Scroll to:
Idiopathic pulmonary arterial hypertension and congenital heart defects: similarities and differences
https://doi.org/10.21886/2219-8075-2025-16-1-46-54
Abstract
Objective: to study the clinical and pathogenetic features of idiopathic pulmonary arterial hypertension (IPAH) and pulmonary arterial hypertension (PAH) in congenital heart defects, based on a 10-year follow-up of patients in the Rostov region. Materials and methods: the analysis included patients over the age of 18 who are registered in the Rostov region with various etiologies of PAH, including with IPAH and PAH-CHD. Two groups of patients were identified: 1st – patients with IPAH (n=32) and 2nd – with PAH in congenital septal heart defects (defects of the atrial or interventricular septa) (n=30). All patients underwent electrocardiography, echocardiography, and condition assessment using the clinical condition assessment scale, as well as the effectiveness of a screening questionnaire for early diagnosis of PAH. Results: it was found that patients with IPAH are older, seek medical help more often, have more pronounced clinical symptoms compared to patients in the 2nd group, and require more aggressive treatment. Patients with PAH in congenital heart defects are less likely to seek help, and have a longer period from the onset of the first symptoms to the detection of pulmonary hypertension. Patients in group 1 required increased PAH-specific therapy in 53% of cases, and patients in group 2 – in 19% (p<0.05). Conclusions: clinical and pathogenetic features of idiopathic PAH and against PAH in congenital heart defects have been identified, which makes it possible to optimize the early diagnosis and treatment of such patients
Keywords
For citations:
Devetyarova E.A., Chesnikova A.I., Dyuzhikov A.A. Idiopathic pulmonary arterial hypertension and congenital heart defects: similarities and differences. Medical Herald of the South of Russia. 2025;16(1):46-54. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-46-54
Introduction
Pulmonary arterial hypertension (PAH) is a hemodynamic and pathophysiological condition characterized by an increase in mean pulmonary artery pressure (mPAP) >20 mmHg at rest, measured by transvenous pacing [1, 2].
Pulmonary hypertension can develop in patients with various diseases and, depending on its etiology, can have a different course. The choice of patient management tactics and prognosis is closely related to the main causes of PAH, which emphasizes the importance of correct and timely diagnosis.
The most common cause of all cases of PAH is a cardiovascular disease, such as acquired and congenital heart defects (CHD), which were not surgically corrected in a timely manner [3].
The true frequency and prevalence of PAH associated with CHD remain unknown, since the necessary tests to verify the diagnosis are not always performed [4, 5]. Among the limited available published data, it is worth noting an epidemiological study conducted in Turkey, which showed that the incidence of PAH associated with CHD was 9.5 cases per million population per year [6].
The diagnosis of “idiopathic PAH (IPAH)” can be made when other causes of PAH were not identified, i.e., “IPAH” is a diagnosis of exclusion [1].
Unfortunately, the prognosis for PAH remains unfavorable. According to research data, the one-, three-, and five-year survival rates are 89%, 85%, and 75%, respectively [7]. There is insufficient information in the current publications about the similarities and differences between IPAH and PAH associated with CHD, which appears to be important for practical healthcare.
The aim of the study was to investigate the clinical and pathogenetic features of idiopathic PAH and PAH associated with CHD, based on 10 years of observation in the Rostov Region.
Materials and Methods
The analysis included patients with PAH over the age of 18 who were in the Rostov Region registry list, which was created in 2014. The registry includes patients with PAH of various etiologies, including IPAH and PAH associated with CHD. Patients with severe CHD and Eisenmenger syndrome were excluded from the analysis. Two groups of patients were identified: Group I – patients with IPAH (n=32) and Group II – patients with PAH associated with septal CHD (atrial or ventricular septal defects), including those corrected in 23% of cases (n=30). The observation period was 12 months. The following research methods were used in the study: electrocardiography, echocardiography, chest X-ray, Holter ECG monitoring, general clinical examination, laboratory diagnostics, including determination of the level of N-terminal polypeptide of brain natriuretic hormone (NT-proBNP), and assessment of the condition of patients using the Clinical Status Assessment Scale modified by V.Yu. Mareev (CSAS). The effectiveness of a screening questionnaire for the early diagnosis of PAH was also evaluated. For the comprehensive assessment of the effectiveness of PAH-specific therapy, clinical symptoms and exercise tolerance, as well as the frequency of hospitalizations for any reason, emergency medical calls, visits to the doctor, and fatalities were evaluated over time.
