Scroll to:
Hypercalciemia in severe kidney failure, differential diagnosis
https://doi.org/10.21886/2219-8075-2024-15-4-21-30
Abstract
Hypercalcemia is a metabolic condition characterized by an increase in total serum calcium concentration above normal. The prevalence of hypercalcemia in the population is about 1–3% according to pilot screening trials, and hypercalcemia is most oſten parathyroid-dependent. In the majority cases hypercalcemia remains asymptomatic, and clinical features are appeared only when the level of total calcium rises to 3,0–3,5 mmol/l. Detection of hypercalcemia in a patient allows the doctor to suspect first the pathology of parathyroid glands - primary hyperparathyroidism or malignant neoplasms, when under the influence of metastases of solid tumors there is destruction of bone tissue. This paper presents a clinical case of hypercalcemia in a patient with severe kidney failure, arterial hypertension and multiple cystic lucencies on radiographs of the leſt shoulder joint. Determination of intact parathyroid hormone concentration within the reference range allows us to reject the hyperparathyroidism and do extend differential diagnostic search. The result of nephrobiopsy was the diagnosis of multiple myeloma, myeloma nephropathy. Unusual for terminal stage of chronic kidney disease hypercalcemia and searching of its cause allows to verify the diagnosis of multiple myeloma in a short period of time.
For citations:
Gafurova N.A., Gorbatova E.V., Strelkova A.V., Postoeva A.V. Hypercalciemia in severe kidney failure, differential diagnosis. Medical Herald of the South of Russia. 2024;15(4):21-30. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-4-21-30
Introduction
Assessment of calcium and phosphorus metabolism is an important component of the examination of a patient with clinically significant renal pathology. The development of renal failure introduces changes in the rigid system of maintaining normocalcemia regulation, disturbing the balance between the intake of calcium (~ 1000 mg) and its excretion by the gastrointestinal tract (~ 900 mg) and the kidneys (~ 100 mg) (Fig. 1) [1].
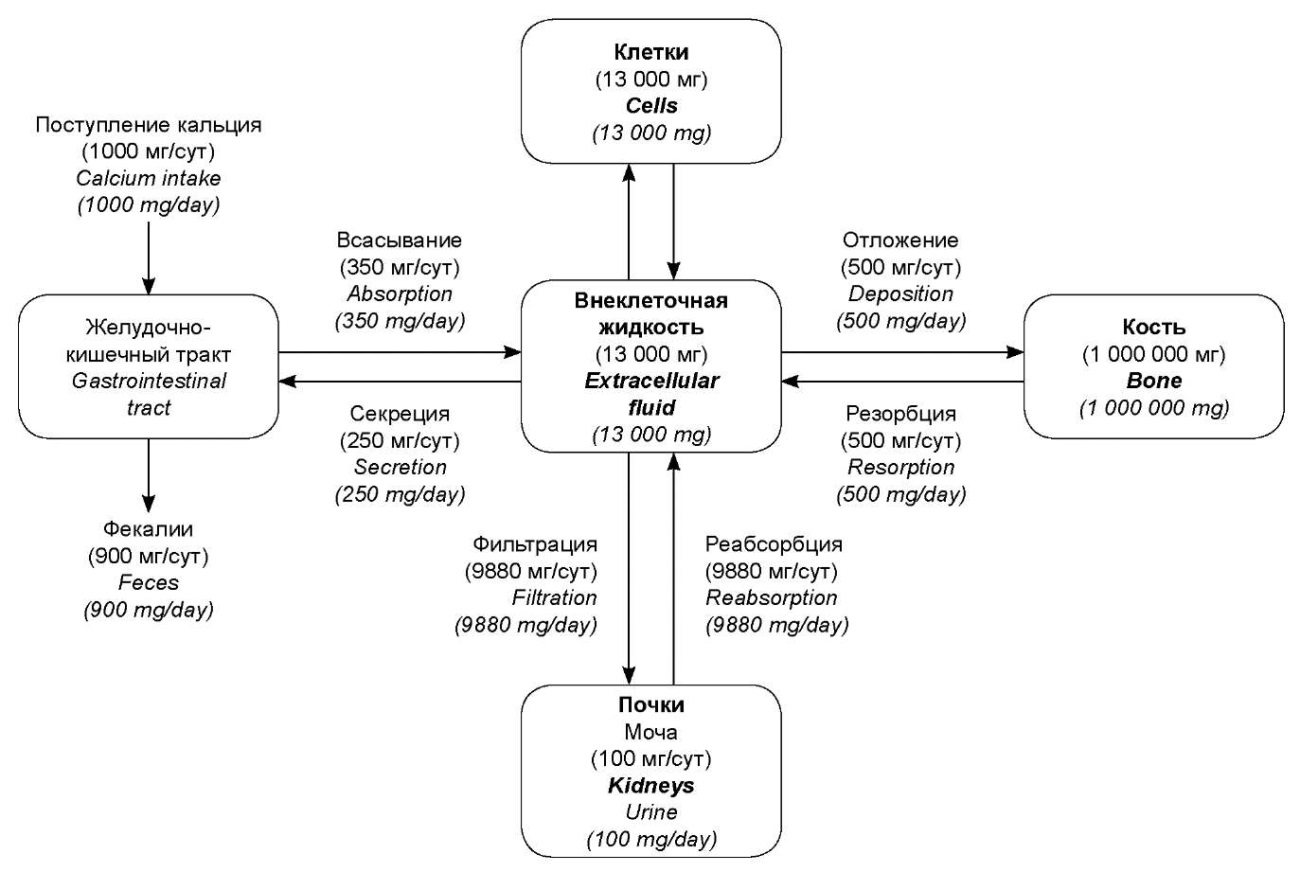
Рисунок 1. Распределение кальция между различными тканями и органами у человека, получающего 1000 мг кальция ежедневно (адаптировано из учебника «Медицинская физиология по Гайтону и Холлу». Пер. с англ.; Под ред. В.И. Кобрина, М.М. Галагудзы, А.Е. Умрюхина. 2-е изд., испр. и доп.)
