Scroll to:
Epizootological situation and epidemiological risks for natural focal infections in the territory of new subjects of the Russian Federation (Donetsk People's Republic, Lugansk People's Republic, Zaporozhye and Kherson regions)
https://doi.org/10.21886/2219-8075-2024-15-1-7-18
Abstract
An epizootological inspection of the liberated territories of the Donetsk and Lugansk People's Republics, Zaporozhye and Kherson regions was carried out. It was established that the faunal complex of small mammals, hare-like and ectoparasites, as well as their number, make it possible to ensure the circulation of natural focal infections identified by the results of laboratory studies: tularemia, Crimean hemorrhagic fever (CHF), West Nile fever (WNF), ixodic tick-borne borreliosis (ITB), rickettsiosis, human granulocytic anaplasmosis (HGA), leptospirosis, hemorrhagic fever with renal syndrome (HFRS), Q fever, intestinal yersiniosis, Batai, Inco, Sindbis, Tyaginya fevers. For the first time, the PCR method in field material from the Zaporozhye region (Berdyansk and Melitopol regions) detected the Crimean-Congo hemorrhagic fever virus, on the territory of the Donetsk and Lugansk People's Republics in the populations of small mammals (rodents and insectivores), the circulation of viruses Inco, Sindbis, Tyagin, Batai was established. In the territories of Kherson and Zaporozhye regions, the presence of combined and conjugated natural foci of ixodic tick-borne borreliosis, rickettsiosis and human granulocytic anaplasmosis is determined. According to the results of molecular genetic analysis, it was shown that the causative agents of natural focal infections (Crimean hemorrhagic fever, West Nile fever, ixodic tick-borne borreliosis, rickettsiosis) identified in 2023 are genetically close to strains circulating in the regions of the south European part of Russia.
Keywords
For citations:
Popova A.Yu., Kulichenko A.N., Noskov A.K., Efremenko D.V., Volynkina A.S., Tsapko N.V., Kotenev E.S., Maletskaya O.V., Kurcheva S.A., Vasilyeva O.V., Gazieva A.Yu., Dobrovolsky O.P., Zabashta M.V., Khametova A.P., Panasyuk N.V., Chemisova O.S., Tsai A.V., Ananyeva N.Ye., Dokashenko D.A., Khattatova N.V., Turov V.M. Epizootological situation and epidemiological risks for natural focal infections in the territory of new subjects of the Russian Federation (Donetsk People's Republic, Lugansk People's Republic, Zaporozhye and Kherson regions). Medical Herald of the South of Russia. 2024;15(1):7-18. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-7-18
Introduction
The affiliation of new regions with the Russian Federation, based on the results of referendums held in 2022, necessitated further integration of the liberated territories of the Donetsk and Lugansk People’s Republics (DPR and LPR), as well as the Zaporozhye and Kherson Regions, in particular, in terms of implementing standards of the sanitary and epidemiological well-being of the population.
Organizing the activities of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing in new territories is being performed with both available and attracting additional necessary human, material, and technical resources. Considering the potential risks of complications of the epidemiological situation due to natural focal infections, as well as due to the lack of systematic monitoring of the causative agents of these diseases over recent decades, implementing an operational epizootological survey of these territories is one of the top priorities.
During the planning of this work, the following features should be considered carefully:
- restrictions on the possibilities for surveying territories stipulated by a special military operation;
- the possibility of complications in the epizootological and epidemiological situation as a consequence of changes in social factors including the movement of the population and military contingents, as well as the deterioration of living conditions.
In order to arrange activities, a Comprehensive Action Plan was established for reducing the risks of complications of the epidemiological situation with especially dangerous and natural focal infectious diseases in the territories of the DPR, LPR, Zaporozhye and Kherson Regions for 2023, which included the following items:
- conducting an epizootological survey of territories for natural focal infections;
- diagnostic investigations of material from different field sources and clinical material from sick individuals;
- genomic epidemiological monitoring based on molecular analysis of identified pathogen strains;
- implementation of planned epidemic controlling and preventing measures against natural focal infections.
According to the available retrospective data, including those dating back to the times of the USSR, natural foci of tularemia, Q fever, leptospirosis, and West Nile fever (WNF) are located in the territories of the new regions of the Russian Federation [1–3]. In addition, there is information on detecting pathogen markers for Crimean hemorrhagic fever (CHF), ixodic tick-borne borreliosis (ITBB), rickettsioses, tick-borne encephalitis (TBE), hemorrhagic fever with renal syndrome (HFRS), human granulocytic anaplasmosis (HGA), and human monocytic ehrlichiosis (HME) [4–7]. Information on most of these infections is fragmentary, which does not allow obtaining the true picture and assessing the real risks.
