options for the homeostatic balance of each of them [8].
The available data on the sexual dimorphism influence on the formation of obstetric pathology indicate that in 2014, the International Federation of Gynecology and Obstetrics recognized the male fetal gender (MFG) as a risk factor for the threat of premature birth [9][11]. Data are presented that certain forms of chromosomal aberrations are associated with sexual dimorphism which is manifested in the biochemical and sonographic marker features [12][13][14]. It has been established that gestational diabetes mellitus (GDM) is more often recorded in cases of male childbearing [15][16]. MFG is recognized as a risk factor for placental dysfunction [15][17] and the female fetal gender (FFG) is accompanied by an increased risk of toxicosis and moderate preeclampsia, while its severe forms are most typical for MFG [8].
Biorhythms with varying frequency have particular importance in gestational tolerance formation: almost all functional processes in the MPFFS are cyclical. The greatest importance belongs to the “sleep-wake” circadian rhythm [18][19][20].
The circadian periodicity of labor, which is most dependent on the day/night cycle, is of the greatest interest from the gestational process standpoint. Thus, there is evidence of the existence of a clear relationship between the act of delivery duration and the day length: within different seasons, rapid labor occurs with greater frequency as the daylight increases [21][22][8].
In connection with the above, it is of considerable interest to study the relationship of the fetus gender during pregnancy and labor, taking into account the chronophysiological characteristics of the birth act.
The goal of the research was to study the nature of the daily frequency of labor, the characteristics of melatonin metabolism, and labor outcomes in women with physiological and complicated pregnancies, depending on the sex of the fetus.
Material and methods
The studies were carried out based on the FSBEI of Higher Education Rostov State Medical University of the Ministry of Health of the Russian Federation.
Before conducting the research, informed consent was obtained from each woman (“Rules for conducting high-quality clinical trials in the Russian Federation” dated December 29, 1998). There is an Ethics Committee Protocol of the Research Institute of Obstetrics and Pediatrics of Rostov State Medical University containing information on the approval of these studies (Protocol No. 23/2 dated April 25, 2013).
A total of 1101 women were examined: 584 primigravidas with physiological gestation and childbirth (Group I). Two hundred eighty of them had a female fetus (Group Ia) and 304 pregnant women had a male fetus (Group Ib). Group II included 517 primigravidas with placental dysfunction: 253 of them had a female fetus (group IIa), 264 – a male fetus (Group IIb). In Groups I and II, all patients had vaginal births without labor induction and stimulation.
Entry criteria in clinical Group I were uncomplicated singleton full-term pregnancy; 18 to 28 years old range; absence of obstetric pathology based on the results of clinical, hormonal, chemical, ultrasound, and Doppler studies. Exclusion criteria from Group I were pregnancies resulting from assisted reproductive technology programs; chromosomal aberrations and fetus congenital malformations; congenital malformations of organs and systems in women; extragenital and endocrine pathology; women refused to participate in the study. Criteria for inclusion in Group II were singleton full-term pregnancy; 18 to 28 years old range; Doppler signs of blood circulatory disturbances in the uteroplacental complex arteries; the asymmetric form of fetal growth retardation; deviations in the indicators of the fetus biophysical profile [23][24].
The groups were randomized by the method of random numbers and “Flipping coin” [25].
Melatonin was determined based on the 6-sulfatoxymelatonin (6-SOMT) level in the morning urine.
The study of the outcome of labor was carried out based on the analysis of 1980 birth histories and the histories of newborns born from January 1 to December 31, 2016, at the maternity department of the FSBEI of Higher Education of the Rostov State Medical University of the Ministry of Health of Russia. The neonatal functional status was assessed using the Apgar scale.
Statistical data processing included the use of descriptive statistics (medians and Q1 and Q3). Statistical significance was calculated based on a 95% confidence level and a 0.05 accuracy of calculating statistical data.
