##article.goto##
Morphological verification of the first missed abortion
https://doi.org/10.21886/2219-8075-2021-12-1-62-67
##article.abstract##
Objective. The study aimed to compare the level of INF a2 immunoexpression in tissues obtained during medical abortion with the corresponding level of IFNa2 expression in a retained fetal egg tissues after the first missed abortion. The authors compared the anamnestic data on previous inflammatory diseases of the genital tract with the results of an extended morphological study of the material obtained during the evacuation of the contents of the uterine cavity during the first non-developing pregnancy in the first trimester.
Materials and methods. The study included 15 patients with first-time missed abortions caused by a viral infection (6-8 weeks of pregnancy). All patients demonstrated either recurrent herpes simplex labialis/genitalis or PCR confirmed HSV, HPV, CMV. Exclusion criteria were recurrent miscarriage, blighted ovum, endocrinopathies, male factor infertility, and other causes of miscarriage. The comparison group included 20 women of the same age that chose to undergo a medical abortion.
Results. In patients from the comparison group, the main producer of IFN a2 was syncytiotrophoblast as well as maternal decidual cells in the parietal endometrium and uteroplacental area. In the main group, manifested hematogenous infection (microabscesses, vasculitis, lymphocytic and macrophage infiltration) with dystrophy and necrosis of decidual maternal cells and secondary pathological changes in the placental villi were diagnosed, which led to a significant decrease in the IFN a2 immunoexpression in all the studied cells.
Conclusion. The lack of anamnestic data on previous urogenital infections does not exclude the etiological role of the inflammatory component in the genesis of non-developing pregnancy. First-time occurred pregnancy loss requires adequate postoperative interferon therapy and a thorough examination of a couple.
##article.subject##
##article.forCitation##
Lebedenko E.Y., Milovanov A.P., Sablina N.V., Fokina T.V., Gaida O.V. Morphological verification of the first missed abortion. Medical Herald of the South of Russia. 2021;12(1):62-67. https://doi.org/10.21886/2219-8075-2021-12-1-62-67
Introduction
Non-developing pregnancy (NP) is a complication associated with the death of the embryo or fetus in the uterine cavity. The term «undeveloped pregnancy» used in Russian-speaking practice has been replaced in English-speaking countries by the definition of «miscarriage» (missed abortion), which more accurately reflects the nature of this pathology as no pregnancy progression when the embryo (fetus) is retained in the uterine cavity.
In modern obstetrics and gynecology, the generally accepted causes of reproductive losses on the mother’s side are structural uterus disease, antiphospholipid syndrome, hormonal and metabolic disorders. Genetic disorders provoke 50–60% of reproductive losses early in gestation [1]. The role of mothers’ medical conditions as to the termination of pregnancies mainly increases in the second and third trimesters of pregnancy. In 26–40% of cases, the causes of miscarriage are considered unknown.
It should be noted that the established concept of the significant role of chromosomal abnormalities as the main cause of gestational losses in the first trimester of pregnancy has formed a passive position among clinicians, which even in the absence of chromosomal abnormalities in fetal cells does not induce clinicians to verify other reproductive failure causes. As a result, the couple’s desire to realize their reproductive function in the near term after their first loss early in gestation, associated with obstetricians’ lack of a cautious attitude towards the first reproductive failure, translates the obstetric situation into habitual miscarriage.
The NP proportion in the structure of reproductive losses varies but remains at a fairly high level at 10–20%. According to Milovanov and Serova (2011), among the losses of the first trimester, NP was 4–10% of all confirmed pregnancies [2]. At the same time, 108 (60%) of 180 surveyed women with NP had inflammatory (most often viral) causes in the presence of subchronic forms or carriage of viruses. A combination of parietal and basal deciduitis, vasculitis, microabscesses, and other signs of viral cell damage was recorded in the abortive material.
Thus, when confirming NP, the most informative way of capturing the reasons is a detailed specification of anamnestic data and a detailed morphological study of the uterine cavity evacuated contents [3, 4].
The goal of the research was to compare the anamnestic data on previous inflammatory diseases of the genital tract with the results of an extended morphological study of vacuum aspirates of the uterine cavity during the first NP.
Material and methods
Group I included 15 nulliparous women (mean age, 29.4 ± 2.3 years) with the first NP at 6–8 weeks diagnosed by the progressive assessment of the blood β-hCG dynamics, as well as by ultrasound. The experimental group (II) included 20 healthy women of comparable age (27.1 ± 3.1 years) who wished to terminate an unplanned pregnancy by an artificial abortion at the same term.