Statistical analysis of the data was performed using the Statistica 12 software package developed by StatSoft (USA).
The distribution of the studied indicators was checked for normality using Shapiro-Wilk’s and Kolmogorov-Smirnov’s tests.
Given that the distribution of the studied indicators in the sample was both normal and abnormal, the median and interquartile range (Me [Q1; Q3]) were used to present the data.
Qualitative variables are presented as absolute (n) and relative (%) values.
Quantitative data for each indicator are presented in the form of a sample mean value with an indication of its standard error, confidence interval limits for the mean value with a confidence level of 95%, median distribution, minimum and maximum values, lower and upper quartile limits, quartile range, and the coefficient of variation.
The significance of differences in quantitative indicators between groups was tested using nonparametric statistical methods. A pairwise comparative analysis was performed using the Mann-Whitney test for independent variables. Frequency analysis and contingency tables were used to analyze qualitative indicators.
Results
Analysis of registry data showed that patients in Group I were older than those in Group II (mean age – 53.5 [42.5; 61.0] years old and 33.533 [18.0; 50.0] years old, respectively, p<0.05). The age at the time of diagnosis also differed (mean age – 53.5 [39.0; 61.0] years old and 17.0 [11.0; 43.0] years old, respectively, p<0.05). Women predominated in each group: 87.5% in the IPAH group and 73.3% in the group of patients with PAH associated with CHD. More overweight patients were identified among patients in Group I (43.75% vs. 20%, p<0.05), although no statistically significant difference was found when comparing obese and normal-weight patients (p>0.05).
An assessment of the medical history data revealed that the following risk factors predominated in Group I: dyslipidemia (31.25% vs. 10%, p<0.05), arterial hypertension (65.625% vs. 36.67%, p<0.05), and use of hormonal contraceptives (28% vs. 7%, p<0.05). Among concomitant pathologies, patients in Group I were more likely to have thyrotoxicosis (9.375% and 0%, p<0.05), varicose veins of the lower extremities, and thrombosis of the lower extremities (34.375% and 15.625% vs. 6.67% and 0%, p<0.05), as well as a history of pulmonary embolism (PE) after a confirmed diagnosis of PAH (12.5% and 0%, p<0.05). Most likely, the development of in situ thrombosis as a pathological characteristic of endothelial dysfunction was more pronounced in patients with IPAH.
When evaluating clinical data, it was found that shortness of breath was observed in 100% of cases in both groups. Patients with IPAH reported fatigue (100% vs. 83.33%, p<0.05), chest pain (75% vs. 50%, p<0.05), and lower limb edema (100% vs. 73%, p<0.05), including anasarca (31.25% vs. 10%, p<0.05), more often.
Analysis of exercise tolerance showed that, when included in the study, exercise tolerance was lower in patients with IPAH according to the 6-minute walk test (6MWT): in patients with IPAH – 255.0 [154.5; 325.0] m, in the Group with CHD – 376.5 [240.0; 423.0] m (p<0.05).
The assessment of the functional class of PAH (WHO) showed that both groups predominantly had FC III PAH (WHO) (56% and 67%, respectively, p>0.05), while patients with FC IV were only in Group I (15.625% vs. 0%, p<0.05) (Fig. 1). It is important to note the greater need for oxygen therapy in patients with IPAH (16% vs. 0%, p<0.05), which is associated with a more severe course of the disease.
A comparative analysis of ECG data revealed that patients in Group II showed signs of right heart overload (84.375% in Group I vs 100% in Group II, p<0.05) more often. According to Holter ECG monitoring data, transient 2–3-degree atrioventricular block was recorded in the group of patients with CHD, which was not detected in patients with IPAH (10% vs. 0%, p<0.05).
The assessment of the structural and functional parameters of the left and right ventricles in patients in Groups I and II did not reveal any significant differences, except for the parameter of right ventricular stroke volume, which was significantly higher in patients with CHD (72.4 ml vs. 45.9 ml, p<0.05).
