Scroll to:
Problems of choosing antimicrobial therapy in young children with community-acquired pneumonia
https://doi.org/10.21886/2219-8075-2025-16-1-74-80
Abstract
Objective: to analyze the prescribed antimicrobial therapy in young children with non-severe community-acquired pneumonia. Materials and methods: a sample of 219 young children with community-acquired pneumonia admitted to the pediatric department of the State Children's Clinical Hospital №17 in Ufa for the period from September 2022 to August 2023 was formed. Results: the clinical and anamnestic characteristics of children with community-acquired pneumonia are described. Analysis of antimicrobial therapy at the outpatient stage revealed in 51,24% of cases its compliance with clinical recommendations. There was a large share of parenteral drugs (73,97%) in the treatment of community-acquired pneumonia in a hospital setting. Preference was given to ceftriaxone (43,38%) due to the convenience of the method of administration. Inhibitor-protected aminopenicillins were prescribed half as often (23,29%). Conclusion: The authors believe that well-planned educational interventions will improve the effectiveness of antimicrobial therapy for community-acquired pneumonia in children.
Keywords
For citations:
Shangareeva Z.A., Faizullina R.M., Sannikova A.V., Mananova A.F., Berezina O.L., Islamgulova O.V., Pererva L.V., Larina A.V. Problems of choosing antimicrobial therapy in young children with community-acquired pneumonia. Medical Herald of the South of Russia. 2025;16(1):74-80. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-74-80
Introduction
Rational antimicrobial therapy (AMT) “still raises many questions and concerns in real clinical practice” [1–3]. The latest version of clinical guidelines for community-acquired pneumonia (CAP) “reflects the leading role of Streptococcus pneumoniae (up to 70–88% of cases) in the development of the disease in children over 3 months of age” [1–3] and mentions “amoxicillin as the optimal drug of choice” [1][3]. According to experts, “beta-lactam antimicrobial drugs (AMD) exhibit high activity against infection caused by Streptococcus pneumoniae” [1][3]. “The high level of resistance of Streptococcus pneumoniae to 14- and 15-membered macrolides” [1][3] stipulates “inexpediency of using azithromycin and clarithromycin in children under 5 years of age” [3]. Macrolide antibiotics are considered “the drugs of choice for proven/assumed atypical etiology of CAP” [1, 3]. Numerous studies demonstrate “an increase of up to 40–45% in resistance of Streptococcus pneumoniae to macrolides, lincosamides, and classical antipneumococcal beta-lactams” [2][3]. Among children with risk factors for the presence of pathogens producing β-lactamases (Haemophilus influenzae, Staphylococcus aureus, Escherichia coli), the drugs of choice are inhibitor-protected aminopenicillins, cephalosporins of the second- and third generations, and carbapenems [1][4].
Thus, according to experts, in the AMT of CAP, “the choice of drugs should be made considering the regional situation of antimicrobial resistance, which reduces the efficacy of therapy” [1][3]. “The phenomenon of antimicrobial resistance” [1–3] complicates the choice of empirical therapy for CAP in patients and explains the close attention of researchers to this problem.
The purpose of the study was to assess the prescribed AMT in infants with CAP at the outpatient and hospital stages.
Materials and methods
A sample of 219 children with moderate CAP was formed for the investigation. The children were examined and treated at the pediatric department of the State Budgetary Healthcare Institution of the Republic of Bashkortostan City Children’s Clinical Hospital No. 17 in Ufa from September 2022 to August 2023. All anamnesis, examination, and treatment data were copied from medical records, including the inpatient medical record and child development history.
Inclusion criteria were the following: 1) children aged 3 months to 5 years; 2) X-ray-confirmed diagnosis of CAP; 3) parental consent for hospitalization, examination, and treatment of the child.
Exclusion criteria were the following: 1) patient age under 3 months and over 5 years; 2) absence of a radiologically confirmed diagnosis of CAP; 3) absence of parental consent for hospitalization, examination, and treatment of the child.
In hospitalized children with suspected pneumonia or an established diagnosis of pneumonia”, before prescribing empirical AMT, a microbiological (cultural) investigation of secretions from the tonsils and posterior pharyngeal wall on aerobic and facultative anaerobic microorganisms was performed with determination of the pathogen sensitivity to antibiotics and other drugs, according to SanPiN 3.3686-21 “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases”, Chapter XL. Prevention of Community-Acquired Pneumonia – p. 3065 [5].