The natural focus of tularemia is located in the territory of all four new regions. There is data on the isolation of Francisella tularensis strains from samples of field material and environmental objects collected in the Lugansk, Donetsk, Zaporozhye, and Kherson Regions during the 1940–1990 periods. The natural focus is located in the steppe and forest-steppe zones, mainly in the floodplains of rivers and near lakes. Its biocoenosis structure is represented by more than 20 species of rodents and insectivores, as well as by more than 10 species of ixodid ticks. Background species, found in almost all identified foci, include the wood mouse (Sylvaemus sylvaticus), the house mouse (Mus musculus), and the common vole (Microtus arvalis) [2].
In recent years, sporadic incidence of tularemia has been registered, mainly among residents of rural areas. Cases of the disease, as a rule, were associated with professional activities. Infection occurred during threshing grain crops, harvesting hay, and stacking, as well as during hunting water rats, muskrats, nutria, and hares. Moreover, the level of vaccination among risk groups was low. The most unfavorable epidemiological situation has developed in the DPR, where the incidence of tularemia in people has been registered almost every year since 2016. In particular, there were 9 cases in 2016, 2 cases in 2017, 5 cases in 2019, 2 cases in 2021, and 10 cases in 2022. In 2022 in the DPR, four cultures were isolated from samples of small mouse-like rodents and identified as F. tularensis, subspecies holarctica, biovar II EryR [8].
Through the period from 2006 to 2017, 51 cases of WNF disease were identified in the Zaporozhye Region but in 2021 there was only a case. During the period of 2019–2021, in the territory of the DPR, some cases of the disease were revealed in Donetsk and Makeevka, as well as in the Starobeshevsky and Telmanovsky Districts, including one fatal case.
The natural focus of Q fever, according to the available information, covers 5 administrative districts (12 settlements) in the DPR, 2 districts (2 settlements) in the Zaporozhye Region, and 7 districts (8 settlements) in the Kherson Region. Information on the presence of a focus in the territory of the LPR was not publicly available.
In 2014, in the DPR, within an epizootological survey of the natural focus of Q fever, 393 specimens of ixodid ticks were examined using immunofluorescence analysis and polymerase chain reaction (PCR) methods. Coxiella burnetii markers were found in samples of Dermacentor marginatus ticks from the town of Novoazovsk, as well as from the Amvrosievsky District, including Lisichye village, Alekseevskoye village, Uspenka village, and from the town of Makeevka including Yasinovka settlement. Besides, pathogen markers were found in 6 out of 16 (37.5%) samples of Hyalomma plumbeum ticks and in 2 out of 22 (9.09%) samples of D. marginatus ticks.
In more recent times, sporadic incidence of Q fever was registered annually mainly in the DPR. In particular, there was 1 case in 2018, 16 cases in 2019, 1 case in 2020, and 4 cases in 2021. In 2022, 37 cases were identified; and the incidence was noted in territories that had not been previously classified as enzootic. They included one of the villages in the Novoazovsky District, as well as the city of Donetsk, the towns of Gorlovka, Enakievo, Snezhnoye, and Khartsyzsk, and the Yasinovatsky District. All cases were recorded from May to November and had no epidemiological interconnection among them; infection mainly resulted from the air-dust transmission path of the pathogen and was accompanied by injury of the respiratory system. Patients indicated the presence of rodents at their place of residence or place of permanent location (military personnel).
In the Zaporozhye Region, two cases of leptospirosis were revealed in 2016, and a case in 2018; the latter one was fatal. In 2021, two cases of the disease were registered in the Kherson Region and a case in the DPR with a fatal outcome. In these new regions, the gray rat (Rattus norvegicus) and the house mouse (M. musculus) are the main carriers of Leptospira pathogens. The incidence of leptospirosis was recorded mainly in the forest-steppe and steppe zones. The fatality rate reached 10%. Most cases of infection were associated with the recreational use of pieces of water located within the boundaries of anthropurgic foci. In particular, in some areas near pieces of water, farms have been built in violation of sanitary and veterinary rules [3].
The incidence of CHF in the territories that became part of Russia in 2022 has not been previously registered. During the epidemiological examination, markers of the Congo-Crimean hemorrhagic fever virus (CCHF) were not detected by PCR. However, antigens (Ags) of the virus were detected in two administrative regions of the DPR and in two regions of the LPR. In addition, by one case of Ag detection was revealed in the Zaporozhye and Kherson Regions. This identifies the presence of a natural focus of CCHF in the steppe zone and, partially, in the forest-steppe zone [6]. In 2022, Ag of the CCHF virus was detected in the DPR; three positive samples were found in Donetsk, and one sample was detected in Porokhnia village.