The evaluation of the average score on the clinical status assessment scale modified by V.Yu. Mareev (CSAS) showed that in Group I, it was significantly higher than in Group II (9.5 [5.0; 12.0] vs. 6.0 [5.0; 8.0] (p<0.05)). In addition, a higher score in patients with IPAH was also noted in the analysis of the PAH screening questionnaire, which was developed by specialists from the E.I. Chazov National Medical Research Center of Cardiology. The questionnaire consists of six sections, including clinical symptoms, medical history data, physical examination data, the presence of diseases associated with PAH, ECG and chest X-ray data, as well as echocardiographic examination (Table 1).
The PAH screening questionnaire was evaluated in two ways: the first method included all sections of the questionnaire except for echocardiography, while the second method involved using the questionnaire in its entirety, including the results of ultrasound examination of the heart. Both groups of patients received high scores, proving the effectiveness of this screening method even without the use of echocardiography results. According to the recommendations, a score of more than 11 on the questionnaire determines the need for immediate referral of patients to a PAH expert center for diagnosis verification [9]. In this study, the results of the questionnaire without echocardiography analysis showed that patients in Group I scored 14.0 [12.0; 16.5] points, while patients in Group II scored 12.0 [9.0; 14.0] points (p<0.05), i.e., patients with IPAH had a higher score. The obtained data allow the authors to conclude that the use of the questionnaire, even in this version, based on the number of obtained points, allows suggesting a high probability of pulmonary hypertension. Analysis of the results, taking into account the data of the ultrasound examination of the heart, also revealed a higher score in patients in Group I (15.0 [13.0; 17.5] vs. 13.0 [10.0; 15.0] points (p<0.05)). Thus, it can be concluded that the careful collection of symptoms, medical history, and attentive clinical examination using ECG and chest X-ray may allow a primary care physician to suspect pulmonary hypertension in a patient, even without the possibility of performing an echocardiographic examination.
The study did not reveal any significant differences between the groups in the analysis of basic laboratory parameters: common and biochemical blood tests, and coagulation data. It should be noted that the level of the heart failure marker NTproBNP also did not differ significantly: 380.0 [220.0; 1314.0] pg/ml versus 276.0 [101.0; 643.0] pg/ml (p>0.05).
During the 12-month observation period, the dynamics of clinical signs, physical exercise tolerance, frequency of hospitalizations, visits to medical facilities, calls to emergency services, and fatalities were assessed. More frequent hospitalization (25% and 6.67%, p<0.05) and emergency calls (68.75% and 43.33%, p<0.05) in patients with IPAH compared to patients with PAH associated with CHD indicated a more severe course of the disease. In addition, patients in Group I applied for outpatient care significantly more often (96.875% vs. 80%, p<0.05), including for decompensated heart failure (43.75% vs. 13.33%, p<0.05), as well as for other concomitant pathologies (65.625% vs. 30%, p<0.05). On average, the number of visits for outpatient care was 10.0 [6.0; 12.0] and 6.0 [5.0; 8.0], respectively (p<0.05). It should be emphasized that patients in Group II more often sought outpatient care on a scheduled basis for dynamic observation (43.3% vs. 15.6%, p<0.05).
Special attention in the study was paid to the analysis of ongoing PAH-specific therapy. At the time of inclusion in the study, 56% of patients in Group I and 40% of patients with PAH associated with CHD received monotherapy with PAH-specific drugs (p>0.05). However, only patients in Group II received combined dual PAH-specific therapy (0% vs. 20% (p<0.05) (Figs. 2, 3).
After the initial examination, therapy escalation occurred in 87.5% of patients with IPAH and in 66.67% of patients with PAH associated with CHD (p<0.05) (Fig. 4).
The change in therapy was predominantly double sequential, which prevailed in patients with PAH associated with CHD (19% in Group I versus 53% in Group II, p<0.05). No significant difference was found in the use of different drugs for PAH-specific therapy. At the time of inclusion in the study, patients were taking sildenafil, bosentan, and macitentan. After one year of follow-up following the escalation of therapy, it became more diverse: such drugs as ambrisentan, riociguat, and selexipag were added (Fig. 5). However, the distribution of drugs was comparable in both groups (p>0.05).
