Scroll to:
Pulse wave propagation rate and dynamics of matrix metalloproteinase in patients with myocardial infarction and arterial hypertension in the presence or absence of diabetes mellitus
https://doi.org/10.21886/2219-8075-2025-16-1-55-61
Abstract
Objective: to study the pulse wave propagation rate and the level of matrix metalloproteinase type 9 in patients with ST-segment elevation myocardial infarction who underwent percutaneous coronary intervention in the presence of hypertension and diabetes mellitus. Materials and methods: the study included 136 patients of both sexes. The first group consisted of 69 patients with STEMI+AH, the second group included 67 patients with acute STEMI+AH+DM2. The patients underwent analysis of the level of matrix metalloproteinase type 9, analysis of pulse wave propagation velocity, Statistical processing of the study results was carried out using Excel spreadsheets and the Statistica 10 statistical software package (StatSoftInc.). Results: at the stationary stage, the level of MMP-9 in the first group ranged from 28 ng/ml to 1340 ng/ml with a median of 297 ng/ml. The level of this indicator above 600 ng/ml is interpreted by statistical analysis as emissions in this distribution. In the second group of patients with DM2, a similar pattern was observed in the distribution of the indicator, but the median value of the indicator was at the level of 387 ng/ml and the observed spread of the indicator was 28–1560 ng/ml statistically significantly higher compared with the first group (p<0.001). The values of MMP-9 are characterized in both the first and second groups by a direct correlation with the level of HbA1c. The analysis of pulse wave propagation velocity revealed a statistically significant difference in pulse wave propagation velocity in muscle-type vessels and the CM ratio/SE between patients of the compared groups. Pulse wave propagation velocity for vessels of the classical type was also statistically significantly higher in the group of patients with diabetes mellitus. Conclusions: the determination of MMP9 and pulse wave propagation velocity levels may become an important method for determining the prognosis in patients with DM2 who have undergone STEMI. The analysis of pulse wave propagation velocity revealed a statistically significant difference in pulse wave propagation velocity in muscle-type vessels and the CM ratio/SE between patients of the compared groups. pulse wave propagation velocity for vessels of the classical type was also statistically significantly higher in the group of patients with diabetes mellitus.
Keywords
For citations:
Suroedov V.A., Pirozhenko A.A., Khaisheva L.A., Khaishev K.A. Pulse wave propagation rate and dynamics of matrix metalloproteinase in patients with myocardial infarction and arterial hypertension in the presence or absence of diabetes mellitus. Medical Herald of the South of Russia. 2025;16(1):55-61. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-55-61
The high prevalence of cardiovascular diseases (CVD) worldwide is prompting the scientific community to seek new solutions that enable the early detection of pathological processes and provide more accurate diagnoses based on multifactorial assessment [1].
PWV (pulse wave velocity) is considered one of the most important clinical parameters for assessing the risk of cardiovascular disease, vascular adaptation, and therapeutic efficacy [2]. Studies devoted to identifying the relationship between PWV measurement and pathological status in various diseases proved the relevance of this parameter [3].
It should be noted that in patients with arterial hypertension (AH), an increase in PWV above 12 m/s is recognized as an independent marker of risk for an unfavorable prognosis, and this indicator is already recommended in the comprehensive diagnosis of hypertension [1]. Increased aortic stiffness, measured by PWV in the aorta, is an independent predictor of mortality in patients with diabetes mellitus.
The Complior study demonstrated that PWV values (in the carotid-femoral segment or in the aortic trunk) increased with age regardless of the patient’s gender [4]. An analysis of the determinants of PWV in the carotid-femoral segment of the vasculature in 2,000 untreated patients with AH from 19 countries revealed that age was the second main determinant influencing PWV (after systolic blood pressure). With age, there is a progressive increase in PWV: from 5.1 m/s in young children, 6.3 m/s at age 22, to 9.6 m/s by age 65 [5]. The study also revealed an increase in PWV in patients with obesity and T2DM, independent of age, gender, and blood pressure levels. In addition, a decrease in body mass index (BMI) is associated with a reversal of arterial stiffness.
Woolam et al. described PWV in the carotid-radial segment in patients with T2DM undergoing oral hypoglycemic therapy or insulin therapy compared to healthy individuals. A significantly higher PWV was recorded in patients with T2DM after adjusting for age. It was noteworthy that a higher PWV was recorded even in very young patients with T2DM [6].