Thus, information on the factors, development mechanisms, and manifestations of epidemic and epizootic processes of natural focal infections, as well as the characteristics of circulating pathogen strains, are fragmentary. To assess existing risks and arrange effective anti-epidemic measures, an examination of the features of the epizootic process should be performed involving methods of genomic epidemiological surveillance [9].
An epizootological examination of natural foci of infections in the territory of new administrative subjects was carried out in compliance with the following provisions:
- uniform coverage of the territory as far as it is possible;
- comprehensive analysis of field material aimed to search for markers of all current natural focal infections;
- collection of field material from all types of biotopes existing in the territory.
Thus, the distribution and contamination by pathogens of natural focal infections among small mammals (SMs), ticks collected from wild and farm animals, as well as mosquitoes, collected on the flag, were investigated in this study.
Samples of field material were collected in the territory of five administrative districts of the Kherson Region including the Genichesky, Novotroitsky, Verkhnerohachiksky, Skadovsky, and Kakhovsky ones; six districts of the Zaporozhye Region including the Melitoposkyl, Berdyansky, Vasilyevsky, Pologovsky, Kuibyshevsky, and Tokmaksky ones; six districts of the DPR including the Novoazovsky, Mangushsky, Telmanovsky, Starobeshevsky, Amvrosievsky, and Shakhtersky ones; and seven districts of the LPR including the Krasnodonsky, Lutuginsky, Antratsitovsky, Sverdlovsky, Stanichno-Lugansky, Belovodsky, and Melovsky ones.
In total, 102 points (8700 km²) were surveyed in the Kherson and Zaporozhye Regions; 5670 trap-nights were set. In addition, 712 specimens of animals, 1715 specimens of ectoparasites, 60 samples of cattle blood serum, 10 samples of small cattle blood serum, and 1 sample from poultry were taken for examination. In the DPR and LPR, 127 points (9900 km²) were surveyed; 14,050 trap-nights were set; 1847 specimens of SMs and 1623 specimens of ectoparasites were collected. A total of 20,925 mosquitoes were collected on the flag in the territory of all four regions (Table 1).
Таблица / Table 1
Общие сведения об эпизоотологическом обследовании в 2023 г.
территории ДНР, ЛНР, Запорожской области (ЗО) и Херсонской области (ХО)
General information on epizootological examination
of the territory of the Donetsk (DPR) and Lugansk People's Republics (LPR),
Zaporozhye region (ZR) and Kherson region (KR) in 2023
|
Даты проведения обследования / Dates of examination |
Субъекты / Subjects |
Автомобильные и пешие учетные маршруты (км) / Road and foot accounting routes (km) |
Количество точек забора проб полевого материала / Number of sampling points for field material |
Площадь обследования (км²) / Inspection area (km²) |
Выявлены возбудители (маркеры) следующих природно-очаговых инфекций / The causative agents (markers) of the following natural focal infections were identified |
|
19.02.2023—25.02.2023 |
ЗО (ХО*) / ZR (KR*) |
722 |
4 |
550 |
— |
|
ДНР / DPR |
250 |
8 |
800 |
ИКБ, иерсиниоз, туляремия / ITB, yersiniosis, tularemia |
|
|
ЛНР / LPR |
300 |
12 |
900 |
иерсиниоз, туляремия / yersiniosis, tularemia |
|
|
03.04.2023—14.04.2023 |
ЗО / ZR |
620 |
12 |
900 |
риккетсиоз / rickettsiosis |
|
ХО / KR |
230 |
12 |
1200 |
ИКБ / ITB |
|
|
ДНР / DPR |
420 |
25 |
1300 |
КГЛ, ИКБ, ГАЧ, иерсиниоз, лептоспироз, ГЛПС, туляремия / CHF, ITB, HGA, yersiniosis, leptospirosis, HFRS, tularemia |
|
|
ЛНР / LPR |
210 |
15 |
1100 |
ИКБ, иерсиниоз, туляремия / ITB, yersiniosis, tularemia |
|
|
16.05.2023—27.05.2023 |
ЗО / ZR |
— |
27 |
2700 |
КГЛ, ИКБ, риккетсиоз / CHF, ITB, rickettsiosis |
|
ХО / KR |
— |
15 |
1500 |
ИКБ, риккетсиоз / ITB, rickettsiosis |
|
|
ДНР / DPR |
— |
24 |
2100 |
туляремия (культура), ИКБ, ГАЧ / tularemia (culture), ITB, HGA |
|
|
ЛНР / LPR |
— |
32 |
2300 |