Statistical processing of the data was performed using the Statistica 10.0 software (StatSoft, USA). All results were presented as median and 25% and 75% quartiles: Me [Q1; Q3, considering the lack of normality in the distribution of variables.
Results
The number of infants with CAP hospitalized in the pediatric department No. 1 of the State Healthcare Institution of the Republic of Bashkortostan City Children’s Clinical Hospital No. 17 was maximal for the December–April period and minimal for the June–September period, respectively (Fig. 1).
The main parameters of the studied sample of patients are presented in Table 1.
The etiological structure, based on the results of microbiological (cultural) examination of discharge from the tonsils and the back wall of the pharynx, is presented in Table 1.
Empirical Antimicrobial Therapy for Community-Acquired Pneumonia in Children
According to the anamnesis collected at the outpatient stage, up to 73.97% (n=162) of children received AMT for 5 [ 3; 6] days. Empirical AMT for CAP in children is presented in Table 2.
Assessment of initial AMT showed (Table 2) that before admission to hospital, only half of patients with CAP (51.24%) received rational therapy represented by oral forms of aminopenicillins, including inhibitor-protected ones. The remaining 48.76% of cases of CAP treatment at the outpatient stage required critical revision.
AMT in hospital conditions was carried out for 8 days [ 7; 10]. Empirical AMT at the hospital stage also required critical re-thinking (Table 2).
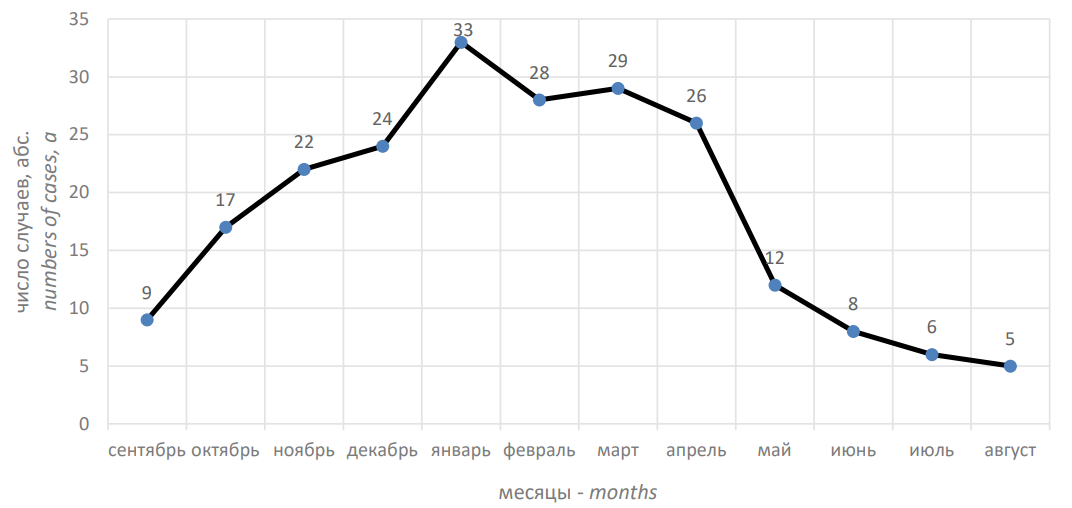
Рисунок 1. Число детей с внебольничной пневмонией за 12 месяцев
Figure 1. Number of children with community-acquired pneumonia over 12 months
Таблица / Table 1
Клинико-анамнестическая характеристика детей
Clinical and anamnestic characteristics of children
|
Параметры детей Children's parameters |
Медиана и значения 25% и 75%-квартилей: Ме [Q1; Q3] Median and 25% and 75% quartile values: Me [Q1; Q3] |
Количество детей (абс./%) Amount of children (abs./%) |
|
Демографические Demographic |
|
|
|
возраст age |
2,2 [ 8 мес.; 3,0 года] года |
|
|
пол sex |
|
|
|
мальчики boys |
|
114 / 52,05 |
|
девочки girls |
|
105 / 47,95 |
|
Перинатальный анамнез Perinatal history |
|
|
|
срок родов due date |
40 [ 37;40] недель |
|
|
вес при рождении birth weight |
3305 [ 2890; 3850] г |
|
|
естественный способ родов natural way of childbirth |
|
152 / 69,41 |
|
роды путем кесарева сечения birth by cesarean section |
|
67 / 30,59 |
|
грудное вскармливание breast-feeding |
|
172 / 78,54 |
|
искусственное вскармливание artificial feeding |
|
47 / 21,46 |
|
Сопутствующие заболевания Accompanying illnesses |
|
|
|
дефицитная анемия различной степени тяжести deficiency anemia of varying severity |
|
57/ 26,03 |
|
отит otitis |
|
31 / 14,16 |
|
риносинусит rhinosinusitis |
|
25/ 11,42 |
|
синдром прорезывания зубов teething syndrome |
|
14 / 6,39 |
|
ГИП ЦНС GIP CNS |
|
12 / 5,48 |
|
атопический дерматит atopic dermatitis |
|
11 /5,02 |
|
ВПС без нарушений гемодинамики CHD without hemodynamic disturbances |
|
6 / 2,74 |
|
Данные рентгенографии (инфильтрация лёгочной ткани) органов грудной клетки X-ray data (infiltration of pulmonary tissue) of the chest organs |
|
|
|
очаговая focal |