According to data from the international registry “VASOTENS” (Vascular Health Assessment Of The Hypertensive), there is a direct correlation between increased arterial stiffness and the presence of coronary heart disease (CHD) [7]. An increase in aortic PWV was found in patients with CHD in all age groups (≥ 40 years), where the average PWV values in patients with CHD differed from those in patients without CHD by 1.68 m/s.
In addition to the use of instrumental diagnostic methods, the identification of new markers of cardiovascular damage, such as MMP 9, has become widespread in recent years [8].
Matrix metalloproteinases (MMPs) are extracellular enzymes that play an important role in many physiological and pathological processes. Their activity is mainly regulated by tissue inhibitors of metalloproteinases (TIMPs). The expression of MMPs is associated with classic risk factors for CVD, as well as with inflammation. They play a central role in the development of atherosclerosis, plaque formation, platelet aggregation, acute coronary syndrome, restenosis, aortic aneurysms, and peripheral vascular disease [9].
The aim of the study was to investigate the PWV and matrix MMP9 levels in patients with STEMI who underwent percutaneous coronary intervention (PCI) in the presence of AH and DM2.
Materials and Methods
The total sampling consisted of 136 patients. The median age was 60 [ 54; 66] years. The study included a total of 74 men (67.5%), with a median age of 60 [ 54; 65] years, and 62 (32.5%) women, with a median age of 60 [ 54; 67] years.
Patients were divided into two groups in accordance with the study aims and tasks. The first group consisted of 69 patients with acute STEMI+AH, with a median age of 61 [ 55; 66] years. The first group included 47 men (68.1%), with a median age of 60 [ 54; 66] years, and 22 (31.9%) women, with a median age of 61 [ 59; 69] years.
The second group included 67 patients with acute STEMI+AH+T2DM, whose median age was 61 [ 55; 66] years. In this group, there were 51 men (76.1%) with a median age of 61 [ 56; 65] years and 16 women (23.9%) with a median age of 60 [ 54; 69] years.
Criteria for inclusion in the study:
- written informed consent signed by the patient to participate in the study;
- patients of both sexes over the age of 18 with a diagnosis of STEMI verified on the basis of the recommendations for the management of patients with STEMI (2020), who have undergone primary PCI with restoration of blood flow in the syndrome-related artery and implantation of no more than two stents;
- patients of both sexes over the age of 18 with previously diagnosed AH, diagnosed based on clinical guidelines for the treatment of adult patients with AH (2020);
- patients of both sexes over the age of 18 with previously diagnosed T2DM (Dedov, 2020). Elevated glucose levels were defined as fasting venous blood glucose levels above 6.1 mmol/L and HbA1c levels above or equal to 6.5%.
Criteria for exclusion from the study:
- BP above 180/100 mmHg, symptomatic forms of AH;
- chronic heart failure, NYHA FC IV;
- hemodynamically significant arrhythmias (atrial fibrillation, atrial flutter, II and III degree AV block);
- severe kidney and liver diseases;
- chronic obstructive and interstitial lung diseases;
- chronic heart failure classified by NYHA as FC III–IV with an ejection fraction of less than 40%;
- chronic diseases of internal organs in the stage of subcompensation or decompensation and/or during exacerbation;
- cancer;
- mental disorders;
- occlusive diseases of the limb arteries;
- psychic illness and incapacity.
Serum was used as the material for the evaluation of MMP9 levels. The level of MMP9 was determined using a standard test kit (Cloud-Clone Corp., China) in accordance with the manufacturer’s instructions. The intensity of the solution color was measured as optical density on an automatic vertical scanning photometer (Multiskan FC 1/00/79) at a wavelength of 450 nm. A calibration curve was constructed, which was used to find the desired MMP-9 levels in the serum samples.
The PWV study was conducted using the Poly-Spectrum-10 sphygmographic attachment (Neurossoft LLC, Ivanovo). Three sphygmograms (carotid, radial, and femoral arteries) and one ECG lead were recorded and analyzed simultaneously. PWV analysis was performed for muscular type arteries (MT) [m/s], arteries of elastic type (ET) [m/s], and the ratio of PWV for MT arteries to PWV for ET arteries (MT/ET)
Statistical processing of the research results was performed using Excel spreadsheets and the Statistica 10 software package (StatSoft Inc.). The nonparametric Kruskal-Wallis test was used, and pairwise comparisons were performed using the median rank comparison method. Related groups were compared using Wilcoxon’s test. Numerical data are presented as the median, first, and third quartiles Me (Q1; Q3). In pairwise comparisons of groups, the significance of differences between groups was adjusted using the Holm-Bonferroni adjustment for multiple comparisons. The threshold level of significance was p<0.05.