туляремия (культура), ИКБ, ГАЧ / tularemia (culture), ITB, HGA |
|
|
08.07.2023—16.07.2023 |
ХО / KR |
— |
6 |
200 |
ЛЗН, туляремия / WNF, tularemia |
|
13.08.2023—25.08.2023 |
ЗО / ZR |
5 |
8 |
800 |
ЛЗН, ИКБ, ГАЧ / WNF, ITB, HGA |
|
ХО / KR |
2 |
2 |
200 |
риккетсиоз, лептоспироз, туляремия / rickettsiosis, leptospirosis, tularemia |
|
|
ДНР / DPR |
5 |
26 |
2800 |
ИКБ, иерсиниоз, Ку-лихорадка / ITB, yersiniosis, Q fever |
|
|
ЛНР / LPR |
5 |
30 |
2500 |
ИКБ, ГАЧ, лептоспироз, Ку-лихорадка / ITB, HGA, leptospirosis, Q fever |
|
|
22.10.2023—04.11.2023 |
ЗО / ZR |
— |
13 |
700 |
туляремия (культура), ИКБ, риккетсиоз, ГАЧ / tularemia (culture), ITB, rickettsiosis, HGA |
|
ХО / KR |
— |
3 |
200 |
ИКБ / ITB |
|
|
ДНР / DPR |
10 |
30 |
2900 |
ИКБ, ГАЧ, иерсиниоз, лептоспироз, ГЛПС, туляремия, лихорадки Батаи, Инко, Тягиня, Синдбис / ITB, HGA, yersiniosis, leptospirosis, HFRS, tularemia, Batai, Inco, Tyaginya, Sindbis fevers |
|
|
ЛНР / LPR |
5 |
34 |
2200 |
ИКБ, лептоспироз, ГЛПС, туляремия, лихорадки Инко, Тягиня, Синдбис / ITB, leptospirosis, HFRS, tularemia, Inco, Tyaginya, Sindbis, fevers |
|
|
Итого по ЗО / Total for ZR |
986 |
62 |
5350 |
туляремия, КГЛ, ЛЗН, ИКБ, риккетсиоз, ГАЧ / tularemia, CHF, WNF, ITB, rickettsiosis, HGA |
|
|
Итого по ХО / Total for KR |
593 |
40 |
3350 |
ЛЗН, ИКБ, риккетсиоз, лептоспироз, туляремия (ПЦР, РНАт) / WNF, ITB, rickettsiosis, leptospirosis, tularemia (PCR, RNAb) |
|
|
Итого по ДНР / Total for DPR |
685 |
113 |
7600 |
туляремия, КГЛ, ИКБ, ГАЧ, иерсиниоз, лептоспироз, Ку-лихорадка, ГЛПС, лихорадки Батаи, Инко, Тягиня, Синдбис / tularemia, CHF, ITB, HGA yersiniosis, leptospirosis, Q fever, HFRS, Batai, Inco, Tyaginya, Sindbis fevers |
|
|
Итого по ЛНР / Total for LPR |
520 |
123 |
7500 |
туляремия, ИКБ, ГАЧ, иерсиниоз, лептоспироз, Ку-лихорадка, ГЛПС, лихорадки Инко, Тягиня, Синдбис / tularemia, ITB, HGA, yersiniosis, leptospirosis, Q fever, HFRS, Inco, Tyaginya, Sindbis fevers |
|
Примечание: * — обследование проводилось методом маршрутно-визуального учёта.
Note: * — the examination was carried out by route-visual accounting.
When performing the survey, the GIS QGIS was used for cartographic analysis. In fact, the following territories were examined: the southeastern part of the Kherson Region, the central and southern parts of the Zaporozhye Region, the southeastern part of the DPR, and the southeastern part of the LPR.
Assessing the number of SMs revealed their mosaic distribution in different biotopes. In the Zaporozhye and Kherson Regions, the number of SMs increased from February to April and August. Examining biotopes adjacent to pieces of water, including forest belts, fields, and coastal strips, revealed that the rate of penetration into animal trapping tools was higher in these areas than in dry biotopes, including forest belts and fields. Analysis of the sex and age composition of the populations showed that in the summer, a cessation of population growth was noted, presumably, due to drought. However, in the autumn, there was a sharp jump leading to maximum values for the percentage of animals caught in trapping tools. The average number of SMs in the DPR and LPR from the beginning of the year until the end of summer was noticeably higher than in the Kherson and Zaporozhye Regions. Moreover, from February to August, an increase in the corresponding indices was found in the territory of the LPR by 1.65 times and in the territory of the DPR by 2.23 times. In autumn, a further increase in the number of SMs was noted.
The number of brown hares remained unchanged and was assessed at an average level in all new subjects.
The number of ticks, collected on the flag, was determined in the spring, since ticks were inactive in summer due to the heat. In the Zaporozhye Region, the number was at an average level (33 individuals per flag/hour), in the Kherson Region, it ranged from low (up to 10 individuals per flag/hour) to high (98 individuals per flag/hour); and in the DPR and LPR, this index was assessed as low (up to 10 individuals per flag/hour).