Figure 1. Calcium distribution between different tissues and organs in a person who receives 1000 mg of calcium daily (adapted from the Guyton and Hall Textbook of Medical Physiology (13th ed.))
Specifically, a decrease in the functioning nephron mass is accompanied by a decrease in the hydroxylation of 25-hydroxycholecalciferol into the active form of vitamin D (1,25-dihydroxycholecalciferol) – calcitriol in the mitochondria of the proximal tubules of the kidneys at chronic kidney disease (CKD). This limits the absorption of calcium in the intestine (normally ~ 350 mg/day, Fig. 1) and forms adaptive mechanisms for eliminating hypocalcemia due to secondary hyperparathyroidism while maintaining the feedback between calcium and parathyroid hormone (PTH) levels [2]. Systemic disorders of mineral and bone metabolism develop due to vitamin D deficiency, disturbance of phosphate excretion, a secondary increase in PTH levels, and hyperplasia of parathyroid cells; development of calcification of vessels and soft tissues as a result of deposition of calcium phosphate; disturbance of renewal and mineralization with prevailing resorption of bone tissue [2].
Hypercalcemia in CKD develops at the stage of tertiary hyperparathyroidism (autonomy of the parathyroid gland/glands) or has a different etiology and developmental mechanisms, usually worsening the course of CKD. For example, it may be a consequence of immobilization (in the first days/weeks), thyrotoxicosis, vitamin D overdose, administration of thiazide-like diuretics, paraneoplastic process, primary hyperparathyroidism, etc. (Table 1) [3].
Таблица / Table 1
Заболевания, ассоциированные с гиперкальциемией
Diseases associated with hypercalcemia
|
Повышенная резорбция костной ткани / Increased bone resorption |
|
|
Паратиреоид-зависимая гиперкальциемия / Parathyroid hormone mediated hypercalcemia |
Спорадический первичный гиперпаратиреоз / Sporadic primary hyperparathyroidism |
|
Семейный / наследственный первичный гиперпаратиреоз / Familial / inherited primary hyperparathyroidism a) Множественные эндокринные неоплазии / Multiple endocrine neoplasia b) Семейный изолированный гиперпаратиреоз / Familial isolated hyperparathyroidism c) Синдром гиперпаратиреоза с опухолью челюсти / Hyperparathyroidism jaw-tumor syndrome d) Семейная гипокальциурическая гиперкальциемия (варианты CASR, GNA11, AP2S1 и так далее) / Familial hypocalciuric hypercalcemia (CASR, GNA11, AP2S1 variants, etc) e) Тяжёлый неонатальный первичный гиперпаратиреоз (вариант CASR) / Neonatal severe hyperparathyroidism (CASR variant) |
|
|
Рак паращитовидной железы / Parathyroid cancer |
|
|
Третичный гиперпаратиреоз / Tertiary hyperparathyroidism |
|
|
Вызванный лекарственными препаратами — литий / Drugs-induced — lithium |
|
|
Злокачественные новообразования, приводящие к эктопической выработке ПТГ (редко) / Malignancy ectopic parathyroid hormone production (rare) |
|
|
Паратиреоид-независимая гиперкальциемия / Non-parathyroid hormone mediated hypercalcemia |
Злокачественные новообразования (паранеопластическая гиперкальциемия) / Malignant neoplasms (paraneoplastic hypercalcemia) a) Гуморальная гиперкальциемия злокачественных опухолей (секреция паратиреоидного гормон-родственного белка) / Humoral hypercalcemia of malignancy (parathyroid hormone–related protein) b) Остеолитические метастазы или миелома / Osteolytic metastases or myeloma |
|
Иммобилизация / Immobilization |
|
|
Гипервитаминоз витамина А / Hypervitaminosis of vitamin A |
|
|
Гипертиреоз / Hyperthyroidism |
|
|
Приём лекарственных препаратов — терипаратид, абалопаратид, рикошетный остеолиз после отмены деносумаба / Drugs-induced –teriparatide, abaloparatide, rebound after denosumab discontinuation |
|
|
Метафизарная хондродисплазия, тип Янсена (вариант активации PTH1R) / Jansen-type metaphyseal chondrodysplasia (activating PTH1R variant) |
|
|
Гуморальная гиперкальциемия доброкачественного характера (секреция паратиреоидподобного белка доброкачественной тканью) / Humoral hypercalcemia of benignancy (parathyroid hormone-related protein secretion by benign tissue) |
|
|
Повышенное всасывание в желудочно-кишечном тракте / Increased gastrointestinal absorption |