Intergroup differences were determined using the nonparametric Kruskal-Wallis test; statistically significant differences were the basis for a posteriori analysis using the Mann-Whitney test. The initial data were processed using the IBM SPSS 26.0.0.1 and Excel 2016 programs. The circadian periodicity of the end of labor was carried out by Fourier time series analysis.
Results
An age distribution analysis of pregnant women in the examined sample revealed the predominance of 23–27-yearold women (60.9%), which is probably due to socioeconomic transformations. An intragroup comparative analysis showed a similar prevalence of patients aged 23– 27 years. In Group I, those were more by 25.4%, and in Group II – by 17.4% than younger pregnant women (p = 0.271).
The average age of menarche in the studied patients from Groups I and II was comparable and amounted to 11.7 ± 1.5 and 12.2 ± 1.3 years, respectively (p = 0.402).
Anamnestic data indicated that in both clinical groups, in most cases the menstrual cycle duration was 27–30 days (in Group I – 29 ± 1.2 and Group II – 28 ± 1.1 days) (p = 0.612).
Abnormal uterine bleeding characterized by an increase in the menstruation frequency (more than 38 days) was recorded in 7.0% of all women studied (42.1 ± 2.5 days in Group I and 38.3 ± 1.9 patients in Group II).
In most of the examined patients (75.0%, p = 0.021), the menstrual cycle duration was less than 8 days (in pregnant women in Group I – 4.7 ± 1.4, in Group II – 4.1 ± 2.1 days). There were no significant differences in the menstruation regularity and volume in the studied women (p = 0.513).
In the structure of past gynecological diseases, regardless of the course of pregnancy, chronic inflammatory diseases of the pelvic organs (PID) prevailed – 73.4% of cases (p = 0.0213). The remaining women’s history was aggravated by abnormal uterine bleeding (8.0%), intraepithelial cervical neoplasia (7.1%), genital endometriosis (0.7%), and polycystic ovary syndrome (0.3%). The analysis data were consistent with the all-Russian structure of gynecological morbidity in women of reproductive age indicating the stable leading position of PID (60-65%), non-inflammatory diseases of the genital tract took second place (21%), benign neoplasms (in the third place – 17%), and abnormal uterine bleeding (the fourth place – 7%) [2].
In Group II patients, pregnancy was aggravated by exacerbations of chronic extragenital diseases and hemodynamic disturbances in the uteroplacental complex which were noted in 58.3% and 89.1% of cases. According to ultrasound, the placental system state showed placenta thinning in 24.7% of women, thrombosis of the intervillous space – in 21.5%, oligohydramnios – in 43%. Medical records of Group II women indicated a high frequency of moderate preeclampsia (33.3%), threatened miscarriages (46.6%), partial chorionic detachment (42.6%), and anemia in pregnant women (58.6%). In 29.2% of pregnant women with placental dysfunction (Group II), an asymmetric form of fetal growth retardation was diagnosed.
In the daily frequency analysis of the time to labor activity onset, a statistically significant relationship to the fetal gender was not revealed (p = 10.069) in any of the clinical groups, apparently due to medical and social factors. However, the labor completion time was significantly associated with the “fetal gender” factor: in the total sample, boys were born 39.4% more often than girls (p = 0.041) associated with a decrease in daily illumination intensity (in the period from 18 to 24 hours); girls were born mainly 35.8% more often with a decrease in illumination intensity (in the morning period from 24 to 6) (p = 0.026).
Depending on the gestation course, the same pattern persisted: in Group I patients, girls were born statistically significantly more often (by 41.2%) (p = 0.019) from 00:00 to 06:00, while the birth of boys was recorded by 25.7% more often in the period from 18:00 to 00:00 hours (p = 0.038). The biorhythm period in the case of both variants of sexual dimorphism was 6 hours.
In clinical Group II, the same pattern persisted: girls were born more often (by 21.6%) in light-saturated hours of the day from 03:00 to 12:00 (the biorhythm period was 9 hours). On the contrary, more boys (by 24.8%) were born as the light-saturated time of day decreased from 16:00 to 00:00 hours (the biorhythm period was 8 hours). The prolongation of the biorhythm period in the case of placental dysfunction is worthy of note.
