Scroll to:
Association of rs622342 SLC22A1 with the short-term changes of lipid and carbohydrate metabolism indicators in different variants of early carbohydrate metabolism disorders management in women
https://doi.org/10.21886/2219-8075-2025-16-1-39-45
Abstract
Objective: to analyze the association of rs622342 SLC22A1 with the short-term changes in fat and carbohydrate metabolism parameters in various types of early carbohydrate metabolism disorders therapy. Materials and methods: the results of management of 89 patients with excess body weight and risk factors for type 2 diabetes mellitus development were analyzed. For 3 months, 53 patients followed diet therapy, 36 patients took metformin in addition to the diet. All of the patients were genotyped for rs622342 SLC22A1. Also initially and 3 months after the beginning of therapy fasting plasma glucose, glycosylated hemoglobin, total cholesterol, low-density and high-density lipoproteins, tryglicerides were measured. Statistical processing was carried out using parametric and non-parametric criteria. Results: 3 months after the start of diet therapy, homozygous СС-carriers of the rs622342 SLC22A1 showed a significant decrease in total cholesterol and triglycerides compared with A-allele carriers. These changes were not observed when metformin was added to diet therapy. There were no significant differences in changes of fasting plasma glucose and glycated hemoglobin levels in groups with different management. Conclusions: carriers of CC rs622342 SLC22A1 are characterized with the significant decrease in total cholesterol and triglycerides levels compared to the A-allele carriers after 3 months of standard diet therapy; when metformin is added to the treatment regimen, these changes are not observed.
For citations:
Valeeva F.V., Medvedeva M.S., Kiseleva T.A., Khasanova K.B., Nabiullina R.M. Association of rs622342 SLC22A1 with the short-term changes of lipid and carbohydrate metabolism indicators in different variants of early carbohydrate metabolism disorders management in women. Medical Herald of the South of Russia. 2025;16(1):39-45. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-39-45
Introduction
Diabetes mellitus (DM) is one of the four priority non-infectious diseases that represent a serious medical and social problem in all countries of the world [1]. According to the results of the epidemiological study NATION, 19.2% of the Russian Federation population has preceding DM prediabetes, diagnosed by the HbA1C level [2]; however, when using the criterion of lean glycemia, the number of patients with early carbohydrate metabolism disorders (CMD) can reach 28.1% of the Russia population [3]. It is known that preventive measures already at the stage of prediabetes can prevent or slow down the development of type 2 DM, as well as its micro- and macrovascular complications [4]. These measures, in addition to normalizing the level of glycemia, should be aimed at reducing body weight and normalizing blood pressure and lipid metabolism. According to the “Algorithms of Specialized Medical Care for Patients with Diabetes Mellitus” of the 11th revision (2023), upon early detection of CMD, in addition to educating patients on the principles of nutritional therapy, metformin can be prescribed [5]. In addition to the sugar-lowering effect, this drug has a number of pleiotropic effects, such as cardioprotection, neuro- and oncoprotective effects, as well as the normalization of lipid metabolism due to the suppression of the production and activity of SREBP-1 protein and apolipoprotein V-48, which regulate fatty acid metabolism [6]. However, the therapeutic response to metformin is variable, which can be due to the genetic characteristics of the patient [7].
One of the genes that can modulate response to metformin therapy is SLC22A1. This gene encodes a cation transporter type 1 (organic cation transporter type 1, OCT1), which transports metformin from the bloodstream into liver cells [8]. Currently, more than 34 polymorphisms of this gene are known [9]; however, the greatest interest for this study is the polymorphic marker rs622342 SLC22A1.
The purpose of the work was to study the relationship of the rs622342 SLC22A1 polymorphism with short-term changes in fat and carbohydrate metabolism in various therapeutic options for early CMD.