|
|
Опосредованное витамином D / Vitamin D mediated |
Гипервитаминоз витамина D / Hypervitaminosis of vitamin D |
|
Опосредованный 1,25(OH)2D3 / 1,25-dihydroxyvitamin D mediated a) Гематологические злокачественные новообразования (повышение активности 1α-гидроксилазы) / Hematologic malignancies (increased 1α-hydroxylase activity) b) Гранулематозные болезни / Granulomatous disorders - Инфекционные — туберкулёз, болезнь кошачьих царапин, гистоплазмоз, криптококкоз, кандидоз, проказа, пневмоцистоз и т.д. / Infectious — tuberculosis, cat-scratch disease, histoplasmosis, cryptococcosis, candidiasis, leprosy, pneumocystosis, etc - Воспалительные — саркоидоз, гранулематозВегенера, болезнь Крона, синдром Блау, гистиоцитоз из клеток Лангерганса и т. д. / Inflammatory—sarcoid, Wegener granulomatosis,Crohn disease, Blau syndrome, Langerhans cell histiocytosis, etc |
|
|
Инородное тело — силикоз, тальк, лечение палочки Кальметта-Герена, бериллиоз, парафиновое масло / Foreign body — silicosis, talc, bacille Calmette-Guérin therapy, berylliosis, paraffin oil |
|
|
Другие причины / Other causes a) подкожный адипонекроз новорождённых / subcutaneous fat necrosis of the newborn b) липоидная пневмония / lipoid pneumonia |
|
|
Не связанное с витамином D / Non–vitamin D mediated |
Лактазная недостаточность / Lactase deficiency |
|
Сахарозо-изомальтазная недостаточность / Sucrase-isomaltase deficiency |
|
|
Влияние на почечный клиренс/реабсорбцию кальция / Effect on renal clearance/reabsorption of calcium |
|
|
Приём тиазидов / Drugs-induced – thiazides |
|
|
Молочно-щелочной синдром (поступление избыточного количества карбоната кальция) / Milk-alkali syndrome (concomitant with excess calcium carbonate ingestion) |
|
|
Острая почечная недостаточность / Acute kidney failure |
|
|
Синдром Барттера (в первую очередь гипокалиемия) / Bartter syndrome (especially hypokalemia) |
|
|
Надпочечниковая недостаточность / Adrenal insufficiency |
|
|
Другой или неясный механизм / Other or unclear mechanism |
|
|
Снижение депонирования кальция в скелете / Reduced deposition of skeletal calcium |
Адинамическая костная болезнь / Adynamic bone disease |
|
Гипофосфатазия / Hypophosphatasia |
|
|
Перераспределение / Redistribution |
Рабдомиолиз / Rhabdomyolysis |
|
Другие заболевания и состояния / Other diseases and conditions |
Акромегалия / Acromegaly |
|
Феохромоцитома / Pheochromocytoma |
|
|
Кетогенная диета / Ketogenic diet |
|
|
Почечный канальцевый ацидоз / Kidney tubular acidosis |
|
|
COVID-19 / COVID-19 |
|
|
Синдром Вильямса (генетический дефект синтеза эластина) / Williams syndrome (genetic defect of elastin synthesis) |
|
|
Лекарственные препараты / Drugs |
Теофиллин / Theophylline |
|
Фоскарнет / Foscarnet, |
|
|
Омепразол / Omeprazole |
|
|
Ингибиторы ароматазы / Aromatase inhibitors |
|
|
Ингибиторы натрий-глюкозного ко-транспортера 2-го типа / Sodium-glucose cotransporter 2 protein inhibitors |
|
|
Ингибиторы иммунных контрольных точек / Immune checkpoint inhibitors |
|
|
Гранулы сульфата кальция / Calcium-sulfate beads |
|
In contrast, acute renal injury (ARI) can be primarily accompanied by hypercalcemia, associated with a relative decrease in renal calcium clearance.
Regulation of calcium excretion by the kidneys depends on the body's needs, but it is difficult to modify renal macronutrient loss. Normally, only 60% of plasma calcium, not associated with proteins and phosphates, passes through the renal filter, but this is quite a lot – ~ 10 grams of calcium (9980 mg/day). Of this amount, 99% is reabsorbed: 65% – in the proximal tubules, PTH-independently; 25–30% – in the Henle loop, and 4–9% – in the distal tubules and collecting tubules. Only 1% (or 100 mg/day) is urine losses, which is 9 times less than through the gastrointestinal tract. Factors that reduce calcium secretion include high levels of PTH, hypovolemia, hypotension, hyperphosphatemia, metabolic alkalosis, and vitamin D3. Factors that increase calcium secretion include low PTH levels, a large volume of extracellular fluid, arterial hypertension (AH), hypophosphatemia, and metabolic acidosis. As a rule, the presence/absence of hypercalcemia in ARI is determined by the ARI etiology.