The regulatory role of melatonin metabolism in maintaining the chronoperiodicity of functional processes has been proven [8][27], in connection with which the morning 6-SOMT fraction in pregnant clinical groups was analyzed.
It was found that in trimester II in Group I pregnant women regardless of the fetus gender, there was a decrease in 6-SOMT by an average of 31.4% (p = 0.031, p = 0.026) compared with trimester I. In trimester III, compared with trimester II, the 6-SOMT was characterized by an increase: in pregnant women with female fetuses by 3.4 times, with male fetuses by 2.6 times (p = 0.015, p = 0.048), which perhaps is due to the physiological “summation” of maternal, placental, and fetal melatonin (Table 1).
Таблица / Table 1
Levels of 6-sulfatoxymelatonin in the urine of women at different stages of physiological pregnancy, depending on the «sex of the fetus»
Уровни 6-сульфатоксимелатонина в моче пациенток I группы по триместрам гестации в зависимости от фактора «половая принадлежность плода»
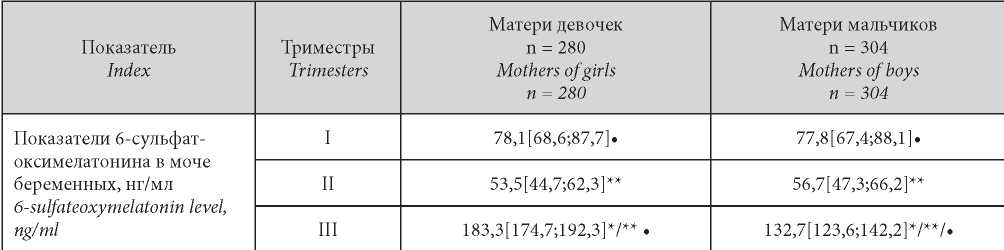
Note: * — statistical significance of the differences (p < 0.05) in the level of 6-sulfate-oxymelatonin in the subgroups examined in dependence on fetus sex during one trimester; ** — statistical significance of differences in the level of 6-sulfatoxymelatonin in one group in different trimesters of pregnancy; • — statistical significance of differences (p < 0.05) in the level of 6-sulfate-oxymelatonin in the group of the same gender within one trimester between physiological and complicated pregnancy
Примечание: * — статистическая значимость отличий (p < 0,05) показателей 6-сульфат-оксимелатонина в моче у беременных клинических групп в течение одного триместра в зависимости от половой принадлежности плода; ** — статистическая значимость отличий показателей 6-сульфатоксимелатонина в моче в одной клинической группе в разные триместры гестации; • — статистическая значимость отличий (p < 0,05) показателей 6-сульфат-оксимелатонинав моче в одноимённой по половой принадлежности группе в пределах одного триместра между физиологическим и осложненным течением беременности.
There were no statistically significant differences in the levels of 6-SOMT between Group I pregnant women with different fetus genders during trimesters I and II (p = 0.057, p = 0.062), however, in trimester III, 6-SOMT levels were significantly higher in pregnant women with FFG (p = 0.018).
A somewhat different example was noted in the placental dysfunction group (Group II) of pregnant women: already in the first trimester, 6-SOMT was 25.8% lower than in uncomplicated birth (Group I) regardless of sexual dimorphism (p = 0.041, p = 0.025) (Table 2).