Results
The main clinical data of the patients included in the study are presented in Table 1. All patients included in the study, regardless of their group affiliation, were comparable in terms of age. The BMI of all patients with T2DM included in the study ranged from 23.0 to 47 kg/m2, with a median BMI of 29.0 kg/m2, which was not significantly different (p=0.35) compared to patients without T2DM. BMI ranged from 16.0 to 43.3 kg/m2, with a median of 28.0 kg/m2. Patients with T2DM had a significantly lower EPI glomerular filtration rate (p<0.001) and a significantly higher HbA1C levels (p<0.001). Troponin I in patients with STEMI and T2DM was also significantly higher (p<0.001) than in patients with STEMI without T2DM.
At the hospital stage, the range of MMP-9 values in the first group was from 28 to 1340 ng/ml, with a median value of 297 ng/ml. A value above 600 was interpreted by statistical analysis as an outlier in this distribution. In the second group of patients with T2DM, a similar pattern was observed in the distribution of the indicator, but the median values of the indicator were 387 ng/ml, and the observed range of the indicator was 28–1560 ng/ml, which was higher than in the first group (p<0.001).
The values of the studied biomarkers were compared with the indicators of patients during the hospital period of the study (Table 3). The MMP9 values in both groups I and II showed a direct correlation with the HbA1C level. For group I, the Spearman correlation coefficient was 0.371, p=0.002, while for group II, the correlation was more pronounced, r=0.50, p<0.001 (Fig. 1). For group II, a significant relationship was found between MMP9 levels and the duration of CHD, p=0.018. This relationship is associated with the presence of long-term low-intensity inflammation in patients with T2DM and CHD. In addition, many studies showed that MMP9 levels increased as coronary atherosclerosis progressed.
A significant difference in PWV in MT vessels was found, as well as in the MT/ET ratio between patients in the compared groups. It should be noted that PWV in classical-type vessels was also significantly higher in the group of patients with T2DM. According to the published data, elevated PWV is strongly associated with the presence of T2DM [9]. This is associated with changes in connective tissue proteins. In patients diagnosed with T2DM, the elastin protein of the vascular wall is replaced by stiffer collagen fibers at an earlier stage than in patients without T2DM [10]. Vessels lose their elasticity and become more rigid.
Discussion
It is known that vascular damage caused by previous prolonged hyperglycemia begins to predominate at HbA1c values ≥7.5%, which is a likely starting point for predicting increased vascular risk [11]. Important factors in the development of vascular complications in T2DM include increased glycation, degradation, and/or accumulation of elastin and collagen in the vascular wall. MMP9, which hydrolyzes protein components of the vascular extracellular matrix, is actively involved in this process [12]. A subgroup of MMP9, known as gelatinase B, can destroy collagen (COL), denatured COL (gelatin), elastin (EL), laminin, fibronectin, and other substrates. Disruption of gelatinase activity regulation is associated with vascular inflammation, remodeling, and fibrosis, and may contribute to the pathophysiology of diabetic complications. A study of patients with AH and T2DM showed that elevated serum PWV levels may reflect early structural changes in the extracellular matrix of blood vessels [13].
Statistically significant associations between MMP9 and HbA1c obtained by us are confirmed by the available published data. Elevated advanced glycation end products and extracellular matrix remodeling by matrix MMPs, in particular MMP9, are associated with vascular complications in T2DM.
There is a link between aortic stiffness, cardiovascular risk factors, and prognosis in patients who have recently suffered an acute myocardial infarction. Our study analyzed the relationship between cardiovascular risk factors and arterial stiffness and assessed its prognostic significance in patients with recently diagnosed STEMI.
Aortic PWV may be a more useful method for measuring changes in arterial stiffness over a long period of time, while wave reflection methods may be particularly useful for measuring short-term changes following therapeutic interventions.