After the escalation of PAH-specific therapy, after 1 year, an improvement in exercise tolerance was observed according to 6MWT in patients in both groups, with patients with CHD maintaining better exercise tolerance: 300.0 [200.0; 400.0] m and 380 [300.0; 441.0] m (p<0.05). However, a more significant increase in 6MWT was observed in patients with IPAH (an increase of 45 m versus an increase of 3.5 m in patients with PAH associated with CHD (p<0.05)).
In the group of patients with CHD, a longer time was observed from the onset of signs to the diagnosis of PAH (8 months [3.5; 20.0] vs. 13.5 months [4.0; 42.0] (p<0.05)) and receiving PAH therapy (13.5 months [8.0; 30.0] vs. 35.5 months [12.0; 79.0] (p<0.05)).
All patients in Groups I and II showed symptoms and signs of chronic heart failure in 100% of cases. At the same time, differences were found between the groups in the treatment of heart failure: more frequent use of torasemide was observed in patients in Group I (65.625% vs. 33.33%, p<0.05), which is associated with the more frequent development of edema syndrome. The use of drugs from other groups (ACE inhibitors, ARBs, MRAs, and STGLT2 inhibitors) in patients from both groups did not differ significantly (p>0.05). It should be noted that warfarin was prescribed more frequently in patients in Group I (25% vs. 3.33%, p<0.05), which is apparently associated with the peculiarities of IPAH therapy in those years.
During the observation period, the mortality rate in both groups did not differ significantly (25% and 17%, respectively, p>0.05).
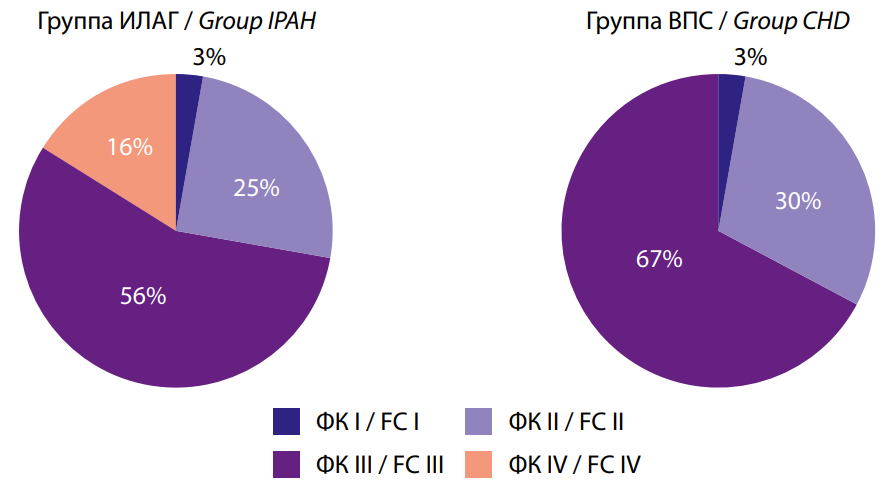
Рис. 1. Функциональный класс ЛАГ (ВОЗ) в исследуемых группах
Fig. 1. PAH functional class (WHO) in the studied groups
Таблица/ Table 1
Скрининговый опросник для ранней диагностики легочной гипертензии
Валиева З.С., Мартынюк Т.В., Чазова И.Е.и соавт., 2014г, [10]
Screening questionnaire for early diagnosis of pulmonary hypertension
Valieva Z.S., Martynyuk T.V., Chazova I.E. et al., 2014, [10]
|
Блок/Chapter |
Признаки/Signs |
Баллы/Scores |
|
1. Клинические симптомы/ Clinical symptoms |
Одышка при физ.нагрузке или в покое/ Shortness of breath during exercise or at rest |
|
|
Повышенная утомляемость/ Increased fatigue |
|
|
|
Сердцебиение/Heartbeat |
|
|
|
Головокружение/Dizziness |
|
|
|
Обмороки/Fainting spells |
|
|
|
Боль в грудной клетке/Chest pain |
|
|
|
Кашель, кровохарканье/ Cough, hemoptysis |
|
|
|
Отеки голеней и стоп/ Swelling of the shins and feet |
|
|
|
2. Анамнез/ Medical history |
Наличие легочной гипертензии у родственников/ The presence of pulmonary hypertension in relatives |
|
|
Случай внезапной смерти у родственников/ The case of sudden death in relatives |
|
|
|
Появление симптомов в период беременности, после родов или абортов/The appearance of symptoms during pregnancy, after childbirth or abortion |