Таблица / Table 2
Levels of 6-sulfatoxymelatonin in the urine of pregnant women with placental dysfunction depending on the «sex of the fetus»
Уровни 6-сульфатоксимелатонина в моче пациенток II группы по триместрам гестации в зависимости от фактора «половая принадлежность плода»
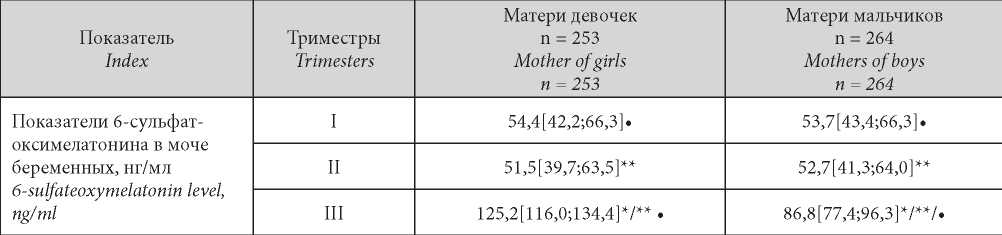
Note: * — statistical significance of the differences (p < 0.05) in the level of 6-sulfate-oxymelatonin in the subgroups examined in dependence on fetus sex during one trimester; ** — statistical significance of differences in the level of 6-sulfatoxymelatonin in one group in different trimesters of pregnancy; • — statistical significance of differences (p < 0.05) in the level of 6-sulfate-oxymelatonin in the group of the same gender within one trimester between physiological and complicated pregnancy.
Примечание: * — статистическая значимость отличий (p < 0,05) показателей 6-сульфат-оксимелатонина в моче у беременных клинических групп в течение одного триместра в зависимости от половой принадлежности плода; ** — статистическая значимость отличий показателей 6-сульфатоксимелатонина в моче в одной клинической группе в разные триместры гестации; • — статистическая значимость отличий (p < 0,05) показателей 6-сульфат оксимелатонинав моче в одноимённой по половой принадлежности группе в пределах одного триместра между физиологическим и осложненным течением беременности.
In this group, in the pregnancy second trimester, a decrease in 6-SOMT specific to a similar period in uncomplicated birth (Group I) was not revealed. In trimester III, an increase in 6-SOMT was recorded in the case of both fetus gender variants more pronounced in girls’ mothers as in the uncomplicated birth case (p = 0.039, p = 0.016).
It should also be noted that in placental dysfunction the absolute values of 6-SOMT were significantly lower compared to the Group I subjects (uncomplicated birth) in both boys’ and girls’ mothers (p = 0.024, p = 0.037) which appeared to be due to placental dysfunction.
Analysis of the frequency and structure of complications accompanying the pregnancy and childbirth period in the examined patients showed the following.
In Group II patients, taking into account the “fetal gender” factor, the antiphospholipid syndrome was more often (98.4%) recorded in pregnant women with FFG (p = 0.047). Cervical insufficiency with a comparable frequency was recorded in pregnant women with MFG and FFG was 25.1% and 18.7%, respectively (p = 0.031, p = 0.046).
GDM cases were significantly more frequent (82.8%) in pregnant women with MFG (p = 0.037) than in pregnant women with FFG. A similar pattern was observed when analyzing type 2 diabetes mellitus cases: in pregnant women with MFG, type 2 diabetes mellitus was recorded in 71.3% of cases (p = 0.045). Among fetuses with diabetic fetopathy, boys were also more common (63.6%, p = 0.049), while fetal growth retardation was detected in 63.7% of girls’ mothers (p = 0.023).
The occipitoposterior position of the vertex and pre-labor rupture of membranes also prevailed in MFG (78.4% and 53.6%, respectively). When analyzing birth complication in Group II patients, premature detachment of normally situated placenta was more often (in 75.4%) (p = 0.048) detected in women in labor with boys. An unripe cervix was more often diagnosed in pregnant women with FFG (65.7%, p = 0.024).
In the analysis of the state of newborns using the Apgar scale, it was found that the lowest scores (6 points and lower at the 1st minute after birth) were observed in 64.7% of boys (p = 0.023). Fetal macrosomia was found statistically significantly more often (2 times) in mothers of boys (p = 0.031), regardless of the gestation course.