Materials and methods
Overweight or obese women (BMI ≥ 25 kg/m2), having also other risk factors for CMD (family history, type 2 DM, impaired fasting glycemia or history of impaired glucose tolerance, high-density lipoprotein ≤ 0.9 mmol/L and/or history of triglycerides ≥ 2.82 mmol/L, gestational DM or history of large fetal birth, cardiovascular disease, polycystic ovary syndrome) were included in the study. Residence in the Republic of Tatarstan for at least three generations, established by a questionnaire, was an important condition for inclusion in the study. The study protocol was approved by the Local Ethics Committee of FSBEI HE Kazan State Medical University of the Ministry of Health of the Russian Federation (meeting minutes No. 10 of December 18, 2018). The objectives and methods of the study were explained to all patients, on the basis of which each patient signed a voluntary informed consent to participate in the study. At the beginning of the study, all patients underwent venous blood sampling to determine the levels of fasting glucose, total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), and triglycerides (TG) by the enzymatic colorimetric method (Olvex Diagnosticum reagent kit, St. Petersburg; biochemical analyzer Stat Fax 4500, USA). Glycated hemoglobin (HbA1C) was also determined by the express method (Abbott NycoCard Reader II analyzer, USA). Additionally, to detect CMD, all patients underwent an oral glucose tolerance test (OGTT) with a standard load of 75 g of anhydrous glucose dissolved in 300 ml of water. The rs622342 SLC22A1 polymorphism was determined using real-time polymerase chain reaction (PCR) (Synthol reagent kit, Moscow; amplifier CFX96, USA). DNA obtained from whole blood lymphocytes of patients by the sorbent method (DNA-sorb-B reagent kit; LLC ILS, Moscow) was the substrate for the reaction. Patients were randomized into two therapeutic groups. Participants in the first group followed the nutritional diet generally accepted at CMD, which implies the exclusion of simple carbohydrates from the diet and the restriction of the use of complex carbohydrates and fats. Participants in the second group took metformin at a therapeutic dose in addition to compliance with dietary therapy. Compliance with the recommendations was monitored by face-to-face meetings once every two weeks, with checking the diet diary. After three months, the lipid spectrum, the level of fasting glucose, and HbA1C were evaluated again in all patients. Statistical data processing was carried out using the IBM SPSS Statistics 26.0 and Microsoft Excel 2016 programs. The χ2 test was used to assess the correspondence of the genotype distribution to the Hardy-Weinberg equilibrium. When studying the relationship of rs622342 SLC22A1 with changes in the lipid profile, a recessive inheritance model was taken into account, namely, the indicators of groups of patients with the absence and presence of allele A were compared. The compliance of the studied samples with the normal distribution was previously checked using the Shapiro-Wilk test. If the distribution was different from normal, then the Mann-Whitney U test was used when comparing the groups. Statistical data are presented as M ± σ in the case of normal distribution and as Me [Q25; Q75] if the data were not subject to normal distribution. The results were considered statistically significant at p ≤ 0.05.
Results
A total of 118 women were included in the study, but 29 patients dropped out of follow-up due to non-compliance with recommended therapy. In total, the therapy results of 89 patients aged 25 to 65 years (average age 47 ± 14 years) with excess body weight or obesity (average BMI before the study 33.67 ± 5.81 kg/m2) were analyzed. The “diet therapy” group included 53 patients; 36 patients took metformin in addition to following the diet. Although the prevalence of genotypes and allele A rs622342 SLC22A1 in the studied groups was comparable (Table 1), in the “diet therapy” group, the prevalence of genotypes and allele A did not comply with the Hardy-Weinberg law of genetic equilibrium (χ2 = 4.39; p = 0.04), which can be explained by the higher occurrence of the risk allele C in the sampling. In the group of “diet therapy and metformin”, the prevalence of genotypes and allele A corresponded to the Hardy-Weinberg law (χ2 = 0.57; p = 0.44).
The compared groups were comparable in terms of the CMD distribution, as well as the severity of body weight excess (Table 2.3).
In the absence of accounting for the method of treatment, the association of the A allele rs622342 SLC22A1 carrier with changes in the biochemical blood parameters of the patients was not revealed (Table 4).
In the “diet therapy” group, among homozygous carriers of allele C compared with carriers of allele A rs622342 SLC22A1, a more pronounced decrease in total cholesterol and triglycerides was observed three months after the follow-up start (Figs. 1, 2), while there were no differences in the decrease in HDL and LDL levels. There were no significant differences in the dynamics of glycated hemoglobin and fasting glucose levels (Table 5).