The abundance index of Hyalomma marginatum ticks, which is the specific CCHF virus carrier, collected from cattle and small cattle, was equal to 8.0 in the Kherson Region, 3.2 in the Zaporozhye Region, 3.8 in the DPR, and 8.0 in the LPR, which exceeded the epidemiologically significant level (2.5).
Examining mosquito-borne infections showed that the dominant genus in samples collected in the territories of the Kherson Region, where 12,036 specimens were collected, and Zaporozhye Region, where 1,821 specimens were collected, was Culex with rates of 51.0% and 62.5%, respectively. In the DPR, where 4474 specimens were collected, the dominant species was Aedes caspius accounting for about 95% of the collected samples, and in the LPR, Aedes cantans predominated (51.5%) in 2594 collected specimens.
Thus, the faunal complex of SMs, lagomorphs, and ectoparasites, as well as their numbers, are favorable for the circulation of all pathogens of natural focal infections known for these territories.
Laboratory diagnostic results
The studies were performed with immunoserological, molecular genetic, biological, and bacteriological methods. Suspensions of ixodid ticks, mosquitoes, fleas, rodent organs, and blood of cattle and small cattle were examined using PCR and ELISA for detecting markers of CCHF, WNF, TBE, orthohantaviruses, causative agents of tularemia, Q fever, intestinal yersiniosis, pseudotuberculosis, ITBB, rickettsiosis, HGA, HME, leptospirosis, as well as Bataille, Inco, Sindbis, Tyagin, Chikungunya, and Dengue viruses. Samples of rodent organs (spleen) were used to perform a bioassay to isolate the F. tularensis culture. Detection of Ags of the tularemia microbe in samples from animals, blood-sucking arthropods, and abiotic objects such as bird pellets, water, and nest-burrow substrate, was carried out using indirect hemagglutination reaction and antibody neutralization reaction (RNAb). Washings out of the chest cavity of rodents were analyzed by the microagglutination reaction (RMA) for the availability of antibodies to the causative agent of leptospirosis.
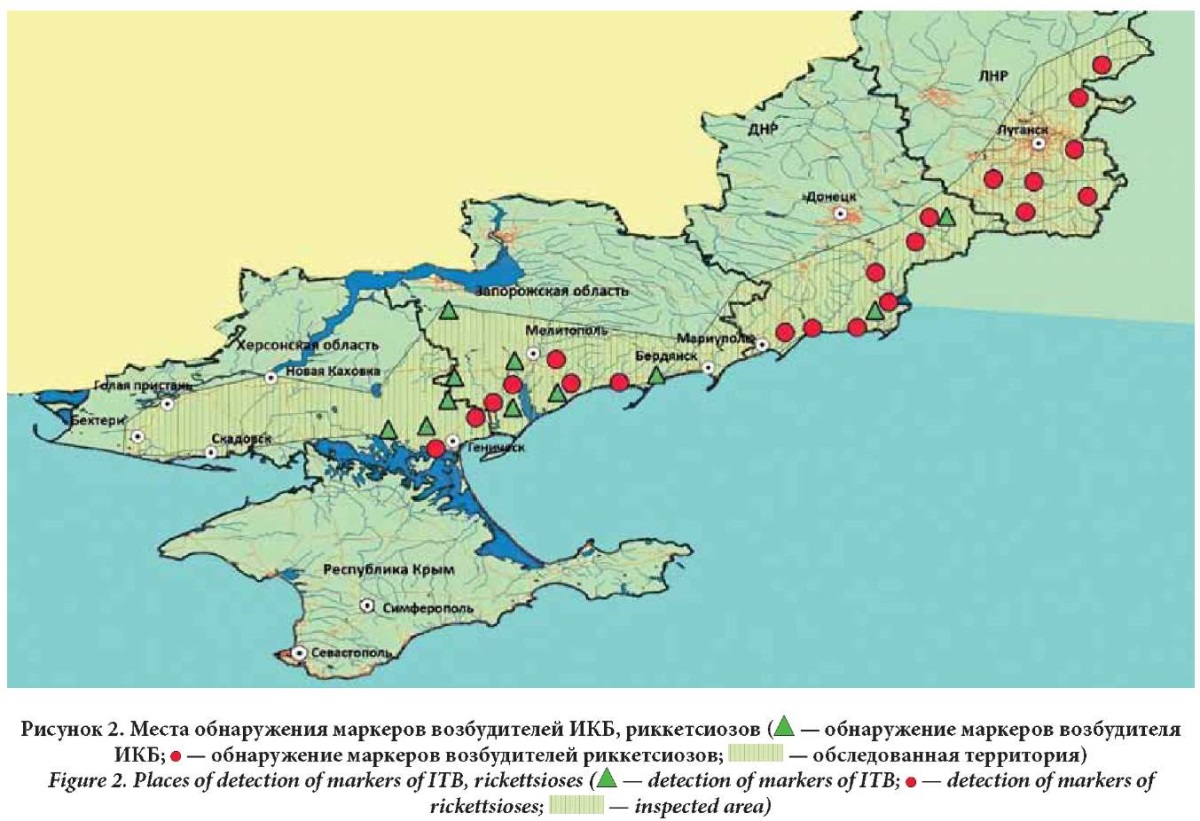
The investigation of samples of field material identified the causative agents of the following infections: tularemia, CCHF, WNF, ITBB, rickettsial infections, HGA, leptospirosis, HFRS, Q fever, intestinal yersiniosis, and Bataille, Inco, Sindbis, and Tyagin fevers (Figs. 1, 2 ).