Discussion
The studies carried out indicate that the structural and functional gestational “footprint” formed as a result of a long 9-month mother-fetus interaction modulates the activity of the functional subsystems responsible for the formation of the labor daily frequency. The fetal gender due to the difference in biochemical and mediator signaling determines the differences in the implementation of this period within the “sleep-wakefulness” daily cycle. A statistically significant dependence of the daily cycle acrophases of the labor completion time on the natural illumination intensity was revealed in both physiological and complicated pregnancies: in the male fetus case, the labor completion time is more often shifted to the dark day time with lower 6-SOMT, while the labor completion time in girls’ mothers is shifted mainly to the daytime with a higher 6-SOMT compared to boys’ mothers. In case of placental dysfunction, a biorhythm period prolongation is registered, which is a manifestation of the chronophysiological compensation of the dysfunctional processes that have arisen in the maternal body: it is known that an increase in biorhythm frequency and decrease in its period, and vice versa, a decrease in frequency and increase in the period of any biorhythm refer to chronophysiological elements of homeostasis maintenance [28][18].
It is also evident that there is a functional relationship between the regulatory structures responsible for the initiation of the delivery duration in the maternal organism carrying fetuses of different genders and the chrono-regulatory structures, in particular, the epiphysis producing melatonin.
As the delivery date approaches, the MPFFS has four sources of melatonin (pineal gland and internal organs of the mother’s body, as well as the placenta and the fetus), which make it possible to achieve its maximum production and ensure maximum mother and fetus adaptation to the delivery. There is also data for the melatonin role as a circadian signal for the onset of labor activity along with oxytocin. As part of the study, it was found that in pregnant women with a female fetus, regardless of the during pregnancy nature, the 6-SOMT was significantly higher. Destructive processes in the placental tissue with placental dysfunction contribute to a decrease in 6-SOMT throughout pregnancy and during childbirth which is a prerequisite for the occurrence of obstetric complications.
Conclusions
The labor completion time in the daily “sleep-wakefulness” cycle regardless of the gestation course is significantly associated with the fetal gender and the natural illumination intensity: in pregnant women with FFG, in the greatest number of cases, the physiological labor completion time is shifted to daylight hours, in the case of MFG – to night-time. The abortion during birth in time in girls’ mothers is accompanied by higher melatonin levels since this labor completion time in the case of FFG falls on the period of its greatest production (from 1 a.m. to 3 a.m.). Lower melatonin in mothers of boys, both in the final stages of pregnancy and during childbirth, is apparently due to the functioning of a protective mechanism preserving the male gonads, since high melatonin can have an aggressive effect on testicular theca, up to male infertility in subsequent stages of extrauterine life [29][30][8][22].
##submission.citations##
1. Zainalova S.A., Sinchikhin S.P., Stepanyan L.V. Placental insufficiency - problems of etiopathogenesis, diagnosis, clinic and treatment. Astrakhan Medical Journal. 2014;9(2):15-23. (In Russ.). eLIBRARY ID: 21794559
2. Savel’eva G.M., Serov V.N., Suhih G.T. Obstetrics and Gynecology. Clinical recommendations. Moscow: GeOTARMedia; 2016. (In Russ.).