The purpose of the publication is to present the case of hypercalcemia detection in a patient examined in connection with severe kidney damage, manifested ~ 8 weeks after coronavirus infection, and the subsequent diagnostic search for its cause.
Clinical observation
Patient T., 64 years old, was taken by a relative to the emergency room of the city clinical hospital with complaints of weakness, decreased urine output within 2 days, swelling of the right lower leg, and pain in the right fibula and tibia. She was hospitalized in the surgical department for observation and further examination with a diagnosis of “Thrombosis of the sural veins of the right lower leg”.
Medical history: the patient experienced deterioration within a month, including general weakness, dyspnea, lack of appetite, decreased urine output, and increased blood pressure up to 220 mm Hg. The patient associated these symptoms with asthenia after SARS-CoV-2 infection. She did not seek medical help even after the fall, as a result of which she believed that she had broken her arm and was forced to fix her limb with a kerchief. Two days ago she noted an increase in swelling of the right lower leg and general pastiness, a sharp decrease in the urine amount, and intensification of the pain syndrome in the right leg. The woman's son brought her to the emergency room.
Patient T. was a non-working pensioner, there was no disability group, and she lived in a rural area. From the age of 23, she noted an increase in blood pressure to high numbers; the patient was haphazardly taking iACE inhibitors and dihydropyridine calcium antagonists. The patient had a history of 2 pregnancies, 2 births, and menopause was since the age of 50; however, extirpation was performed in 57 due to symptomatic fibroids. In that time, 7 years ago, the diagnosis of “Type 2 diabetes mellitus” was established, and therapy with metformin at a dose of 2000 mg per day was prescribed. Two months before hospitalization, the patient had SARS-CoV-2 infection without hospitalization; she had received antiviral and antipyretic drugs. Subjectively, the patient believed that she easily suffered the coronavirus infection.
In the surgical department, the patient's condition was considered moderate. The consciousness was clear; she was oriented in personality, time, and space. She was worried about weakness, her appetite was low, and she was nauseous, but there was no vomiting. The skin was clean and dry. The temperature was 36.6 °C. The breathing was vesicular, wheezing was not heard, respiratory rate was 20 per minute (SpO2 – 95%). Blood pressure was 250/110–160/70 mm Hg st, HR was 57–70 per minute. Heart tones were rhythmic and muffled, noises were not heard, and the accent was 2 tones on the aorta. The abdomen was soft, painless when palpated. The lower limbs were of normal color, warm to the touch, moderate edema of the right lower leg (+ 1 cm) was noted, palpation was moderately painful, sensitivity was preserved. Pulse on the main arteries of the lower extremities was determined at all levels. Movements in the ankle and knee joints were in full volume. The left upper limb was “hanging” fixed with a kerchief. The hand was warm and pink. Height – 156 cm, weight – 100 kg. BMI – 41.09 kg/m ². Diuresis – 400 ml.
As a result of the examination, the probability of deep vein thrombosis of the lower extremities was determined to be low by the clinical signs.
A laboratory examination revealed critical hyperazotemia (urea – 44.4 mmol/L, creatinine – 919 μmol/L) and metabolic (lactate) acidosis (pH – 7.21, BE – 13.8 mmol/L, lactate – 10.7 mmol/L), potassium – 5.23 mmol/L, sodium – 135 mmol/L (the dynamics of azotemia indicators and acid-base equilibrium during hospitalization is presented in Table 2). The urinalysis showed insignificant proteinuria: relative urine density was 1.009, urine color was straw, urine was clear, pH was 5.00, protein was 0.5 g/L, glucose was not detected, white blood cells were not detected, and red blood cells were 0.3 mg/L.
Таблица / Table 2
Динамика показателей азотемии и кислотно-щелочного равновесия в период госпитализации
Dynamics of indices of azotemia and acid-base balance during hospitalization
|
1-е сутки First 24 hours |
2–4-е сутки Second to fourth day |
5–14-е сутки (отделение нефрологии) 5–14 days (nephrology department) |
15–28-е сутки (отделение нефрологии и выписка) 15–28 days (nephrology department and discharge from the hospital) |
|
|
Креатинин, мкмоль/л Сreatinine, μmol/L |
981 |
951 |
565 |
599 |
|
Мочевина, ммоль/л Urea, μmol/L |
47,2 |
45,5 |
29,7 |
10,9 |
|
pH |
7,25 |
7,26 |
7,33 |
7,37 |
|
Бикарбонат актуальный, ммоль/л сНСО, μmol/L |
12,5 |
15,3 |
23,3 |
22,9 |
|
Стандартный избыток оснований, BE, ммоль/л Base excess, BE, μmol/L |
-15,9 |
-11,5 |
-0,9 |
-1,1 |
|
Лактат, ммоль/л Lactate, μmol/L |
10,7 |
2,4 |
1,4 |
1,2 |
|
Калий, ммоль/л Potassium, μmol/L |
5,3 |
5,0 |
5,0 |
4,6 |
|
Натрий, ммоль/л Sodium, μmol/L |
135 |
138 |
135 |
134 |
|
Глюкоза, ммоль/л Glucose, μmol/L |
5,2 |
6,3 |
5,5 |
6,9 |
An ultrasound of the kidneys was performed. The right kidney had a normal position, uneven, fuzzy contour, dimensions – 12.3 × 6.2 cm, parenchyma thickness – 1.2 cm, the parenchyma structure was compacted, pyramids were expressed, the cavity system was expanded due to cups up to 0.8 cm, pelvis – 2.2 × 2.0 cm. The position of the left kidney was ordinary, the contour was uneven, fuzzy, the dimensions were 12.4 × 6.5 cm, the thickness of the parenchyma was 1.7 cm, the structure of the parenchyma was compacted, the pyramids were expressed, the cavity system was expanded due to cups – up to 0.9 cm, pelvis – 2.3 × 2.0 cm, the bladder was empty. Some expansion of the cup-pelvis system was noted, and after examination by a urologist, taking into account contraindications (hyperazotemia) for intravenous urography, spiral computed tomography (SCT) of the kidneys was performed, which excluded obstructive nephropathy as the cause of severe kidney damage.