|
65 / 29,68 |
|
сегментарная segmental |
|
92 / 42,01 |
|
полисегментарная polysegmental |
|
55 / 25,11 |
|
долевая shared |
|
7 / 3,19 |
|
Наличие бронхообструктивного синдрома Presence of broncho-obstructive syndrome |
|
25 / 11,41 |
|
Результаты микробиологического (культурального) исследования отделяемого с миндалин и задней стенки глотки Results of microbiological (cultural) examination of discharge from the tonsils and posterior pharyngeal wall |
|
|
|
Микст бактерий Mixed bacteria |
|
27 / 12,33 |
|
Staphylococcus aureus |
|
25 / 11,42 |
|
Klebsiella pneumoniae |
|
8 / 3,65 |
|
Streptococcus pneumoniae |
|
5 / 2,28 |
|
Pseudomonas aeruginosa |
|
5 / 2,28 |
|
Escherichia coli |
|
5 / 2,28 |
|
Moraxella catarrhalis |
|
5 / 2,28 |
|
Serratia marcescens |
|
5 / 2,28 |
|
Enterobacter aerogenes |
|
5 / 2,28 |
|
Acinetobacter baumannii |
|
5 / 2,28 |
|
Candida albicans |
|
10 / 4,57 |
|
Neisseria perflava |
|
56 / 25,57 |
|
Streptococcus oralis |
|
53 / 24,21 |
|
Staphylococcus epidermidis |
|
5 / 2,28 |
Этиологическая структура, по результатам микробиологического (культурального) исследования отделяемого с миндалин и задней стенки глотки, представлена в таблице 1.
Эмпирическая антимикробная терапия внебольничной пневмонии у детей
Согласно собранному на амбулаторном этапе анамнезу, до 73,97% (n=162) детей получало АМТ в течение 5 [ 3; 6] суток. Эмпирическая антимикробная терапия ВП у детей приведена в таблице 2.
Таблица / Table 2
Эмпирическая антимикробная терапия у детей с внебольничной пневмонией
Empirical antimicrobial therapy in children with community-acquired pneumonia
|
Группы антимикробных препаратов Groups of antimicrobial drugs |
Частота назначения Prescription frequency |
||
|
Абс. (n) Abs. (n) |
Относ. (%) Rel. (%) |
||
|
Амбулаторный этап лечения (n=162) Outpatient stage of treatment (n=162) |
|||
|
Перорально Orally |
Амоксициллин/клавуланат Amoxicillin/clavulanate |
57 |
35,19 |
|
Цефиксим Cefixime |
34 |
20,98 |
|
|
Амоксициллин Amoxicillin |
26 |
16,05 |
|
|
Азитромицин Azithromycin |
23 |
14,20 |
|
|
Кларитромицин Clarithromycin |
5 |
3,09 |
|
|
Парентерально Parenterally |
Цефтриаксон Ceftriaxone |
17 |
10,49 |
|
Госпитальный этап лечения (n=219) Hospital stage of treatment (n=219) |
|||
|
Перорально Orally |
Амоксициллин/клавуланат Amoxicillin/clavulanate |
47 |
21,46 |
|
Амоксициллин Amoxicillin |
12 |
5,48 |
|
|
Парентерально Parenterally |
Цефтриаксон Ceftriaxone |
98 |
44,75 |
|
Ампициллин/сульбактам Ampicillin/sulbactam |
51 |
23,29 |
|
|
Цефепим Cefepime |
6 |
2,74 |
|
|
Амикацин Amikacin |
3 |
1,37 |
|
|
Меропенем Meropenem |
2 |
0,91 |
|
Discussion
“The etiological structure of CAP in children is diverse and closely related to the child’s age” [1][4]. In infants under 3 months, CAP is more often associated with respiratory viruses and bacteria (Escherichia coli, Chlamydophyla trachomatis, Haemophilus influenzae, and Staphylococcus aureus) [1][4]. In children aged 3 months to 5 years, Streptococcus pneumoniae dominates, less often CAP is caused by Haemophilus influenzae and “atypical bacteria” (Mycoplasma pneumonia and Chlamydophila pneumoniae) [1][4]. At the age of over 5 years, Streptococcus pneumoniae dominates as the cause of CAP, and the role of “atypical bacteria” increases [1][4].