3. Belotserkovtseva L.D., Kovalenko L.V., Kasparova A. E., Sus L.A. Placental and cardio-placental insufficiency: functional diagnostics modern methods of fetus pathology. Vestnik SurGU. Medicina. 2016;2(28):17-23. (In Russ). eLIBRARY ID: 28199173
4. Tapilskaya N.I., Mel’nikov K.N., Kuznetsova I.A., Glushakov R.I. Placental insufficiency and fetal growth restriction: etiology, prevention, and treatment. Medical alphabet. 2020;4:6-10. (In Russ). DOI: 10.33667/2078-5631-2020-4-6-10
5. Kuznetsova N.B., Bushtyrova I.O., Zabanova E.A., Barinova V.V., Gugueva A.V. Predictive value of critical disturbances in fetoplacental circulation in intrauterine growth restriction pregnancies. Obstetrics and Gynecology. 2020;6:59-64. (In Russ.). DOI: 10.18565/aig.2020-6.59-64
6. Di Renzo GC, Cabero Roura L, Facchinetti F, Helmer H, Hubinont C, et al. Preterm Labor and Birth Management: Recommendations from the European Association of Perinatal Medicine. J Matern Fetal Neonatal Med. 2017;30(17):2011-2030. DOI: 10.1080/14767058.2017.1323860
7. Kabanova M.A., Tolkach N.M., Kolesnikova N.B., Kalentyeva S.V. The course and outcomes of pregnancy depending on the sex of the fetus. Academic journal of Western Siberia. 2011;2:21-23. (In Russ.). eLIBRARY ID: 20695501
8. Botasheva T.L., Khloponina A.V., Vasil’eva V.V., Zavodnov O.P., Kaushanskaya L.V., Zheleznyakova E.V. Seasonal periodicity of melatonin exchange and hormonal status of pregnant women in dependence on fetus sex. Medical Herald of the South of Russia. 2018;9(3):70-76. (In Russ.). DOI: 10.21886/2219-8075-2018-9-3-70-76
9. Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19-30. DOI: 10.1016/s1550-8579(07)80004-0
10. Ramiro-Cortijo D, de la Calle M, Böger R, Hannemann J, Lüneburg N, et al. Male fetal sex is associated with low maternal plasma anti-inflammatory cytokine profile in the first trimester of healthy pregnancies. Cytokine. 2020;136:155290. DOI: 10.1016/j.cyto.2020.155290.
11. Saoi M, Kennedy KM, Gohir W, Sloboda DM, BritzMcKibbin P. Placental Metabolomics for Assessment of Sexspecific Differences in Fetal Development During Normal Gestation. Scientific Reports. 2020;10(1):9399. DOI: 10.1038/s41598-020-66222-3.
12. Larsen SO, Wøjdemann KR, Shalmi AC, Sundberg K, Christiansen M, Tabor A. Gender impact on first trimester markers in Down syndrome screening. Prenat Diagn. 2002;22(13):1207-8. DOI: 10.1002/pd.493
13. Knippel AJ. Role of fetal sex in amniotic fluid alphafetoprotein screening. Prenat Diagn. 2002;22(10):941-5. DOI: 10.1002/pd.408.
14. Gol M, Tuna B, Dogan E, Gulekli B, Bagci M, et al. Does fetal gender affect cytotrophoblast cell activity in the human term placenta? Correlation with maternal hCG levels. Acta Obstet Gynecol Scand. 2004;83(8):711-5. DOI: 10.1111/j.0001-6349.2004.00491.x
15. Botasheva T.L., Palieva N.V., Khloponina A.V., Vasiljeva V.V., Zheleznyakova E.V., et al. Fetal sex in the development of gestational diabetes mellitus and endothelial dysfunction. Obstetrics and Gynecology. 2020;9:56-64. (In Russ.). DOI: 10.18565/aig.2020.9.56-64
16. Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366-9. DOI: 10.1159/000077967
17. Gonzalez TL, Sun T, Koeppel AF, Lee B, Wang ET, et al. Sex
18. differences in the late first trimester human placenta transcriptome. Biol Sex Differ. 2018;9(1):4. DOI: 10.1186/s13293-018-0165-y
19. Agadzhanyan N.A. Fundamentals of human physiology. Volume 1. Normal human physiology. Moscow .: RUDN; 2012. (In Russ.).