In the “diet therapy and metformin” group, no significant differences were found in the dynamics of fat and carbohydrate metabolism indicators among carriers of the A allele and CC genotype (Table 6).
Таблица / Table 1
Распространённость генотипов, аллеля А rs622342 SLC22A1 в исследуемых группах
Prevalence of genotypes and A-allele of rs622342 SLC22A1 in the study groups
|
|
Генотип АА (%) |
Генотип АС (%) |
Генотип СС (%) |
Аллель А A-allele (%) |
|
Все пациенты |
56 |
31 |
13 |
72 |
|
Группа «диетотерапии» |
58 |
28 |
14 |
73 |
|
Группа «диетотерапии и метформина» |
52 |
36 |
12 |
21 |
Таблица / Table 2
Распределение наличия избытка массы тела и ожирения в исследуемых группах (%)
Distribution of overweight and obesity in the study groups (%)
|
|
Группа «диетотерапии» (n=53) |
Группа «диетотерапии и метформина» "Diet therapy and metformin" group (n=36) |
||
|
Генотип АА+АС (n=45) |
Генотип СС (n=8) |
Генотип АА+АС (n=32) |
Генотип СС Genotype CC (n=4) |
|
|
Избыток массы тела |
40,0 |
37,5 |
28,1 |
50,0 |
|
Ожирение 1 ст. |
35,5 |
25 |
31,2 |
25,0 |
|
Ожирение 2 ст. |
20,0 |
25 |
25,0 |
25,0 |
|
Ожирение 3 ст. |
4,5 |
12,5 |
15,7 |
0,0 |
Таблица / Table 3
Распределение НУО в исследуемых группах (%)
Distribution of early metabolism disorders in the study groups (%)
|
|
Группа «диетотерапии» (n=53) |
Группа «диетотерапии и метформина» "Diet therapy and metformin" group (n=36) |
||
|
Генотип АА+АС (n=45) |
Генотип СС (n=8) |
Генотип АА+АС (n=32) |
Генотип СС Genotype CC (n=4) |
|
|
Норма |
82,2 |
62,5 |
12,5 |
25,0 |
|
Нарушение гликемии натощак |
6,7 |
12,5 |
6,2 |
0,0 |
|
Нарушение толерантности к глюкозе |
8,8 |
12,5 |
37,5 |
25,0 |
|
Впервые выявленный СД2 |
2,3 |
12,5 |
43,8 |
50,0 |
Таблица / Table 4
Взаимосвязь аллеля А rs622342 SLC22A1 с изменениями биохимических показателей крови пациентов без учета метода выбранной терапии
Association of A-allele of rs622342 SLC22A1 with changes in biochemical blood parameters of patients without taking into account the method of chosen therapy
|
Показатель |
Изменение показателей (в % от первого измерения) |
Значение |
|
|
Генотип АА+АС |
Генотип СС (n=11) |
||
|
HbA1C |
-1,87 [-5,88; 3,44] |
-0,29 [-5,76; 5,17] |
0,825 |
|
Глюкоза |
-6,01 [-16,29; 13,55] |
0,02 [-27,12; 12,48] |
0,890 |
|
Общий холестерин |
-2,01 [-19,71; 17,64] |
-9,21 [-36,24; 19,75] |
0,551 |
|
ЛПВП |
7,05 [-21,39; 77,16] |
16,18 [-7,09; 65,11] |
0,634 |
|
ЛПНП |
-9,09 [-34,85; 42,11] |
-41,64 [-49,32; 13,57] |
0,237 |
|
ТГ |
-7,16 [-24,35; 34,86] |
-60,46 [-61,01; -44,68] |
0,131 |
Таблица / Table 5
Взаимосвязь аллеля А rs622342 SLC22A1 с изменениями биохимических показателей крови пациентов в группе «диетотерапии»
Association of A-allele of rs622342 SLC22A1 with changes in biochemical blood parameters of patients in the «diet therapy» group
|
Показатель |
Изменение показателей (в % от первого измерения) Change in indicators (in % from the first measurement) |