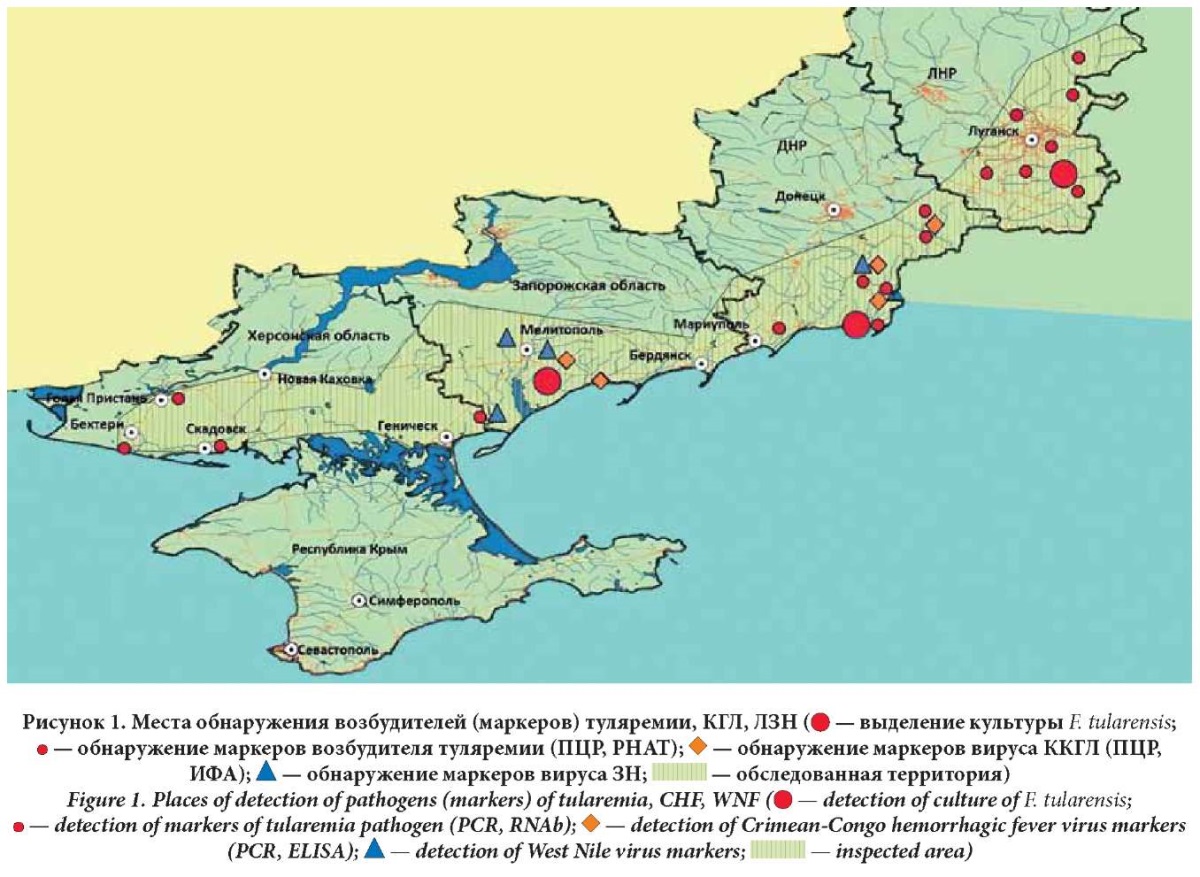
For the first time, the circulation of the CCHF virus was confirmed in the Zaporozhye Region with the PCR method. In particular, virus RNA was detected in three pools from imagoes of ixodid ticks Rhipicephalus rossicus collected from small cattle (in Rainovka village, the Berdyansky District) with the proportion of positive samples of 3.1% over the district and from H. marginatum imagoes collected from cattle (in Spasskoye village, the Melitopolsky District) with the proportion of positive samples of 0.4% over the district.