The exclusion criteria were a history of childbirth, recurrent miscarriage, anembryonic pregnancy, as well as those associated with endocrinopathies and hemostasis disorders, surgical interventions on the uterus and cervix.
In both groups of patients, the emptying of the uterus was carried out at the gynecological department of the Public Funded Health Facility Municipal Clinical Hospital named after S.P. Botkin (Moscow) by vacuum aspiration (by the method regulated by Order No. 572n, as well as by the American College of Obstetricians and Gynecologists manual (2015) [5]), the Eschmann VP 35 apparatus (Great Britain) at an 80 kPa negative pressure or a plastic aspirator with a 60 ml volume using disposable plastic cannulas without prior dilation of the uterine cervix with intravenous anesthesia.
Infectious disease history, as well as the results of previous clinical and laboratory studies, was taken according to a specially developed questionnaire.
At the Research Institute of Human Morphology (Moscow), histologic sections were prepared from vacuum aspirates and stained with hematoxylin and eosin. After viewing in a Leica 2500 microscope (Germany), blocks with samples of placental villi, parietal endometrium, as well as the uteroplacental area, the junction of the villi with the uterine wall, were taken. Additional sections were cut from these blocks and, after dewaxing, were placed on polylysine-coated slides. Epitope retrieval was carried out in a microwave oven for 20 minutes in citrate buffer (pH 6.0). The following antibodies were used by immunoperoxidase technology: 1) IFNa2 polyclonal antibody of assessing the immunoexpression level: 1 point – light brown cytoplasm staining, 2 points – brown cytoplasm staining, 3 points – dark brown cytoplasm staining; 2) rabbit monoclonal vimentin antibody (Vimentin EP21) for the determination of decidual stromal cells; 3) rabbit monoclonal granzyme B antibody (Granzyme B) – a marker of uterine natural killer cells. A detection system was used to visualize the immunoreaction results. Negative reactions to the reagents used were conducted.
The IFNa2 immunoexpression scoring was statistically processed by the Mann-Whitney U test.
Results
In the detailed study of the infectious history in Group I patients, it was established that four patients (26.7%) had acute inflammatory diseases of bacterial etiology only during this pregnancy diagnosed as non-developed. The rest (73.3%) had anamnestic data on acute viral diseases during pregnancy: acute herpesvirus and acute respiratory viral infection. Also, in these patients, herpes simplex virus-2 (HPV-2) – 40.0%, cytomegalovirus (CMV) – 26.6%, rubella virus – 26.6%, and bacterial agents – 26.6% were detected once in the blood before pregnancy by polymerase chain reaction. Seven patients (46.7%) had acute inflammatory diseases of viral and bacterial etiologies.
The histological processing of uterine vacuum aspirate in Group 1 patients showed that all patients had pronounced signs of hematogenous infection, in particular microabscesses, vasculitis, lymphoma-macrophage infiltration in the parietal endometrium, and uteroplacental area showing a pattern of acute or chronic endometritis. At the same time, markers of viral endometrial lesions were diagnosed in every second patient (50%). In 7 cases (46.7%), signs of basal deciduitis with microabscesses were visualized in the endometrium. Local bleeding disorders as arterial microthrombosis were detected in 11 cases (73.3%). At the same time, none of the patients included in the study had any blood coagulation disorders previously diagnosed. In 12 out of 15 cases (80%), rheological disorders were revealed according to the type of development of various age retrochorial hematoma.
Comparison of the anamnestic data on the transferred infectious and inflammatory diseases with the cytomorphological examination showed the following. In 11 (73.3%) patients, the cause of NP was local inflammation that arose by ascending or hematogenous infection, which, when the blastocyst was immersed in the endometrium and formed a chorionic sac, damaged glandular epithelial cells, decidual cells, and invade cytotrophoblast.
In four women (26.7%) who did not have an infectious history, morphological signs of inflammation associated with epithelial proliferation with cellular infiltration of the stroma were interpreted as a consequence of an inflammatory reaction resulting from the dead ovum retention in the uterine cavity and the chorionic villi rejection. In these patients, non-infectious factors were identified that caused the early fetal loss, having an allo-/ autoimmune nature (abnormal activity of natural killer cells, the presence of alloimmune antibodies, human leukocyte antigen incompatibility between partners, thrombophilic conditions).