In the clinical guidelines on CAP, “it is not recommended to conduct a microbiological (cultural) study of discharge from the oral cavity, mucus from the tonsils and the back wall of the pharynx for aerobic and facultative anaerobic microorganisms in children with pneumonia,” since, “firstly, the frequency of carriage of Streptococcus pneumoniae is high and amounts to 20–93.4% (in healthy children under 5 years of age), and secondly, the results of these studies do not always correlate with the etiologic agent that caused CAP” [1]. However, according to the requirements of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor), in accordance with SanPiN 3.3686-21, namely “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases”, Chapter XL. Prevention of Community-Acquired Pneumonia, p. 3065, this examination was carried out on all children with CAP at the time of their hospitalization. The etiological structure based on the results of microbiological (cultural) examination of discharge from the tonsils and posterior pharyngeal wall is presented in Table 1. The aforementioned SanPiN states that “it is preferable to examine clinical material from the lower respiratory tract (sputum, aspirates); if it is impossible to obtain them, a combined smear from the mucous membrane of the nasopharynx and the back wall of the pharynx1 is examined.” Some microorganisms are not characteristic of the development of bronchopulmonary inflammation. Their isolation from smears does not indicate their etiological significance in the development of CAP. Such microorganisms include Neisseria perflava, Streptococcus oralis, and Staphylococcus epidermidis.
Empirical AMT is prescribed taking into account the assumed pathogens of CAP in children [1–3][5]. Unfortunately, “very often specialists incorrectly select the starting antimicrobial drug, prescribe it in doses lower than recommended, and do not adhere to the prescription time for respiratory tract infections caused by atypical flora” [4].
Since “Streptococcus pneumoniae is the causative agent of CAP in children in most cases” [1][4], “the starting AMD in children from 3 months of age is amoxicillin in oral form and in a standard dosage of 45–55 mg/kg/day, divided into 2–3 doses, according to clinical recommendations” [4].
In our study, only 16.05% of patients with CAP received rational therapy through oral forms of aminopenicillins before hospitalization (Table 2).
In children with CAP, if pathogens producing β-lactamases are detected or if initial therapy with amoxicillin is ineffective, priority is given to inhibitor-protected aminopenicillins (amoxicillin/clavulanate, amoxicillin/sulbactam), cephalosporins of second- and third-generation, and carbapenems [1][4]. “Under outpatient conditions, amoxicillin/clavulanate is most often prescribed orally at a dosage of 45–60 mg/kg/day of amoxicillin, divided into 2–3 doses” [4].
In our study, amoxicillin/clavulanate was prescribed at the outpatient stage to 35.19% of children with a history of AMT during the previous 3 months, which should additionally be assessed as rational therapy.
The remaining 48.76% of cases required revision in accordance with clinical recommendations. For example, the fairly frequent prescription of cefixime (20.98%) for the treatment of respiratory pathology was inappropriate due to its “narrow spectrum of activity (no antipneumococcal activity) and widespread resistance of pathogens” [1][6]. The prescription of macrolide antibiotics such as azithromycin (14.20%) and clarithromycin (3.09%) as initial therapy was inappropriate due to the “high level of pneumococcal resistance to 14- and 15-membered macrolides” [1–3][5].