20. Zaslavskaya R.M., Vas’kova L.B., Bolsunovskaya Y.R. Chronopharmacology and chronomedicine as a new methodological approach to optimize the therapy. Space and time. 2012;1(7):195-198. (In Russ.). eLIBRARY ID: 17322355
21. Kovalzon V.M., Dorokhov V.B. Human sleep-waking cycle and biorhythms with the different regimens of natural lightdark alternation. Health and education in the XXI century. 2013;15(1-4):151-162. (In Russ.). eLIBRARY ID: 20387316
22. Komarov F.I., Rapoport S.I. Chronobiology and chronomedicine. Moscow: Triada-X; 2000. (In Russ.).
23. Khloponina A.V., Botasheva T.L., Radzinsky V.E., Zheleznyakova E.V., Zavodnov O.P., Babayan K.T. Daily periodicity of labor depending on fetus sex. The Bulletin of the Adyghe State University. 2018;3(226):76-83. (In Russ.). eLIBRARY ID: 36526536
24. Sidelnikova V.M., Sukhikh G.T. Miscarriage. Moscow: Honey. inform. agency (MIA); 2010. (In Russ.).
25. Sherer DM, Kogan MG. Abnormal nonstress test yet otherwise reassuring biophysical profile in a compromised fetus with severe antepartum intracranial hemorrhage. Gynecol Obstet Invest. 2001;52(1):66-70. DOI: 10.1159/000052944
26. Dvoirin V.V., Klimenkov A.A. Methodology of controlled clinical trials. Moscow. 2004. (in Russ.).
27. Boyarsky A.Ya., Gromyko L.G. General theory of statistics. Moscow: Moscow University; 1985. (In Russ.).
28. Aulamazyan E.K., Evsyukova I.I., Yarmolinskaya M.I. The role of melatonin in development of gestational diabetes mellitus. Journal of Obstetrics and Women’s Diseases. 2018;67(1):85-91. (In Russ.). DOI: 10.17816/JOWD67185-91
29. Zaguskin S.L. Cell rhythms and human health. Rostov on Don: SFU; 2010. (In Russ.).
30. Chekhonatskaya M.L., Vasilevich L.K., Bondarenko N.A. Influence of pregnancy course peculiarities on formation of fetal testicles (review). Saratov Journal of Medical Scientific Research. 2013;9(2):234-240. (In Russ.). eLIBRARY ID: 20203827
31. Komarov F.I., Rapoport S.I., Breus T.K., Chibisov S.M. Desynchronization of biological rhythms in response to environmental factors. Clinical medicine. 2017;95(6):502-512. (In Russ.). DOI: 10.18821/0023-2149-2017-95-6-502-512
##article.authors.about##
T. L. BotashevaРоссия
Tatyana L. Botasheva, Dr. Sci. (Med.), Professor, Chief Researcher, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics
Rostov-on-Don
V. O. Andreeva
Россия
Vera O. Andreeva, Dr. Sci. (Med.), Associate Professor, Chief Researcher, Obstetrics and Gynecology Department
Rostov-on-Don
E. Yu. Lebedenko
Россия
Elizaveta Yu. Lebedenko, Dr. Sci. (Med.), Professor of the Chair of obstetrics and gynecology №3
Rostov-on-Don
A. D. Fabricant
Россия
Anna D. Fabrikant, Resident of the Department of Obstetrics and Gynecology No. 1
Rostov-on-Don
A. V. Khloponina
Россия
Anna V. Khloponina, Dr. Sci. (Med.), Senior Researcher, Obstetric and Gynecological Department
Rostov-on-Don
E. V. Zheleznyakova
Россия
Elena V. Zheleznyakova, Сand. Sci. (Med.), Research Associate, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics
Rostov-on-Don
O. P. Zavodnov
Россия
Oleg P. Zavodnov, Dr. Sci. (Bio.), , Researcher, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics
Rostov-on-Don
##reviewer.review.form##
##article.forCitation##
Botasheva T.L., Andreeva V.O., Lebedenko E.Yu., Fabricant A.D., Khloponina A.V., Zheleznyakova E.V., Zavodnov O.P. Daily periodicity of labor in pregnant women in physiological and complicated pregnancy depending on the sex of the fetus. Medical Herald of the South of Russia. 2021;12(1):46-53. https://doi.org/10.21886/2219-8075-2021-12-1-46-53
JATS XML






