Due to severe hyperazotemia, lactic acidosis (Table 2), and uncontrolled hypertension, the patient was transferred to the intensive care unit, where hemodialysis sessions were started. A day later, during treatment, the lactate level returned to normal, and hemodynamic parameters were stabilized. Diuresis was reduced.
On the 5th day, the patient was transferred to the nephrology department with a diagnosis of “Unspecified nephropathy. Severe ARI with CKD”.
Further diagnostic measures were aimed at clarifying the severe renal injury etiology. The patient denied taking cholecalciferol; however, she reported occasional intake of non-steroidal anti-inflammatory drugs for pain and regular metformin intake, which, of course, could cause acute kidney injury and lactic acidosis, but the “ideal” level of glycated hemoglobin (5.3%) on biguanide monotherapy denied the possibility of diabetic nephropathy. There were also no signs of a “secondary wrinkled kidney” against the background of prolonged uncontrolled hypertension.
Laboratory and instrumental examination data in the nephrology department
In the complete blood count, there were white blood cells – 9.28×10⁹/L (N 3.9–9.0×10¹²/L), red blood cells 3.17×10¹²/L (N 3.6–5.0×10¹²/L), hemoglobin – 77 g/L (N 120–140 g/L), ESR – 28 mm/h, other parameters were within the reference values.
In the general urinalysis with daily urine output not more than 200 ml, there was protein – 1.0 g/L (N up to 0.033 g/L), erythrocytes– 0.30 mg/L (N less than 0.2 mg/L), other indicators were also within the reference values.
There was a decrease in urea and creatinine levels in the biochemical blood study (Table 2).
A slightly decreased sodium level persisted (133.2 mmol/L, N 136–146 mmol/L), as well as normokalemia – 4.63 mmol/L (N 3.4–4.5 mmol/L), uric acid parameters– 267.57 μmol/L (N 140–310 μmol/L), alkaline phosphatase– 55 U/L (N 35–104 U/L). Transaminase was normal: ALT – 14 U/L (N 5–30 U/L), AST – 25 U/L (N 5–31 U/L). The total protein level was reduced (58.4 g/L, N 60–83 g/L) with a preserved albumin level of 41.1 g/L (N 32–52 g/L). There were high ferritin values (372.7 ng/mL. N 10–120 ng/mL) against the background of anemia and iron deficiency (7.9 μmol/L). C-reactive protein was 7.0 mg/L. The glomerular filtration rate according to CKD-EPI was 6.32 mL/min/1.73 m².
The lipid profile suggested dyslipidemia associated with metabolic syndrome: cholesterol was 5.78 mmol/L (N up to 5.2 mmol/L), triglycerides – 2.71 mmol/L (N up to 1.71 mmol/L), high-density lipoprotein cholesterol – 1.47 mmol/L (N 1.2–1.55 mmol/L), low-density lipoprotein cholesterol – 3.41 mmol/L L (N to 2.59 mmol/L).
Hypercalcemia (2.95 mmol/L, N 2.02–2.6 mmol/L) was detected; the phosphorus level was at the upper limit of normal (1.41 mmol/L, N 0.81–1.45 mmol/L). Albumin-corrected calcium was 2.93 mmol/L. The intact PTH (32.21 pg/mL, N 15–65 pg/mL) was determined; it was normal, which excluded the classic secondary hyperparathyroidism in CKD in combination with another cause of hypercalcemia, as well as any other hyperparathyroidism.
However, the radiologist's conclusion on the images of the left shoulder joint indicated changes in bone structure similar to renal osteodystrophy: signs of a decrease in bone density due to osteoporosis, numerous cyst-like enlightenment of the left humerus, deformation of the upper 1/3 of the humerus diaphysis (consolidated fracture?); similar changes in the left scapula, clavicle, and ribs in the visible extent; no significant fresh bone-traumatic changes were found; the joint ratio was acceptable (Fig. 2).