At the hospital stage (Table 2), oral forms of aminopenicillins (including inhibitor-protected ones) were prescribed only in a quarter of cases of CAP (26.94%) in the absence of antibacterial therapy in the anamnesis before admission to the hospital, as well as in the “step therapy” schemes. It is also worthy of note that a large proportion of parenteral drugs was revealed in the course of treatment (73.97%), and preference, unfortunately, was given to ceftriaxone (43.38%) due to the convenience of the mode of administration. Inhibitor-protected aminopenicillins were prescribed half as often (23.29%). In the case of insufficient efficacy of AMT, replacement or combined treatment with cefepime (n=6), amikacin (n=3), and meropenem (n=2) was carried out. Moreover, one should take into account the “resistance of pneumococci to aminoglycosides” [6]; therefore, the use of amikacin in CAP is irrational. “Amikacin is most preferable for the treatment of nosocomial pneumonia” [3][6].
In addition to clinical guidelines for the treatment of CAP, there are specialized Internet resources for monitoring the state of antibiotic resistance in the Russian Federation [5-7], as well as for selecting AMT2. Convenient web products are the online platforms “AMRmap”3 and “AMRbook”. These platforms can be actively used both in training and in the real practice of a pediatrician “for the competent choosing of AMT in the context of increasing antimicrobial resistance” [2][5-7].
The functionality of the online platforms “AMRmap” and “AMRbook” is very extensive, but without a brief explanation of their operating principle, it is difficult to convey the idea of the importance of their application in practice.
By selecting the tab “Community-acquired pneumonia” on the online platform “AMRbook”, one can view the etiology and empirical AMT of patients of different age groups. Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae are “the main causative agents of CAP in the age from 6 months to 5 years”. Further, AMT is described as “oral administration of amoxicillin or amoxicillin with clavulanic acid” [1] or “alternative therapy with cefotaxime, ceftaroline fosfamil, parenteral ceftriaxone in combination with azithromycin” [1].
While working with the platform, one can assess (Fig. 2) the “natural sensitivity of assumed pathogens to AMD”.
It is necessary to remember that Streptococcus pneumoniae is not sensitive to amikacin, cefixime, and the drugs are used in “treatment regimens on CAP in children irrationally” [1][3], that is, without considering this circumstance.
Selecting the parameters of interest on the antimicrobial resistance map (AMRmap) will help to clearly demonstrate the resistance of Streptococcus pneumoniae to various AMPs (Fig. 3).
The diagram (Fig. 3) shows the resistance of Streptococcus pneumoniae in the pediatric population with CAP in the Perm Territory to azithromycin and clarithromycin in 28.57% each, and to erythromycin and clindamycin in 14.29% each, respectively. High resistance of Streptococcus pneumoniae, unfortunately, “does not allow the use of macrolides as drugs of choice” [1][3]. Therefore, they are considered by experts as “drugs of choice for proven/assumed atypical etiology of CAP” [1][3].
In our opinion, the use of the online platforms “AMRmap” and “AMRbook” in the practice of a pediatrician, as well as well-planned educational activities, will increase the efficacy of AMT for CAP in children.