Таблица / Table 1
Клиническая характеристика обследованных пациентов
Clinical characteristics of the examined patients
|
Показатели Indicators |
I группа, n=69 ОИМпST + АГ 1 group, n=69 STEMI+arterial hypertension |
II группа, n=67 ОИМпST + АГ + СД 2 2 group, n=67 STEMI+arterial hypertension+type 2 diabetes mellitus |
p |
|
Возраст, годы, Me [Q1; Q3] Age, years, Me [Q1; Q3] |
61 |
61 |
p=0,13 |
|
Мужчины/женщин, n (%) Men/women, n (%) |
47 (68,1%)/ |
51 (76,1%)/ |
p=0,67 |
|
АГ 1 степень, n (%) Arterial hypertension 1 degree, n (%) |
10 (14,5%) |
10 (14,9%) |
p=0,97 |
|
2 степень, n (%) 2 degree, n (%) |
13 (18,8%) |
14 (20,9%) |
|
|
3 степень, n (%) 3 degree, n (%) |
46 (66,7%) |
43 (64,2%) |
|
|
Длительность АГ Me [Q1; Q3] Duration of arterial hypertension Me [Q1; Q3] |
10 [ 8; 14] |
10 [ 7; 14] |
p =0,31 |
|
САД при поступлении, мм рт. ст., Me [Q1; Q3] Systolic blood pressure at admission, mmHg, Me [Q1; Q3] |
140 |
140 |
p=0,88 |
|
ДАД при поступлении, мм рт. ст., Me [Q1; Q3] Diastolic blood pressure at admission, mmHg, Me [Q1; Q3]
|
85 |
80 |
p=0,92 |
|
ЧСС, уд./мин. Heart rate, beats/min. |
85 |
80 |
p =0,73 |
|
Курение, n (%) Smoking, n (%) |
24 (34,8%) |
17 (25,4%) |
p=0,47 |
|
ИМТ, кг/м2, Me [Q1; Q3] BMI, kg/m2, Me [Q1; Q3] |
28,7 |
29,4 |
p=0,86 |
|
Лодыжечно-плечевой индекс, Me [Q1; Q3] Ankle-shoulder index, Me [Q1; Q3] |
1,21 |
1,20 |
p =0,31 |
|
Killip I, n (%) Killip I, n (%) |
53 (76,8%) |
45 (67,2%) |
p =0,57 |
|
Killip II, n (%) Killip II, n (%) |
12 (17,4%) |
13 (19,4%) |
|
|
Killip III, n (%) Killip III, n (%) |
1 (1,4%) |
6 (9,0%) |
|
|
Killip IV, n (%) Killip IV, n (%) |
3 (4,3%) |
3 (4,5%) |
|
|
ИБС, класс стенокардии ФК1, n (%) Coronary heart disease, functional class 1, n (%) |
13 (18,8%) |
14 (20,9%) |
p =0,28 |
|
ФК2, n (%) Functional class 2, n (%) |
24 (34,8%) |
25 (37,3%) |
|
|
ФК3, n (%) Functional class 3, n (%) |
30 (43,5%) |
27 (40,3%) |
|
|
ФК4, n (%) Functional class 4, n (%) |
2 (2,9%) |
1 (1,5%) |
|
|
ХСН, класс 1 по NYHA, n (%) Chronic heart failure, 1 class NYHA, n (%) |
31 (44,9%) |
32 (47,8%) |
p=0,94 |
|
Класс 2А по NYHA, n (%) 2A class NYHA, n (%) |
38 (55,1%) |
35 (52,2%) |
|
|
СКФ, Me [Q1; Q3] Glomerular filtration rate, Me [Q1; Q3] |
71,0 |
58,5 |
p =0,004 |
Таблица / Table 2
Результаты измерений ММР9
Measurement results of MMR9
|
Показатель Indicators |
I группа, n=69 ОИМпST + АГ 1 group, n=69 STEMI+arterial hypertension |
II группа, n=67 ОИМпST + АГ + СД2 2 group, n=67 STEMI+arterial hypertension+type 2 diabetes mellitus |