In the DPR, CCHF virus RNA was detected in a sample of organs from a gray hamster (in Malaya Shishovka village, the Amvrosievsky District), in a sample of imago of ixodid ticks Dermacentor reticulatus collected from a dog (in Amvrosievka, the Amvrosievsky District), and in a sample of Rh. rossicus imago (in Vasilievka village, the Starobeshevsky District).
In the territory of the new subjects, high activity of the natural focus of tularemia was found in the meadow and water-stream areas.
In the DPR, the circulation of F. tularensis was detected in populations of carriers, including the house mouse, European wood mouse, Kurganchik mouse, small wood mouse, small shrew, social vole, common vole, and gray rat, and transmitters such as ixodid ticks D. marginatus, D. reticulatus, Rh. rossicus, H. marginatum, and blood-sucking mosquitoes Aedes caspius, Anopheles hyrcanus, and Coquilettidia richardii. Five cultures of the pathogen were isolated from samples of ixodid ticks Rh. rossicus in Bezymyanny village, the Novoazovsky District.
In field material collected in the territory of the LPR, Ags and specific DNA fragments of F. tularensis were found in samples from SMs of sensitivity group I such as the common shrew, house mouse, Kurganchik mouse, European wood mouse, yellow-throated mouse, pygmy wood mouse, common vole, and red-backed voles, and SMs of sensitivity group II including the gray hamster and forest dormouse, as well as in samples of blood-sucking arthropods including mosquitoes Aedes excrucians, ticks Rh. rossicus and H. Marginatum. A culture of the pathogen was isolated from a sample of H. marginatum in Svetlichny township, the Krasnodonsky District.
Using a bioassay, the F. tularensis strain was isolated from the corpse of a social vole found in the Melitopolsky District of the Zaporozhye Region.
DNA of the causative agent of tularemia was found in a pool of C. pipiens mosquitoes collected in the outskirts of Novogrigorivka village, the Genichesky District, the Kherson Region. Positive results were obtained in RNAb when examining samples of C. pipiens mosquitoes collected in the Skadovsky and Kakhovsky Districts of the Kherson Region although isolation of the tularemia causative agent culture failed.
The genetic material of the WNF virus was detected in pools of Culex pipiens mosquitoes in several areas. In the Melitopolsky and Tokmaksky Districts of the Zaporozhye Region, the contamination of samples, collected from four surveyed areas, ranged from 4.76% to 25.0%, in the Kherson Region, namely, in the town of Gola Prystan of the Skadovsky District, the contamination of samples reached 3.45%.
Specific DNA fragments of ITBB pathogens were found in field material collected in all new regions. In the Zaporozhye Region (the Melitopolsky and Berdyansky Districts) and the Kherson Region (the Genichsky District), the proportion of positive pools of Ixodes redikorzevii ticks ranged from 3.5% to 5.6%; in samples of mouse-like rodents such as the gray hamster, steppe mouse, small forest mouse, and Kurganchik mouse, this index was from 10.9% to 100%. In the DPR, namely, in the Amvrosievsky, Mangushsky, Novoazovsky, Telmanovsky, Starobeshevsky, and Shakhtarsky Districts, the proportion of positive pools in different representatives of ixodid ticks varied. In particular, in ixodid ticks Ixodes ricinus, this index ranged from 33.3% to 100%; in I. redikorzevii it was 63.6%; in D. reticulatus it was from 1.6% to 25%; in D. marginatus it was 10%; and in Rh. rossicus it was 11.1%. The proportion of positive pools in SM samples, including the house mouse, European wood mouse, small forest mouse, yellow-throated mouse, short-legged mouse, small shrew, social vole, common vole, and gray hamster, varied from 19.8% to 100%. In the LPR, namely in the Antratsitovsky, Belovodsky, Lutuginsky, Sverdlovsky, Krasnodonsky, Melovsky, and Stanichno-Lugansky Districts, the proportion of positive pools in different representative of ixodid ticks also ranged. In particular, in I. ricinus this index varied from 20.3% to 100%; in D. reticulatus it was 33%; in D. marginatus it was 50%; and in Rh. rossicus it varied from 25.0% to 63.3%. The proportion of positive pools in SM samples, including the house mouse, small forest mouse, yellow-throated mouse, Kurganchik mouse, small shrew, common vole, red vole, and gray hamster, ranged from 8.6% to 100%.
Rickettsia of the tick-borne spotted fever group were identified in 55 pools of ixodid ticks of the following species: H. marginatum, Haemaphysalis punctata, Hyalomma scupense, Rh. rossicus, I. redikorzevi, D. reticulates. They were revealed in the Zaporozhye Region, namely, in the Berdyansky District, where the proportion of positive samples was 21.1%, as well as in the Vasilievsky District with 33.3% and the Melitopolsky District with 12.5% of positive samples. They were also found in the Kherson Region including the Genichesky District with the 8.0% index and the Novotroitsky District with the 100% index (in the only examined sample).