Значение |
|
|
Генотип АА+АС |
Генотип СС (n=8) |
||
|
HbA1C |
-0,89 [-4,35; 5,31] |
-3,59 [-10,15; 5,16] |
0,321 |
|
Глюкоза |
-2,11 [-14,81; 16,27] |
-17,06 [-27,41; 1,41] |
0,341 |
|
Общий холестерин |
0,93 [-19,71; 25,14] |
-46,69 [-57,15; -36,24] |
0,036 |
|
ЛПВП |
2,87 [-20,48; 77,68] |
-7,09 [-9,75; 11,41] |
0,739 |
|
ЛПНП |
-15,09 [-36,84; 32,97] |
-47,05 [-48,64; 36,14] |
0,820 |
|
ТГ |
-5,54 [-17,37; 43,41] |
-73,71 [-86,95; -60,46] |
0,038 |
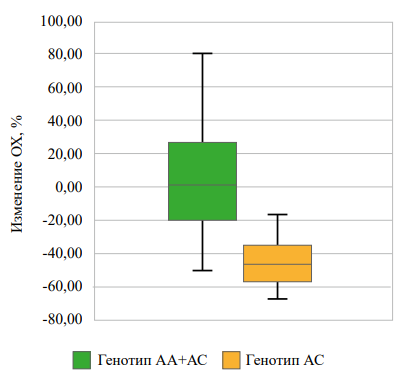
Рисунок 1. Взаимосвязь rs622342 SLC22A1 с изменением уровня ОХ через 3 месяца наблюдений в группе «диетотерапии».
Figure 1. Association of rs622342 SLC22A1 with changes in total cholesterol level after 3 months of observation in the «diet therapy» group.
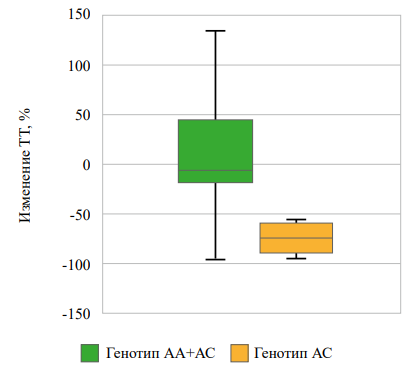
Рисунок 2. Взаимосвязь rs622342 SLC22A1 с изменением уровня ТГ через 3 месяца наблюдений в группе «диетотерапии».
Figure 2. Association of rs622342 SLC22A1 with changes in triglycerides level after 3 months of observation in the «diet therapy» group.
Таблица / Table 6
Взаимосвязь аллеля А rs622342 SLC22A1 с изменениями биохимических показателей крови пациентов в группе «диетотерапии и метформина»
Association of A-allele of rs622342 SLC22A1 with changes in biochemical blood parameters of patients in the «diet therapy and metformin» group
|
Показатель |
Изменение показателей (в % от первого измерения) Change in indicators (in % from the first measurement) |
Значение |
|
|
Генотип АА+АС |
Генотип СС (n=4) |
||
|
HbA1C |
-2,42 [-6,89; -2,39] |
|
0,108 |
|
Глюкоза |
-11,57 [-17,16; 9,15] |
8,53 [-18,59; 31,52] |
0,440 |
|
Общий холестерин |
-4,78 [-21,32; 4,57] |
9,88 [-9,21; 25,22] |
0,289 |
|
ЛПВП |
8,45 [-22,14; 74,24] |
65,11 [ 33,76; 118,63] |
0,353 |
|
ЛПНП |
12,82 [-32,86; 17,77] |
-36,23 [-56,91; -23,11] |
0,128 |
|
ТГ |
-12,63 [-51,08; 19,71] |
-44,68 [-52,84; 24,62] |
0,896 |
Discussion
During the analysis of literature data, we identified several opposing opinions of various groups of authors about the relationship of rs622342 SLC22A1 with changes in blood biochemical parameters. In the course of work conducted among representatives of the Mexican population by Res’endiz-Abarca et al., a significant decrease was revealed in the level of HbA1C among carriers of allele A relative to CC homozygotes rs622342 SLC22A1 at three and six months from the follow-up start [10]. In the study of Tkáč et al., conducted on the European population, rs622342 SLC22A1 was not associated with the dynamics of carbohydrate metabolism indicators [11]. In our study, conducted on the indigenous population of the Republic of Tatarstan, in the genetic composition of which the Caucasian component predominates [12], we also did not find an association of the polymorphic marker rs622342 SLC22A1 with changes in carbohydrate metabolism indicators.