p |
|
ММР9, нг/мл, MMP9, ng/ml, upon admission |
297 [ 124; 332] |
387 [ 278; 640] |
p <0,001; |
Таблица / Table 3
Сравнение связи значений ММР9 с другими показателями в остром периоде инфаркта миокарда
Comparison of the relationship of MMR 9 values with other indicators in the acute period of myocardial infarction
|
Сопоставляемые показатели ММР9 при поступлении Comparable indicators of MMP9 upon admission |
I группа, n=69 ОИМпST + АГ 1 group, n=69 STEMI+arterial hypertension
|
II группа, n=67 ОИМпST + АГ + СД2 2 group, n=67 STEMI+arterial hypertension+type 2 diabetes mellitus |
||
|
r Спирмена Spearman's r |
p |
r Спирмена Spearman's r |
p |
|
|
ПАД, мм рт. ст. Pulse blood pressure, mmHg |
0,020 |
0,868 |
0,153 |
0,215 |
|
Длительность ГБ Duration of arterial hypertension |
0,121 |
0,324 |
-0,217 |
0,078 |
|
Длительность ИБС Duration of coronary heart disease |
0,020 |
0,873 |
-0,287 |
0,018 |
|
Длительность ХСН Duration of chronic heart failure |
0,032 |
0,793 |
-0,054 |
0,666 |
|
Возраст, лет Age, years |
0,080 |
0,516 |
0,229 |
0,062 |
|
HbA1C HbA1C |
0,371 |
0,002 |
0,500 |
0,000 |
|
СРПВ СЭ [м/с] Rate of propagation of the pulse wave through elastic vessels [m/s] |
0,131 |
0,307 |
-0,011 |
0,934 |
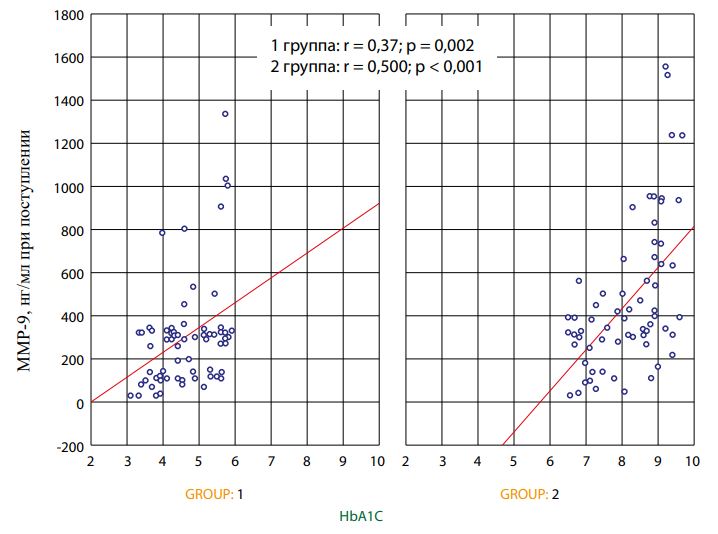
Рисунок 1. Зависимость маркера ММР9 и HbA1C в остром периоде инфаркта миокарда в I и II группах.
Figure 1. Dependence of the MMP9 and HbA1c markers in the acute period of myocardial infarction in groups 1 and 2.
Таблица / Table 4
Изучение скорости распространения пульсовой волны у обследованных пациентов
The study of the pulse wave propagation velocity in the examined patients
|
Показатель Indicators |
I группа, n=69 ОИМпST + АГ 1 group, n=69 STEMI+arterial hypertension |
II группа, n=67 ОИМпST + АГ + СД 2 2 group, n=67 STEMI+arterial hypertension+type 2 diabetes mellitus |
p |
|
СРПВ СМ [м/с] Rate of propagation of the pulse wave through the vessels of the muscular type [m/sec] |
2,38 (2,10; ,22) |
6,48 (4,66; 9,84) |
0,004 |
|
СРПВ СЭ [м/с] Rate of propagation of the pulse wave through elastic vessels [m/sec] |
9,37 (7,23; 12,60) |
12,10 (10,76; 15,80) |
0,016 |
|
Соотн. СМ/СЭ Ratio of vessels of elastic type and muscular type |
0,20 (0,19; 0,22) |
0,75 (0,54; 0,93) |
0,004 |
Conclusion
Together with the determination of such prognostically significant factors as MMP9, the determination of PWV may become a significant method for determining the prognosis in patients with T2DM who have undergone STEMI.