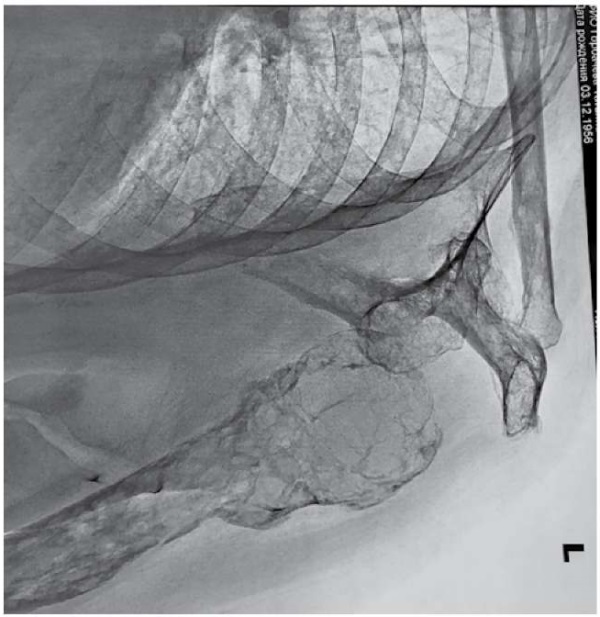
Рисунок 2. Рентгенография левого плечевого сустава
Figure 2. Radiography of the left shoulder joint
Renal osteodystrophy is a disorder of bone morphology in patients with CKD, which is one of the characteristics of the skeletal component of mineral and bone systemic disorders [4]. The presence of fibrotic osteitis in combination with hypercalcemia suggests renal osteodystrophy with high bone metabolism against the background of hyperparathyroidism, which was not found in this patient.
Abdominal SCT also revealed signs of osteoporosis of the lumbar spine and lower thoracic vertebrae. Pelvic SCT showed signs of osteoporosis in the pelvic bones.
Based on the examination data, a preliminary diagnosis was established: “Unspecified nephropathy. Severe acute renal injury. Renal replacement therapy. Parathyroid-independent hypercalcemia. Anemia of moderate severity (total hemoglobin – 77.00 g/l). Complicated total osteoporosis. Grade 3 hypertension”.
Thus, the normal level of PTH excluded hyperparathyroidism as the cause of bone pathology and switched the further diagnostic search to the hypercalcemia genesis, primarily to the paraneoplastic, competing in frequency with primary hyperparathyroidism of 0.86–1% [5].
The most common types of tumors and the corresponding pathogenetic mechanisms that cause hypercalcemia are presented in Table 3 [6]. In the patient, the changes in bone structure described above made it possible to suspect osteolytic metastases in the first place.
Within the myeloma exclusion, blood protein fractions were determined by electrophoresis: total protein was 58.4 g/L, albumin – 58.2% (N 55.80–66.10%), a1-globulins – 6.4% (N 2.90–4.90%), a2-globulins – 12.1% (N 7.10–11.80%), b1-globulins – 6.1% (N4.70–7.20%), b2-globulins – 3.8% (N3.20–6.50%), g1-globulins – 13.4% (N11.10–18.80%); a slight shift towards a1- and a2-globulins was detected; subsequent immunotyping revealed free kappa light chains of immunoglobulin.
Таблица / Table 3
Наиболее распространённые виды опухолей и соответствующий им патогенетический механизм, приводящий к развитию гиперкальциемии
The most common types of tumors and their corresponding pathogenetic mechanism leading to the development of hypercalcaemia
|
Злокачественная гуморальная гиперкальциемия (секреция паратгормонподобного белка) / Humoral hypercalcemia of malignancy (parathyroid hormone–related protein) |
Бронхиальный карциноид / Bronchial carcinoid |
|
Почечно-клеточный рак / Renal cell carcinoma |
|
|
Опухоли мочеполовой системы / Tumors of the urogenital system |
|
|
Карциномы яичников / Ovarian carcinomas |
|
|
Неходжкинские лимфомы / Non-Hodgkin's lymphomas |
|
|
Карциномы молочных желез / Mammary gland carcinomas |
|
|
Сквамозно-клеточная карцинома / Squamous cell carcinoma |
|
|
Хронический миелоидный лейкоз / Chronic myeloid leukemia |
|
|
Остеолитические метастазы / Osteolytic metastasis |
Карциномы молочных желез / Mammary gland carcinomas |
|
Множественная миелома / Multiple myeloma |
|
|
Лейкемия / Leukemia |
|
|
Лимфомы / Lymphomas |
|
|
Эктопическая секреция кальцитриола / Ectopic secretion of calcitriol |
Неходжкинские лимфомы / Non-Hodgkin's lymphomas |
|
Эктопическая секреция паратиреоидного гормона / Ectopic secretion of parathyroid hormone |
Карцинома яичников / Ovarian carcinomas |
|
Рак бронхов / Bronchial cancer |
|
|
Нейроэндокринные опухоли / Neuroendocrine tumors |
|
|
Карциномы щитовидной железы / Thyroid carcinomas |
|
|
Рак поджелудочной железы / Pancreatic cancer |
Free light chains (FLCs) are associated with multiple myeloma, as they are produced in all types of multiple myeloma, their concentration in the blood serum does not depend on the preservation of renal function and directly reflects their secretion by cells, as well as FLCs can be used for prognostic purposes [7]. Detection of FLCs made it possible to accumulate available data on parathyroid-independent hypercalcemia, anemia, multiple bone damage, and renal failure and to suspect stage III of multiple myeloma (according to Durie and Salmon), despite the absence of Bence-Jones protein in the urine, the preserved albumin level, and the low level of total protein in the patient's blood serum1.