The association of rs622342 SLC22A1 with changes in lipid fractions remains poorly understood. During the analysis of the literature, we found only one study, in which this issue was covered. In the course of a study conducted among the Indian population, there was no association of this polymorphism with changes in the levels of TC, TG, LDL, and HDL three months after the beginning of metformin administration [13], which is consistent with our results. At the same time, we found a significant decrease in the levels of TC and TG among homozygous carriers of CC who did not take metformin, which can level out the lipid-lowering effect of diet therapy when prescribing metformin in this category of patients. Further sample enlargement, in particular by including men in the study, will allow determining how applicable the results are for the European population as a whole.
Conclusions
- Among carriers of the rs622342 SLC22A1 CC genotype, there was a significant decrease in the level of total cholesterol and glycerides compared to carriers of the A allele after three months of generally accepted diet therapy for disorders of carbohydrate metabolism.
- There were no changes in the lipidogram between carriers of different variants of rs622342 SLC22A1 when metformin was added to a non-drug treatment regimen of early CMD.
References
1. Global report on diabetes. Geneva: World Health Organization 2018. License: CC BY-NC-SA 3.0 IGO.
2. Dedov I.I., Shestakova M.V., Galstyan G.R. Prevalence of type 2 diabetes mellitus in the adult population of Russia (NATION study). Diabetes mellitus. 2016;19(2):104-112. DOI: 10.14341/DM2004116-17
3. Barbarash O.L., Voyevoda M.I., Galstyan G.R., Shestakova M.V., Boytsov S.A., Aleksandrova O.Yu., Bryzgalina S.M., Druk I.V., Karetnikova V.N., Kashtalap V.V., Kvitkova L.V., Korennova O.Yu., Ogarkov M.Yu., Plotnikova E.Yu., Tsygankova O.V., Svarovskaya A.V., Chumakova G.A. Pre-diabetes as an interdisciplinary problem: definition, risks, approaches to the diagnostics and prevention of type 2 diabetes and cardiovascular complications. Russian Journal of Cardiology. 2019;(4):83-91. (In Russ.) DOI: 10.15829/1560-4071-2019-4-83-91
4. Grineva E.N., Mazurov V.I., Khalimov Yu.Sh., Bakulin I.G., Panov A.V., Tyrenko V.V., Novikova I.A., Babenko A.Yu., Karonova T.L., Banshikov G.T., Leboeva M.M., Lole O.Yu., Ryabova N.Yu., Toinov A.A., Semko A.A., Sheinskaya I.M. Draft resolution of the expert council of the сhief specialists of the North-West Federal District on the identification of prediabetes and the prevention of type 2 diabetes mellitus and related diseases of cardiovascular system, liver, musculoskeletal system. "Arterial’naya Gipertenziya" ("Arterial Hypertension"). 2019;25(6):693-699. (In Russ.) DOI: 10.18705/1607-419X-2019-25-6-693-699
5. Dedov I., Shestakova M., Mayorov A., Mokrysheva N., Andreeva E., Bezlepkina O., Peterkova V., Artemova E., Bardiugov P., Beshlieva D., Bondarenko O., Burumkulova F., Vikulova O., Volevodz N., Galstyan G., Gomova I., Grigoryan O., Dzhemilova Z., Ibragimova L., Kalashnikov V., Kononenko I., Kuraeva T., Laptev D., Lipatov D., Melnikova O., Mikhina M., Michurova M., Motovilin O., Nikonova T., Rozhivanov R., Smirnova O., Starostina E., Surkova E., Sukhareva O., Tiselko A., Tokmakova A., Shamkhalova M., Shestakova E., Jarek-Martynowa I., Yaroslavceva M. Standards of Specialized Diabetes Care / Edited by Dedov I.I., Shestakova M.V., Mayorov A.Yu. 11th Edition. Diabetes mellitus. 2023;26(2S):1-157. (In Russ.) DOI: 10.14341/DM13042
6. Teplova A.S., Titova V.V., Tenchurina A.I. Biochemical bases of the organoprotective properties of metformin. FOCUS. Endocrinology. 2024;5(1):59-64. (In Russ.) DOI: 10.62751/2713-0177-2024-5-1-08
7. Pozdnyakov N.O., Kagarmanyan I.N., Miroshnikov A.E., Emelyanov E.S., Gruzdeva A.A., Sirotkina A.M., Dukhanina I.A.,
8. Milkina A.A., Khokhlov A.A., Pozdnyakov S.O. Pharmacogenetic aspects oftype 2 diabetes treatment. Acta biomedica scientifica. 2020;5(3):13-23. DOI: 10.29413/ABS.2020-5.3.2.
9. Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98. DOI: 10.2165/11534750-000000000-00000
10. Mato EPM, Guewo-Fokeng M, Essop MF, Owira PMO. Genetic polymorphisms of organic cation transporter 1 (OCT1) and responses to metformin therapy in individuals with type 2 diabetes. Medicine (United States). 2018;97(27). DOI: 10.1097/MD.0000000000011349
11. Reséndiz-Abarca CA, Flores-Alfaro E, Suárez-Sánchez F, Cruz M, Valladares-Salgado A, del Carmen Alarcón-Romero L, et al. Altered Glycemic Control Associated With Polymorphisms in the SLC22A1 (OCT1) Gene in a Mexican Population With Type 2 Diabetes Mellitus Treated With Metformin: A Cohort Study. J Clin Pharmacol. 2019;59(10):1384–90. DOI: 10.1002/jcph.1425
12. Tkáč I, Klimčáková L, Javorský M, Fabianová M, Schroner Z, Hermanová H, et al. Pharmacogenomic association between a variant in SLC47A1 gene and therapeutic response to metformin in type 2 diabetes. Diabetes Obes Metab. 2013;15(2):189–91. DOI: 10.1111/j.1463-1326.2012.01691.x
13. Kravtsova, O. A. (2007). Structure of the nuclear gene pool of the Volga Tatars (according to autosomal microsatellite loci). Scientific notes of Kazan University. Natural Science Series, 149(2), 138-147.
14. Umamaheswaran G, Praveen RG, Damodaran SE, Das AK, Adithan C. Influence of SLC22A1 rs622342 genetic polymorphism on metformin response in South Indian type 2 diabetes mellitus patients. Clin Exp Med. 2015;15(4):511–7. DOI: 10.1007/s10238-014-0322-5
15.
About the Authors
F. V. ValeevaРоссия
Farida V. Valeeva, Dr. Sci. (Med.), Professor, Head of Endocrinology Department
Kazan
Competing Interests:
Authors declare no conflict of interest
M. S. Medvedeva
Россия
Maria S. Medvedeva, endocrinologist
Kazan
Competing Interests:
Authors declare no conflict of interest
T. A. Kiseleva
Россия
Tatyana A. Kiseleva, Cand. Sci. (Med.), Associate Professor of Endocrinology Department
Kazan
Competing Interests:
Authors declare no conflict of interest
K. B. Khasanova
Россия
Kamilya B. Khasanova, Cand. Sci. (Med.), Assistant of Endocrinology Department
Kazan
Competing Interests:
Authors declare no conflict of interest
R. M. Nabiullina
Россия
Roza M. Nabiullina, Cand. Sci. (Med.), Associate Professor of Biochemistry and Clinical Laboratory Diagnostics Department
Kazan
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Valeeva F.V., Medvedeva M.S., Kiseleva T.A., Khasanova K.B., Nabiullina R.M. Association of rs622342 SLC22A1 with the short-term changes of lipid and carbohydrate metabolism indicators in different variants of early carbohydrate metabolism disorders management in women. Medical Herald of the South of Russia. 2025;16(1):39-45. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-39-45
JATS XML







