Histological processing of 20 vacuum aspirates from Group II patients showed no signs of an inflammatory reaction (decidual tissue, chorionic villi, progressive uterine pregnancy) which corresponded to the absence of anamnestic data on bacterial and viral infectious diseases before and during pregnancy.
Taking into account the data of some authors on the role of infectious agents as a trigger mechanism in the subsequent induction of autoimmune reactions of the endometrium [6], the development of secondary immunodeficiency and immunosuppressive states, it was of some interest to compare the levels of immunoexpression of interferon alpha-2 (IFNa2) in vacuum aspirate cells after the first NP in terms of 6–8 weeks with the same in Group II (medical abortions).
The main object of the immunohistochemical study was cells producing IFNa2. In scoring, the immunoactivity of all cells producing IFNa2 in the patient group with NP (Group I) was significantly lower (Table 1).
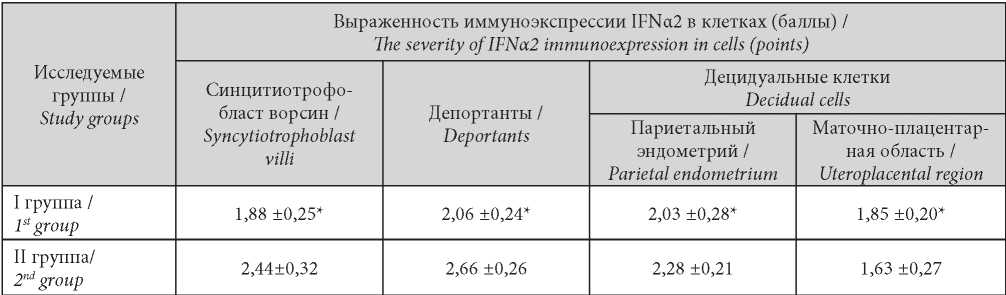
Таблица / Table 1
Scoring of IFNα2 immunoexpression in medical abortion (MA) and first occurred missed abortion caused by inflammation (NB) cells
Балльная оценка иммуноэкспрессии IFNα2 в клетках вакуум-аспиратов медицинских абортов (МА) и неразвивающейся беременности (НБ)
Immunohistochemically, inflammatory infiltration was characterized by microabscesses in the uteroplacental area with the retention of IFNa2 producing cells only within the fibrinoid boundary layer (left) zone and death of decidual
cells in the inflammatory zone (Figure 1). The approach of the villi near the chorionic sac wall and a decrease in the number of deportants were detected, a thinned syncytiotrophoblast was detected in the intervillous space with a reduced immunoexpression of IFNa2, and vasculogenesis was absent in the stroma (Figures 2 and 3).
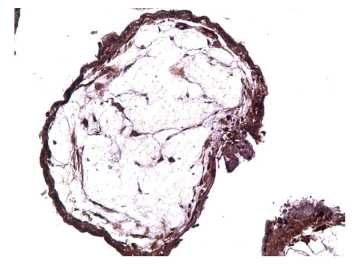
Figure 1. Occurred missed abortion (7 weeks bp): microabscess in the uteroplacental region, retention of IFNa2- producing cells only in the zone of the fibrinoid boundary layer (left) and death of decidual cells in the inflammation zone, immunohistochemistry, x100.
Рисунок 1. НБ (7 нед п.о.): микроабсцесс в маточноплацентарной области, сохранение IFNa2- продуцирующих клеток только в зоне пограничного слоя фибриноида (слева) и гибель децидуальных клеток в зоне воспаления, иммуногистохимия, х100.
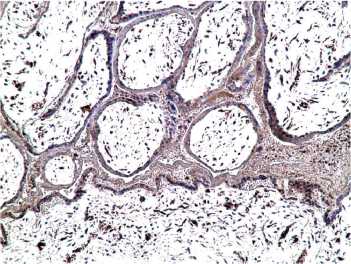
Figure 2. Occurred missed abortion (6 weeks): placental villi with thinned syncytiotrophoblast with reduced immunoexpression of IFN-2, in the stroma absence of vasculogenesis, x200.
Рисунок 2. НБ (6 нед): ворсина плаценты с истонченным синцитиотрофобластом при сниженной иммуноэкспресии IFNa2, в строме — отсутствие васкулогенеза, х200.