Multiple myeloma is a B-cell malignancy, the morphological substrate of which is plasma cells producing monoclonal immunoglobulin1. In 2021, the incidence of multiple myeloma in Russia, according to Kaprin et al., amounted to 1.47 per 100 thousand population. Among all malignant tumors, the incidence of multiple myeloma is approximately 1%, and among all tumors of hematopoietic and lymphoid tissues up to 10–13%2. Patients of the older age group are predominantly ill, the prevalence of multiple myeloma among populations younger than 40 years does not exceed 2%, and the average age of new cases is approximately 70 years [8].
Myeloma osteolysis can affect any bone, but more often bones are affected, in which the content of cortical bone tissues is greater than spongy bone tissues. Osteolytic destruction in multiple myeloma is based on the secretion of osteoclast-activating factors by myeloma cells and suppression of the proliferation of osteoblastic cells, which leads to the destruction of the bone matrix without its subsequent replacement. As a result, myeloma osteolysis on an X-ray looks like a “zone of a completely empty area”, which indicates the absence of bone defect mineralization [9].
The course of myeloma can correspond to smoldering (asymptomatic) or symptomatic form. The patient did not meet the criteria for asymptomatic myeloma due to the presence of organ damage – signs of bone damage. For confirmation of symptomatic multiple myeloma, the presence ≥ 10% of the clonal plasma cells or biopsy-confirmed monoclonal plasma cells in the bone or extramedullary lesions[1] were required, besides hypercalcemia, renal impairment, and osteolytic lesions observed in the patient.
Due to the patient's presence in the nephrology department and inability to perform a sternal puncture in the department condition, on the 14th day of hospitalization, a nephrobiopsy of the left kidney was performed.
The conclusion obtained as a result of nephrobiopsy was “Combined monoclonal paraproteinemic nephropathy: 1. BJCN/ Κ: BenceJones’ (Kappa) diffuse severe monoclonal cylinder nephropathy with pronounced tubulo-interstitial inflammation and total acute tubular necrosis. 2. LHCDD/IgGk: diffuse deposition of monoclonal light (Kappa) and heavy (Gamma) chains in all basement membranes of kidney tissue (GBM, TBM, BMGC); complete glomerulosclerosis (9%); moderate tubulo-interstitial fibrosis (30%); arteriolo-arteriosclerosis of extreme severity with a sharp narrowing of the vascular lumens”.
Taking into account the clinical picture and the results of laboratory and instrumental studies, the following diagnosis was established:
The main one was “Monoclonal gammopathy of renal significance (multiple myeloma)”.
A complication of the main diagnosis was Myeloma nephropathy (combined monoclonal paraproteinemic BJCN/k nephropathy: Diffuse heavy monoclonal cylinder Bence Jones' (Kappa) nephropathy with pronounced tubulointerstitial inflammation and total acute tubular necrosis. LHCDD/lgGk: diffuse deposition of monoclonal light (Kappa) and heavy (Gamma) chains in all basement membranes of kidney tissue (GBM, TBM, BMGC); complete glomerulosclerosis (9%); moderate tubulointerstitial fibrosis (30%); arteriolo-arteriosclerosis of extreme severity with a sharp narrowing of the vascular lumens. Acute renal injury of renal C3 genesis, oliguric form with outcome in chronic C5 D kidney disease. Anemia of moderate (Hb 77 g/L) severity of combined genesis. Hypoproteinemia. Bone and mineral disorders. Secondary total osteoporosis. Consolidated fracture of the upper third of the diaphysis of the left humerus.
Thus, metabolic acidosis, low PTH, and uncontrolled AH were partly mechanisms of adaptation to severe osteolytic hypercalcemia, as much as possible all mentioned above increased calcium secretion till the total invasion of the glomeruli by monoclonal plasma cells and till renal tubular necrosis.
During the treatment, the patient was hemodynamically stable, did not have a fever, and had persistent anuria, hemodialysis sessions were well tolerated. For further treatment, she was transferred to the specialized hematology department. An immunochemotherapy program was prescribed in the hematology department, including cyclophosphamide, bortezomib, and dexamethasone. Pathogenetic treatment, in particular with bortezomib, not only has a pronounced effect on tumor cells, but also reduces the differentiation and functional activity of osteoclasts, thus reducing bone resorption [10]. The patient tolerated the first course of the program satisfactorily. In the future, to control hypercalcemia and bone resorption, taking into account reduced kidney function, denosumab3 therapy is possible.
Conclusion
Hypercalcemia is a relatively rare and most commonly laboratory finding associated first of all with primary hyperparathyroidism.