|
|
|
Появление симптомов после ОРВИ или пневмонии/The appearance of symptoms after acute respiratory viral infections or pneumonia |
|
|
|
Появление симптомов после стресса или чрезмерной физ.нагрузки/ The appearance of symptoms after stress or excessive physical activity |
|
|
|
Прием лекарственных препаратов (аноректики, горм.контрацептивы, химиотерапия)/Taking medications (anorexics, hormonal contraceptives, chemotherapy) |
|
|
|
3. Данные физикального осмотра/ Physical examination data |
Акцент (расщепление) II тона над ЛА, шумы, ритм галопа/Accent (splitting) of II tone over pulmonary artery noises, gallop rhythm |
|
|
Цианоз –центральный, периферический/ Cyanosis –central, peripheral |
|
|
|
Расширение и пульсация шейных вен/Expansion and pulsation of the cervical veins |
|
|
|
Асцит, гепатомегалия/Ascites, hepatomegaly |
|
|
|
Периферические отеки/Peripheral edema |
|
|
|
Хрипы в легких/Wheezing in the lungs |
|
|
|
Признаки тромбоза вен нижних конечностей или перенесенная ТЭЛА/Signs of venous thrombosis of the lower extremities or pulmonary embolism |
|
|
|
4. Наличие заболевании, ассоциированных с легочной гипертензией/ The presence of diseases associated with pulmonary hypertension |
Вич-инфекция/HIV infection |
|
|
Портальная гипертензия/Portal hypertension |
|
|
|
Системные заболевания соединительной ткани/Systemic connective tissue diseases |
|
|
|
Саркоидоз/Sarcoidosis |
|
|
|
Заболевания щитовидной железы/Thyroid disease |
|
|
|
Заболевания крови/Blood diseases |
|
|
|
Редкие генетические заболевания/Rare genetic diseases |
|
|
|
5. Данные ЭКГ Данные рентгенографии грудной клетки/ ECG data Chest X-ray data |
Признаки ЛАГ/Signs of PAH |
|
|
Признаки ЛАГ/Signs of PAH |
|
|
|
6. УЗИ сердца/ Echocardiography |
Скорость трикуспидальной регургитации от 2,8 и выше/Tricuspid regurgitation rate from 2.8 and above |
|
|
Систолическое давление в ЛА от 36 мм.рт.ст. и выше/Systolic blood pressure in PA from 36 mmHg and above |
|
|
|
Примечание: каждому из выше указанных симптомов можно присудить по одному баллу. Если пациент набрал: до 5 баллов – диагноз легочная гипертензия отсутствует от 5 и до 10 баллов – есть вероятность легочной гипертензии и необходимо направить к специалисту от 11 и выше баллов - легочная гипертензия высоковероятна и необходимо направить к специалисту/ Note: Each of the above symptoms can be awarded one point. If the patient has typed: up to 5 points – there is no diagnosis of pulmonary hypertension from 5 to 10 points – there is a possibility of pulmonary hypertension and it is necessary to refer to a specialist from 11 and above points - pulmonary hypertension is highly probable and it is necessary to refer to a specialist |
||
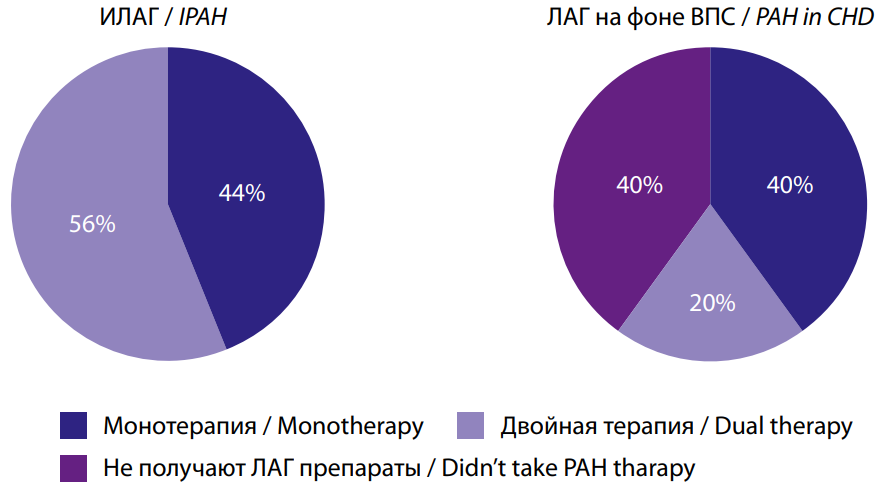
Рис. 2. Наличие ЛАГ-специфической терапия до включения в исследование.