References
1. Balanova Yu.A., Shalnova S.A., Imaeva A.E., Kapustina А.V., Muromtseva G.A., Evstifeeva S.V., Tarasov V.I., Redko A.N., Viktorova I.A., Prishchepa N.N., Yakushin S.S., Boytsov S.A., Drapkina O.M. Prevalence, Awareness, Treatment and Control of Hypertension in Russian Federation (Data of Observational ESSERF-2 Study). Rational Pharmacotherapy in Cardiology. 2019;15(4):450-466. (In Russ.) https://doi.org/10.20996/1819-6446-2019-15-4-450-466
2. Brodskaya T.A., Nevzorova V.A., Shakhgeldyan K.I., Geltser B.I., Vrazhnov D.A., Kistenev Yu.V. Predictive potential of cardiovascular risk factors and their associations with arterial stiffness in people of European and Korean ethnic groups. Russian Journal of Cardiology. 2021;26(5):4230. (In Russ.) https://doi.org/10.15829/1560-4071-2021-4230
3. Gumerova V.E., Sayganov S.A., Gomonova V.V. Parameters of arterial stiffness in hypertensive patients with and without subclinical carotid atherosclerosis. "Arterial’naya Gipertenziya" ("Arterial Hypertension"). 2021;27(4):427- 435. (In Russ.) https://doi.org/10.18705/1607-419X-2021-27-4-427-435
4. Asmar R, Topouchian J, Pannier B, Benetos A, Safar M; Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens. 2001;19(4):813-818. https://doi.org/10.1097/00004872-200104000-00019
5. Tolkunova K.M., Rotar O.P., Erina A.M., Boiarinova M.A., Alievа A.S., et al. Supernormal vascular aging — prevalence and determinants at population level (the ESSE-RF data). "Arterial’naya Gipertenziya" ("Arterial Hypertension"). 2020;26(2):170-183. (In Russ.) https://doi.org/10.18705/1607-419X-2020-26-2-170-183
6. Jing J, Pan Y, Zhao X, Zheng H, Jia Q, et al. Prognosis of Ischemic Stroke With Newly Diagnosed Diabetes Mellitus According to Hemoglobin A1c Criteria in Chinese Population. Stroke. 2016;47(8):2038-2044. https://doi.org/10.1161/STROKEAHA.116.013606
7. Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, et al. Interaction Between Hypertension and Arterial Stiffness. Hypertension. 2018;72(4):796-805. https://doi.org/10.1161/HYPERTENSIONAHA.118.11212
8. Falkovskaya A.Yu., Mordovin V.F., Pekarskiy S.E., Ripp T.M., Zyubanova I.V., et al. Matrix metalloproteinases in patients with resistant hypertension and type 2 diabetes mellitus: relation with renal blood flow and kidney function. "Arterial’naya Gipertenziya" ("Arterial Hypertension"). 2019;25(1):34-45. (In Russ.) https://doi.org/10.18705/1607-419X-2019-25-1-34-45
9. Nedogoda S.V., Barykina I.N., Salasyuk A.S., Sanina T.N., Smirnova V.O., Popova E.A. The effect of various classes of glucose-lowering medications on the blood vessel elasticity in patients with type 2 diabetes. Russian Journal of Cardiology. 2020;25(4):3766. (In Russ.) https://doi.org/10.15829/1560-4071-2020-3766
10. Koziolova N.A., Chernyavina A.I., Polyanskaya E.A. Selection of antyhyperglycemic agents in high-risk patients with type 2 diabetes mellitus (part 1). "Arterial’naya Gipertenziya" ("Arterial Hypertension"). 2016;22(4):330-348. (In Russ.) https://doi.org/10.18705/1607-419X-2016-22-4-330-348
11. Yozgatli K, Lefrandt JD, Noordzij MJ, Oomen PHN, Brouwer T, et al. Accumulation of advanced glycation end products is associated with macrovascular events and glycaemic control with microvascular complications in Type 2 diabetes mellitus. Diabet Med. 2018;35(9):1242-1248. https://doi.org/10.1111/dme.13651
12.
About the Authors
V. A. SuroedovRussian Federation
Vladislav A. Suroedov, Head of the physiotherapy department; Postgraduate student of the Department of Therapy
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
A. A. Pirozhenko
Russian Federation
Pirozhenko Anna Alexandrovna, Associate Professor of the Department of Therapy
Competing Interests:
Authors declare no conflict of interest
L. A. Khaisheva
Russian Federation
Larisa A. Khaisheva, Professor, Head of the Department of Therapy
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
K. A. Khaishev
Russian Federation
Kirill A. Khaishev, the 6th-year student at a medical and preventive institution
Rostov-on-Don
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Suroedov V.A., Pirozhenko A.A., Khaisheva L.A., Khaishev K.A. Pulse wave propagation rate and dynamics of matrix metalloproteinase in patients with myocardial infarction and arterial hypertension in the presence or absence of diabetes mellitus. Medical Herald of the South of Russia. 2025;16(1):55-61. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-55-61







