However, other diseases associated with hypercalcemia should not be overlooked. In the case of multiple myeloma, its diagnostics can be difficult for a long time, and doctors are more often faced with an already complicated course of this disease. This is due to the fact that in practice, the clinical manifestations of multiple myeloma are extremely diverse, non-specific, and distant from the onset of the disease. At the same time, the definition of PTH in patients with hypercalcemia and CKD allows narrowing the differential search, accelerating the path to diagnosis and necessary treatment.
1. Russian Association of Oncologists, National Hematological Society, Russian Professional Society of Oncohematologists. Clinical Guidelines. Multiple Myeloma. 2020
2. Kaprin A.D., Starinsky V.V., Shakhzadova A.O. Malignant Neoplasms in Russia in 2021 (Morbidity and Mortality). Moscow: P. A. Hertsen Moscow Oncology Research Center – branch of the Federal State Budgetary Institution “National Medical Research Center of Radiology” of the Russian Ministry of Health, 2022.
3. Russian Association of Endocrinologists, Association of Endocrine Surgeons. Clinical Guidelines. Primary Hyperparathyroidism. 2020
References
1. Hall jE. Guyton and Hall Textbook of Medical Physiology. 13th ed. 2015.
2. Mokrysheva NG, Maganeva IS. Mineral-bone disorders in patients with chronic kidney disease and diabetes mellitus: the real possibilities of cardio and nephroprotection. Meditsinskiy sovet = Medical Council. 2018;(4):60-65. (In Russ.) https://doi.org/10.21518/2079-701x-2018-4-60-65
3. Walker MD, Shane E. Hypercalcemia: A Review. JAMA. 2022;328(16):1624-1636. https://doi.org/10.1001/jama.2022.18331
4. Editorial a. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Nephrology (Saint-Petersburg). 2011;15(1):87-95. (In Russ.) https://doi.org/10.24884/1561-6274-2011-15-1-87-95
5. Mokrysheva N.G., Kovaleva E.V., Eremkina A.K. Registries of parathyroid glands diseases in the Russian Federation. Problems of Endocrinology. 2021;67(4):4-7. (In Russ.) https://doi.org/10.14341/probl12803
6. Suttmann Y, Fischereder M. Hyperkalzämie: Häufig steckt eine Tumorerkrankung dahinter : Onkologische Zufallsbefunde [Hypercalcemia]. MMW Fortschr Med. 2020;162(7):35-38. (In German). https://doi.org/10.1007/s15006-020-0388-9
7. Skvortsova N.V., Kovynev I.B., Halzov K.V., Pospelova T.I. The importance of serum immunoglobulin free light chain assessment for predicting outcome in patients with newly diagnosed multiple myeloma in real clinical practice. Oncohematology. 2020;15(3):38-50. (In Russ.) https://doi.org/10.17650/1818-8346-2020-15-3-38-50
8. Yarikov A.V., Boyarshinov A.A., Lobanov I.A., Dubskikh A.O., Perlmutter O.A., Fraerman A.P., Sosnin A.G., Kuznetsov S.F., &Kabardaev R.M. Multiple myeloma: epidemiology, etiology, diagnosis and modern aspects of surgical treatment. VolgaOncologicBulletin. 2021;12(2):53-64. (In Russ.) eLIBRARY ID: 47289133 EDN: ZwjOMq
9. Heltser B.I., Zhilkova N.N., Anufrieva N.D., Kochetkova E.A. Bone lesions in case of multiple myeloma. Pacific medical journal. 2011;(3):11-16. eLIBRARY ID: 22661267 EDN: TBxHVx
10. Smirnov A.V., Dobronravov V.A., Khrabrova M.S., Afanasyev B.V. Kidney involvement in monoclonal gammopathies: multidisciplinary approach in oncohematology and nephrology. Oncohematology. 2020;15(2):49-60. (In Russ.) https://doi.org/10.17650/1818-8346-2020-15-2-49-60
About the Authors
N. A. GafurovaRussian Federation
Natalya A. Gafurova, Assistant of the Department of Hospital Therapy and Endocrinology
Arkhangelsk
Competing Interests:
Authors declares no conflict of interest.
E. V. Gorbatova
Russian Federation
Ekaterina V. Gorbatova, Cand. Sci. (Med.), Associate Professor of the Department of Internal medicine
Arkhangelsk
Competing Interests:
Authors declares no conflict of interest.
A. V. Strelkova
Russian Federation
Aleksandra V. Strelkova, Cand. Sci. (Med.), Associate Professor of the Department of Hospital Therapy and Endocrinology, Northern State Medical University; N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Science
Arkhangelsk
Competing Interests:
Authors declares no conflict of interest.
A. V. Postoeva
Russian Federation
Anna V. Postoeva, Cand. Sci. (Med.), Associate Professor of the Department of Hospital Therapy and Endocrinology
Arkhangelsk
Competing Interests:
Authors declares no conflict of interest.
Review
For citations:
Gafurova N.A., Gorbatova E.V., Strelkova A.V., Postoeva A.V. Hypercalciemia in severe kidney failure, differential diagnosis. Medical Herald of the South of Russia. 2024;15(4):21-30. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-4-21-30







