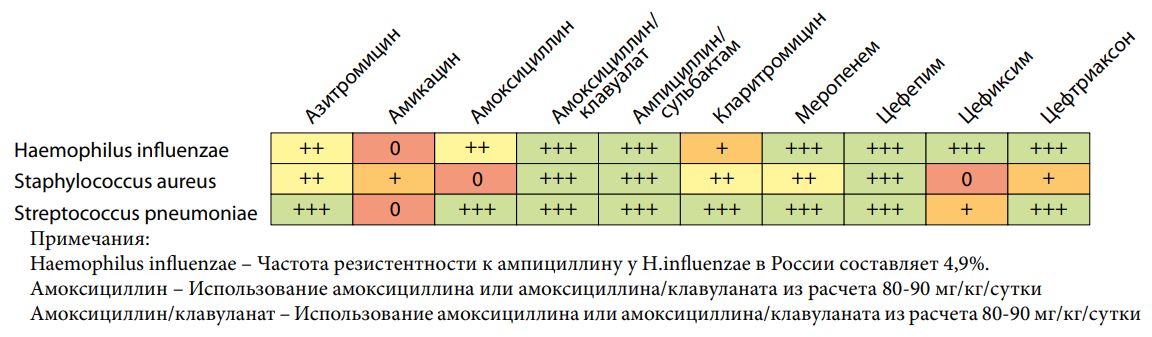
Рисунок 2. AMRbook: Природная чувствительность Haemophilus influenza, Staphylococcus aureus, Streptococcus pneumoniae к антимикробным препаратам, https://amrbook.ru (дата обращения — 04.05.2024)
Figure 2. AMRbook: Natural sensitivity of Haemophilus influenza, Staphylococcus aureus, Streptococcus pneumoniae to antimicrobial drugs, https://amrbook.ru (access date 05.04.2024)
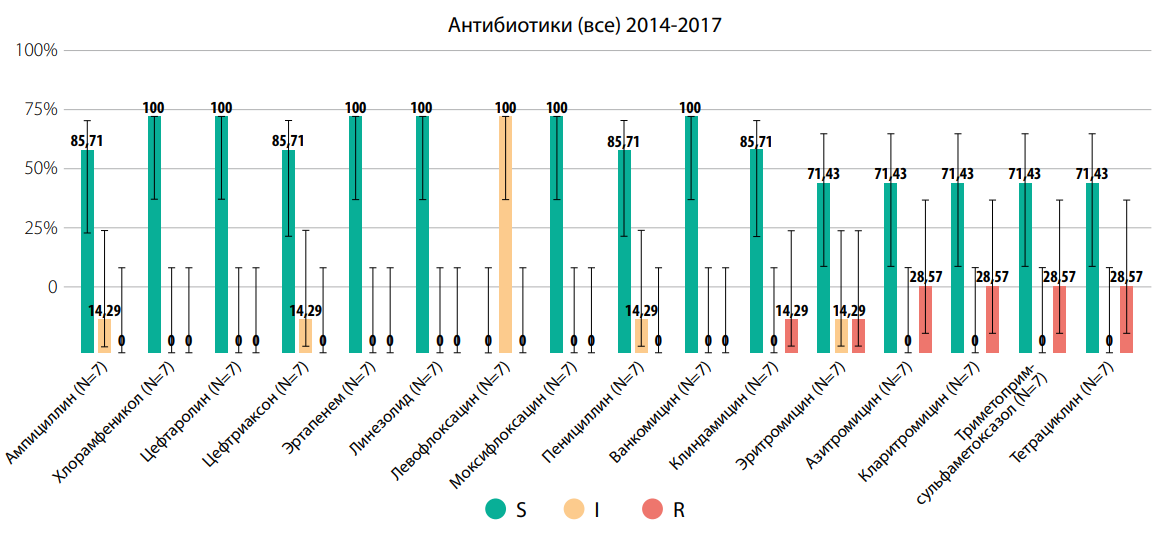
Рисунок 3. AMRmap: Резистентность Streptococcus pneumoniae к антимикробным препаратам на примере детского населения с внебольничной пневмонией г. Пермь Пермского края, https://map.antibiotic.ru (дата обращения — 04.05.2024)
Figure 3. AMRmap: Resistance of Streptococcus pneumoniae to antimicrobial drugs using the example of the child population with community-acquired pneumonia in Perm, Perm region, https://map.antibiotic.ru (access date — 05.04.2024)
Conclusion
Analysis of initial AMT at the outpatient stage showed that the prescription of aminopenicillins (16.05%) and inhibitor-protected aminopenicillins (35.19%) should be assessed as rational therapy. The remaining 48.76% of cases of AMD prescription at the outpatient stage require critical revision. The prescription of cefixime (20.98%) should be considered inappropriate due to the lack of antipneumococcal activity, while the prescription of macrolide antibiotics (azithromycin, clarithromycin) is inadvisable due to the widespread prevalence of pneumococcal resistance to them.
Oral forms of antimicrobial drugs at the hospital stage were prescribed only to a quarter of patients with CAP (26.94%) and only in the absence of antibacterial therapy in the anamnesis or in the “step therapy” schemes. Besides, a large proportion of parenteral drugs was revealed (73.97%) with preference for ceftriaxone (43.38%) due to the convenience of the mode of administration. Inhibitor-protected aminopenicillins were prescribed half as often (23.29%).
The problems of choosing AMT in children with CAP create the requests of specialists for additional educational programs. Well-planned educational activities, including the use of the online platform “AMRmap” and “AMRbook”, their widespread adaptation into the practice of a pediatrician, in our opinion, will increase the efficacy of AMT for CAP in children.