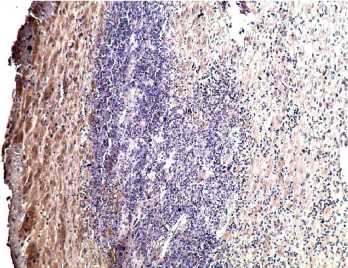
Figure 3. Occurred missed abortion (8 weeks): contiguous villi near the chorionic sac (bottom), lack of immunoexpression of IFN-2, in the stroma individual placental macrophages, x200.
Рисунок 3. НБ (8 нед): сближенные ворсины возле хориального мешка (внизу), отсутствие имууноэкспресии IFNa2, в строме — отдельные плацентарные макрофаги, х200.
In the immunohistochemical study of the cells in Group II patients, the maximum IFNa2 expression was found in the surface epithelium (syncytiotrophoblast) of the placental villi, as well as in its derivatives – deportants (Figure 4).
A pronounced immunoreaction (3 points) was visible in the syncytiotrophoblast cytoplasm and superficial brush border. Also, lateral epithelial diverticulum with many nuclei in the common cytoplasm became typical. The deportants and syncytiotrophoblast connections gradually diminished, and they ended up in the intervillous space, in the venous collectors.
The visualized placental villi were distinguished by thick syncytiotrophoblast and pronounced IFNa2 expression; intense vasculogenesis was found in the stroma (Figure 5).
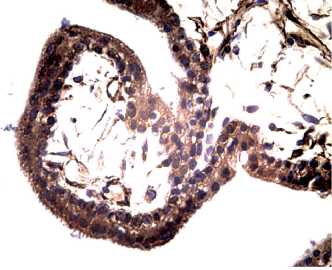
Figure 4. Artificial abortion — vacuum aspiration (6 weeks): placental villi with thick syncytiotrophoblast and pronounced expression of IFN-2, in the stroma intense vasculogenesis, immunohistochemistry, x 200.
Рисунок 4. Артифициальный аборт — вакуумаспирация (6 нед): ворсины плаценты с толстым синцитиотрофобластом и выраженной экспрессией IFNa2, в строме — интенсивный васкулогенез, иммуногистохимия, х 200.
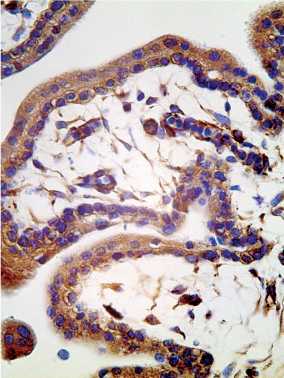
Figure 5. Artificial abortion — vacuum aspiration (7 weeks): full-fledged villi near the chorionic sac, immunoexpression of IFNa2 in syncytiotrophoblast and stromal vessels (angiogenesis), immunohistochemistry, x 200.
Рисунок 5. Артифициальный аборт — вакууумаспирация (7 нед): полноценные ворсины возле хориального мешка, иммуноэкспрессия IFNa2 в синцитиотрофобласте и стромальных сосудах (ангиогенез), иммуногистохимия, х 200.
Discussion
A comparison of IFNa2 immunoexpression between patients after the first NP (Group I) and healthy women with a medical abortion (Group II) confirmed a significant deficiency of interferonogenesis in maternal decidual cells and placental structures such as syncytiotrophoblast and its deportants. During the physiological development of pregnancy in trimester I in Group II patients, significant IFNa2 production by syncytiotrophoblast of villi and its deportants with delivery to the maternal body, as well as maternal decidual cells in the parietal endometrium and uteroplacental region, was registered.
There is no doubt that this phenomenon is causally associated with a massive inflammatory process in the parietal endometrium and the uteroplacental area, as well as with the remote effect of local factors of maternal inflammation on the placental villi including the uterine killer cells’ cytotoxic effect.
Conclusion
The data obtained correlate with the association of NP and chronic endometritis generally accepted at the FIGO 2006 International Congress [10]. However, the routine antibiotic prescription to each patient with NP without a proven causally significant infection must be recognized as irrational, since the use of chemotherapeutic agents for aseptic inflammation can enhance immunosuppression and aggravate structural and functional disorders of the endometrium.