Fig. 2. The presence of PAH-specific therapy before inclusion in the study.

Рис. 3. ЛАГ-специфическая терапия до включения в исследование.
Fig. 3. PAH-specific therapy before inclusion in the study.
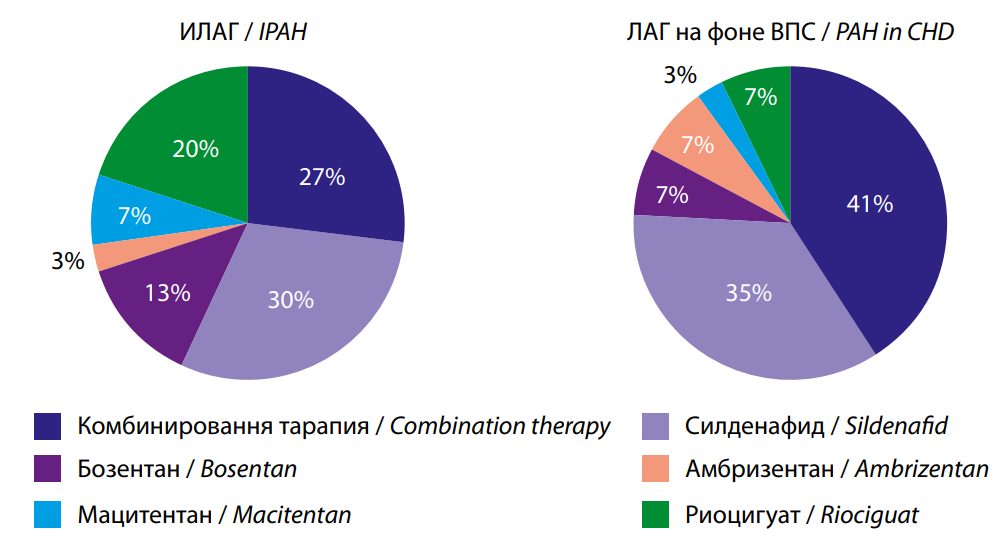
Рис. 4. Эскалация ЛАГ- специфической терапии.
Fig. 4. Escalation of PAH-specific therapy.
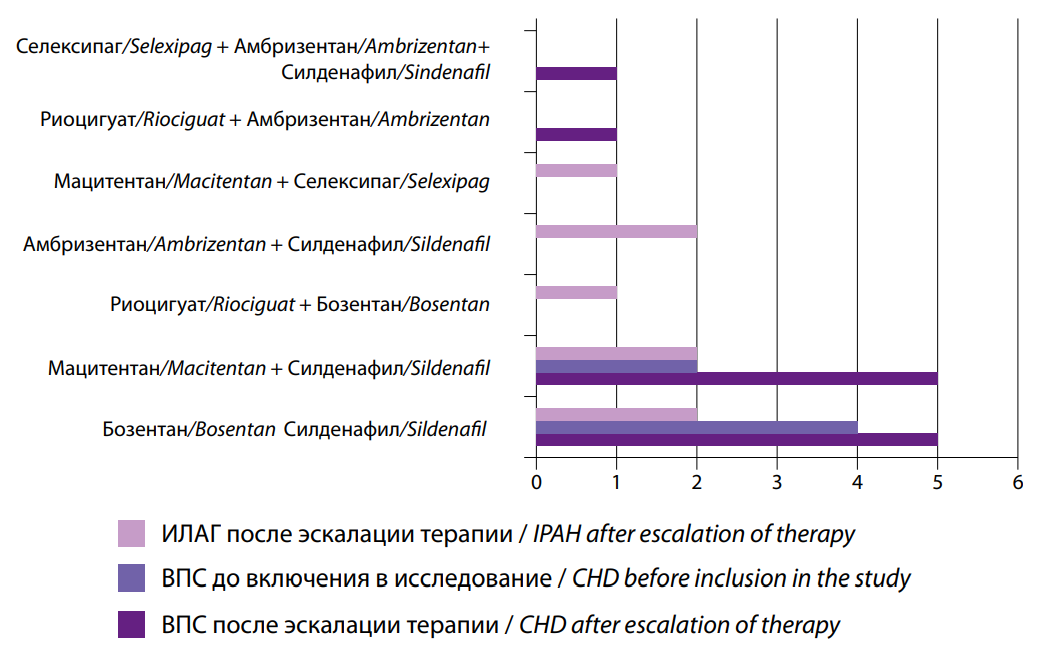
Рис. 5. Комбинированная ЛАГ- специфическая терапия после эскалации.