1. Resolution of the Chief State Sanitary Doctor of the Russian Federation dated January 28, 2021 No. 4 “On approval of sanitary rules and regulations SanPiN 3.3686-21 “Sanitary and epidemiological requirements for the prevention of infectious diseases”.
2. AMRbook: Handbook of Antimicrobial Therapy. [Electronic resource]. https://amrbook.ru/ (date of access 04.05.2024).
3. AMRmap: online platform for analysis of antimicrobial resistance data in Russia. [Electronic resource]. http://map.antibiotic.ru/ (date of access 04.05.2024).
References
1. Klinicheskie rekomendacii. M; 2022. (In Russ.).
2. Ivanchik N.V., Chagaryan A.N., Suxorukova M.V., Kozlov R.S., Dexnich A.V. et al. Antimicrobial resistance of clinical Streptococcus pyogenes isolates in Russia: the results of multicenter epidemiological study «PEHASus 2014–2017». Clinical Microbiology and Antimicrobial Chemotherapy. 2020; 22(1):40-45. (In Russ.). https://doi.org/10.36488/cmac.2020.1.40-45
3. Fajzullina R.M., Shangareeva Z.A., Sannikova A.V., Muxametzyanov A.M., Zajcev S.V., et al. Community-acquired pneumonia in children of the first five years of life. Prakticheskaya pul`monologiya. 2019;(4):30-36. (In Russ.). eLIBRARY ID: 42699180 EDN: CFONYD
4. Zaytseva SV, Zaytseva OV. Current guidelines for the selection of an antibacterial drug in children with communityacquired pneumonia. Meditsinskiy sovet = Medical Council. 2022;(6):158-165. (In Russ.) https://doi.org/10.21518/2079-701X-2022-16-6-158-165
5. Kuzmenkov A.Yu., Vinogradova A.G., Trushin I.V., Edelstein M.V. Avramenko A.A., et al. AMRmap – antibiotic resistance surveillance system in Russia. Clinical Microbiology and Antimicrobial Chemotherapy. 2021;23(2):198-204. (In Russ.). https://doi.org/10.36488/cmac.2021.2.198-204
6. Strachunskij L.S., Kozlov S.N. Sovremennaya antimikrobnaya ximioterapiya. Rukovodstvo dlya vrachej. Moscow: Borges; 2002 (In Russ.).
7. Vinogradova A.G., Kuzmenkov A.Yu. Application of AMRmap: «from the general to the specific» approach by the example of Klebsiella pneumoniae. Clinical Microbiology and Antimicrobial Chemotherapy. 2019;21(2):181-186. (In Russ.). https://doi.org/10.36488/cmac.2019.2.181-186
8.
About the Authors
Z. A. ShangareevaRussian Federation
Ziliya A. Shangareeva, PhD, associate Professor of the Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
R. M. Faizullina
Russian Federation
Reseda M. Faizullina, Dr. Sci. (Med.), Professor of the Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
A. V. Sannikova
Russian Federation
Anna V. Sannikova, PhD, associate Professor of the Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
A. F. Mananova
Russian Federation
Albina F. Mananova, head of the Pediatrics department
Ufa
Competing Interests:
Authors declare no conflict of interest
O. L. Berezina
Russian Federation
Olesya L. Berezina, 2-year resident, Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
O. V. Islamgulova
Russian Federation
Olga V. Islamgulova, 2-year resident of the Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
L. V. Pererva
Russian Federation
Lyudmila V. Pererva, 2-year resident, Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
A. V. Larina
Russian Federation
Anna V. Larina, 1st year resident, Department of Faculty Pediatrics and Neonatology
Ufa
Competing Interests:
Authors declare no conflict of interest
Review
For citations:
Shangareeva Z.A., Faizullina R.M., Sannikova A.V., Mananova A.F., Berezina O.L., Islamgulova O.V., Pererva L.V., Larina A.V. Problems of choosing antimicrobial therapy in young children with community-acquired pneumonia. Medical Herald of the South of Russia. 2025;16(1):74-80. (In Russ.) https://doi.org/10.21886/2219-8075-2025-16-1-74-80







