The results obtained confirm the need to revise the stereotype formed among obstetricians-gynecologists to consider the first NP as a natural selection sporadic element. A reasonable approach to the subsequent recurrent miscarriage prevention among patients with a history of first NP should be the formation of a high-risk group of repeated reproductive failures. When confirming the virus-associated inflammatory cause of the first NP, it is necessary to carry out adequate interferon therapy in the postoperative period and a similar pregravid preparation for the next pregnancy.
Active identification of anamnestic data on persistent viral infection allows verifying its etiological role in the NP genesis, which consists in the development of endometrium morphofunctional changes with impaired normal cyclic transformation and tissue receptivity, impaired implantation, trophism, and early embryo loss. Unjustified antibiotic therapy with broad-spectrum drugs in the rehabilitation framework after NP leaves in the «shadow» the true etiological factors and mechanisms for stopping the pregnancy development. Comparison of infectious history data with a detailed morphological study clarifies the goals of subsequent preconception preparation when planning pregnancy, substantiates the feasibility and range of additional diagnostic measures for the prevention of repeated early reproductive losses.
##submission.citations##
1. Adamyan L.V., Serov V. N. Miscarriage in early pregnancy: diagnosis and management tactics. Clinical guidelines (treatment protocol). 2016. (In Russ.)
2. Milovanov A.P. Serova O.F. Causes and differential treatment of early miscarriage. M. Studio MDV; 2011. ( In Russ.).
3. Radzinsky V.E., Orazmuradov A.A., Eds. Early pregnancy. M; 2005. (In Russ.).
4. Milovanov A.P. Pathology of the mother-placenta-fetus system. M.: Medicine; 1999. (In Russ.)
5. Ailamazyan E. K., Kulakov V. I., Radzinsky V. E., Savelyeva G. M., eds. Obstetrics. National leadership. 2014. (In Russ.)
6. RadzinskyV.E., Zapertova E.Yu., Misnik V.V. Genetic and immunological aspects of habitual miscarriage. Akush. and gin. 2005;6:24-29. (In Russ.). eLIBRARY ID: 9141156
7. Milovanov A.P., Voloshchuk I.N. Deported syncytiotrophoblast and placental microparticles in the mother’s body during normal pregnancy and preeclampsia (28 years later). Archive of pathology. 2017;79(1): 1–6. (in Russ.). DOI: 10.17116/patol201779161-67
8. Papadopoulos N, Simopoulos C, Karamanidis D, Kotini A, Tamiolakis D. Large granular lymphocytes (LGLS) activity in women with spontaneous abortions during the 1st trimester of gestation. Panminerva Med. 2002;44(4):343-7. PMID: 12434116.
9. Lambropoulou M, Tamiolakis D, Venizelos J, Liberis V, Galazios G, et al. Imbalance of mononuclear cell infiltrates in the placental tissue from foetuses after spontaneous abortion versus therapeutic termination from 8th to 12th weeks of gestational age. Clin Exp Med. 2006;6(4):171-6. DOI: 10.1007/s10238-006-0111-x
10. Gupta S., ed. ACOG’s Guide to Managing Miscarriage: Follow Patient Preference. Updated clinical management of early pregnancy loss focuses on patient choice. Medpageto- day, 04.22.2015.
##article.authors.about##
E. Y. LebedenkoРоссия
Elizaveta Yu. Lebedenko, Dr.Sci. (Med.), associate professor, professor of the Department of obstetrics and gynecology №3
Rostov-on-Don
A. P. Milovanov
Россия
Andrey P. Milovanov, Dr. Sci. (Med.), Head Researcher Reproduction Pathology Laboratory
Moscow
N. V. Sablina
Россия
Natalya V. Sablina, obstetrician-gynecologist, gynecologic department
Moscow
T. V. Fokina
Россия
Tatyna V. Fokina, Cand. Sci. (Med.), Senior Researcher Reproduction Pathology Laboratory
Moscow
O. V. Gaida
Россия
Gaida Oksana Vladimirovna, Cand. Sci. (Med.), associate professor of the Department of Obstetrics and Gynecology
Rostov-on-Don
##reviewer.review.form##
##article.forCitation##
Lebedenko E.Y., Milovanov A.P., Sablina N.V., Fokina T.V., Gaida O.V. Morphological verification of the first missed abortion. Medical Herald of the South of Russia. 2021;12(1):62-67. https://doi.org/10.21886/2219-8075-2021-12-1-62-67
JATS XML






