Fig. 5. Combined PAH-specific therapy after escalation.
Таблица/ Table 2
Динамика теста 6-минутной ходьбы
Dynamics of the 6-minute walk test
|
Группы/Groups
Период наблюдения/ Observation period |
Пациенты с ИЛАГ (1 группа, n=32)/ Patients with IPAH (group 1, n=32) |
Пациенты с ВПС (2 группа, n=30)/ Patients with CHD (Group 2, n=30) |
р |
|
Исходно (м)/ Initial (m) |
255 [ 154,5; 325,0] |
376,5 [ 240,0; 423,0] |
0,022 |
|
Через 1 год наблюдения (м)/ After 1 year of follow-up (m) |
300 [ 200,0; 400,0] |
380,0 [ 300,0; 441,0] |
0,099 |
Примечание: р - достоверность различий
Note: p - the reliability of the differences
Discussion
Pulmonary hypertension is a serious and rapidly progressive disease that can develop in various forms of cardiovascular pathology. In this study, we investigated differences in the course of pulmonary hypertension in patients with IPAH and PAH associated with CHD.
A study of PAH aimed at identifying differences between the idiopathic form and the form associated with CHD emphasizes the importance of in-depth diagnosis and an individualized approach to treatment. The obtained data indicate that patients with IPAH are generally older, have more pronounced symptoms, and require more aggressive therapy compared to patients with PAH associated with CHD. Most likely, patients with CHD, having a longer history of the disease and being monitored by a doctor from early childhood, receive treatment for heart failure for a longer period of time. The presence of a congenital heart defect forces the child to live in a state of moderate hypoxia from birth. These factors seem to explain the significantly greater severity of clinical manifestations of right ventricular failure in patients with IPAH compared to patients with PAH associated with CHD.
However, differences in the pathogenesis and structural-functional state of the right heart chambers and pulmonary circulation vessels became the basis for differences in response to treatment and, as a result, affected patients’ quality of life.
In addition, the study results emphasize the need to use screening questionnaires for early detection of PAH, especially in remote regions where access to specialized care is limited. A simple and accessible method for examining patients with suspected PAH was developed by specialists at the E.I. Chazov National Medical Research Center of Cardiology of the Russian Ministry of Health for early detection and extended examination to verify the diagnosis [8]. Most often, patients seek medical help at an advanced clinical stage of the disease. It takes a long time to diagnose a disease accompanied by such a nonspecific symptom as shortness of breath [3]. It should be noted that if shortness of breath occurs in a young patient, and most often in female patients, the body’s compensatory abilities allow them to ignore their condition and other clinical manifestations for some time. With a clear algorithm for collecting information and a minimum set of instrumental studies to suspect PH, the time from the onset of symptoms to the diagnosis of PH will be significantly shorter. According to the published data, screening questionnaires are one of the tools used to detect PAH in its early stages and are particularly effective in high-risk groups [9, 10].
The study once again demonstrated the effectiveness of the PAH screening questionnaire when the physician has a limited set of diagnostic tools, such as ECG and chest X-ray, but carefully collects data on symptoms, medical history, and course of the disease during a physical examination, even when echocardiography data is not yet available, they can suspect the presence of pulmonary hypertension in the patient and refer them to a specialist at a PAH center. This approach will improve the level of diagnosis and reduce the time from the onset of symptoms to the start of treatment.
The differences identified in the clinical picture and response to treatment between patients with IPAH and CHD are important for practicing physicians, as they can help in more accurate assessment of the condition of patients and selecting the optimal treatment strategy, which in turn can improve the quality of life of patients with PAH.
Conclusions:
- Patients with IPAH had more frequently such symptoms as chest pain and lower limb edema, including anasarca, and were more likely to be classified as having functional class III–IV pulmonary hypertension compared to patients with CHD.
- Earlier detection of pulmonary hypertension in patients with IPAH compared to patients with CHD resulted in a shorter time from symptom onset to the start of IPAH-specific treatment.
- The effectiveness of the PAH screening questionnaire was clearly demonstrated. It can be actively used by primary care physicians in areas remote from PAH centers, where the only available diagnostic tools are ECG and chest X-ray.
- In most cases, patients in the IPAH group required escalation of therapy.
- Patients with PAH associated with CHD were less likely to require hospitalization and emergency medical services. They were also less likely to seek medical attention at their place of residence than patients with IPAH, which is probably due to the less severe clinical manifestations of pulmonary hypertension, a long history of the disease, and better adaptation to hypoxia.
References
1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. https://doi.org/10.1183/13993003.01913-2018
2. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618- 3731. Erratum in: Eur Heart J. 2023;44(15):1312. https://doi.org/10.1093/eurheartj/ehac237.
3. Chazova IY, Martynyuk TV, Valieva ZS, Gratsianskaya SY, Aleevskaya AM, et al. Clinical and Instrumental Characteristics of Newly Diagnosed Patients with Various Forms of Pulmonary Hypertension according to the Russian National Registry. Biomed Res Int. 2020;2020:6836973. https://doi.org/10.1155/2020/6836973
4. Chazova I.E., Martynyuk T.V., Shmalts A.A., Gramovich V.V., Danilov N.M., et al. Eurasian guidelines for the diagnosis and treatment of pulmonary hypertension (2023). Eurasian heart journal. 2024;(1):6-85. (In Russ.) https://doi.org/10.38109/2225-1685-2024-1-6-85
5. Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):2045894020977300. https://doi.org/10.1177/2045894020977300
6. Pektas A, Pektas BM, Kula S. An epidemiological study of paediatric pulmonary hypertension in Turkey. Cardiol Young. 2016;26(4):693-697. https://doi.org/10.1017/S1047951115001043
7. Chang KY, Duval S, Badesch DB, Bull TM, Chakinala MM, et al. Mortality in Pulmonary Arterial Hypertension in the Modern Era: Early Insights From the Pulmonary Hypertension Association Registry. J Am Heart Assoc. 2022;11(9):e024969. https://doi.org/10.1161/JAHA.121.024969
8. Valieva Z.S., Valeeva E.G., Glukhova S.I., Martynyuk T.V., Chazova I.E. The development of a screening questionnaire to improve the early detection of pulmonary arterial hypertension. Systemic Hypertension. 2014;11(4):62-67. eLIBRARY ID: 22808067 EDN: TFCVLL
9. Valieva Z.S., Glukhova S.I., Martyniuk T.V., Chazova I.E. The validation of the questionnaire for the early detection of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Systemic Hypertension. 2016;13(1):34-38. (In Russ.) eLIBRARY ID: 26001737 EDN: VWZXCB
10. McGoon M, Gutterman D, Steen V, Barst R, McCrory DC, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S. https://doi.org/10.1378/chest.126.1_suppl.14S
11.
About the Authors
E. A. DevetyarovaRussian Federation
Elena A. Devetyarova, Department of Internal Medicine No. 1
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. I. Chesnikova
Russian Federation
Anna I. Chesnikova, Dr. Sci. (Med.), Professor, Head of the Department of Internal Medicine No. 1
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. A. Dyuzhikov
Russian Federation
Alexander. A. Dyuzhikov, Dr. Sci. (Med.), Professor
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Devetyarova E.A., Chesnikova A.I., Dyuzhikov A.A. Idiopathic pulmonary arterial hypertension and congenital heart defects: similarities and differences. Medical Herald of the South of Russia. 2025;16(1):46-54. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-46-54







