Markers of the HGA pathogen were detected in four pools of ixodid ticks I. redikorzevi collected in the Berdyansky and Melitopolsky Districts of the Zaporozhye Region. The proportion of positive pools was 2.8% and 1.5%, respectively. In the DPR, specific DNA fragments of Anaplasma phagocytophilum were found in six samples of SMs trapped in the Shakhtarsky, Novoazovsky, Mangushsky, and Telmanovsky Districts, namely, in the gray hamster, the small wood mouse, and the house mouse, as well as in three samples from the common vole and in a pool of imago of ixodid ticks D. marginatus collected from cattle. In the LPR, the genetic material of the HGA pathogen was detected in four pools of ixodid ticks I. ricinus collected on the outskirts of the city of Lugansk with 33.3% of the positive pools.
In the Novoazovsky District of the DPR, the DNA of the causative agent of Q fever was detected in two samples from the European forest mouse. In the LPR, its genetic markers were identified in a sample from the pygmy wood mouse in the Lutuginsky District. The proportion of positive samples was 10% and 4.3%, respectively.
Antibodies to the causative agent of leptospirosis were detected using the RMA method in the pygmy wood mouse trapped in the territory of the Kherson Region in the outskirts of Sivashskoe village, the Genichesky District; the sample contamination was 10%. In addition, leptospires were detected with the PCR method in samples from the Kurganchik mouse and a common vole collected in the territory of the Starobeshevsky District of the DPR, as well as in a sample from the small wood mouse in the Stanichno-Lugansky District of the LPR. The proportion of positive samples was 25%, 23%, and 14.3%, respectively.
In the territory of the DPR specific DNA fragments of Yersinia enterocolitica were found in a sample from a small shrew in the Mangushsky District and in a sample from a wood mouse in the Telmanovsky District. In the LPR, markers of the causative agent of intestinal yersiniosis were detected in four samples from SMs including the common vole (the Lutuginsky District), the small forest mouse (the Sverdlovsky and Krasnodonsky Districts), and the forest dormouse (the Krasnodonsky District).
Markers of the HFRS pathogen were detected in samples from SMs, including the small shrew, house mouse, and European wood mouse, trapped in the territory of the DPR in the Starobeshevsky, Telmanovsky, Shakhtarsky, Novoazovsky, and Amvrosievsky Districts. The proportion of positive samples ranged from 6.6% to 20% over the region. In the LPR, orthohantavirus Ags were found in samples from rodents and insectivores including the common vole, the small shrew, the gray hamster, the European wood mouse, and the small wood mouse, trapped in the biotopes of the Sverdlovsky and Lutuginsky Districts. The proportion of positive samples ranged from 5.8% to 16.6%.
For the first time in the territory of the DPR in the Mangushsky District, spontaneous infection of SMs with the Inko, Sindbis, Batai, and Tyagin viruses was detected. The Sindbis virus Ag was found in a sample from the common vole; the Bataille virus Ag was revealed in samples from the small shrew and the Kurganchik mouse; the Inko virus Ag was in samples from the yellow-throated mouse and the common vole; and the Tyagin virus Ag was in a sample from the European wood mouse.
The circulation of the Inko, Sindbis, and Tyagin viruses was registered for the first time in the territory of the LPR. Inco virus Ag was detected in five samples from SMs including the bank vole, common vole, Kurganchik mouse, and small shrew. Sindbis virus Ag was found in a sample from a small shrew. Tyagin virus Ag was revealed in a sample from the small shrew.
Genetic markers of the TBE virus and HME were not detected during the examination of field material samples.
Molecular genetic analysis
Herein, the genomic nucleic acid sequences in isolates of F. tularensis, Borrelia sp., Rickettsia sp., and CCHF and WNF viruses were examined.
Whole-genome sequencing of the RNA isolate of the CCHF virus identified in a pool of H. marginatum ticks collected in the Berdyansky District of the Zaporozhye Region revealed that this sample belonged to the Europe-1 genetic line, namely, the VaVaVa genovariant, which is widespread in the territory of the natural focus of CCHF in the Russian Federation.
Examining of variants of the WNF virus identified in four pools of C. pipiens mosquitoes collected in the Zaporozhye Region showed that RNA isolates of the virus belonged to genetic line 2, a group of Russian strains.
Whole-genome sequencing of F. tularensis strains, isolated in 2023 in the territory of the DPR in the Novoazovsky and Telmanovsky Districts and in the territory of the LPR in the Krasnodonsky district, showed that they belonged to the B.203 genotype according to the canonical SNP scheme. Thus, these strains coincide with the genotype of the strains isolated in the territory of the Rostov Region [10][11].
The analysis of the nucleotide sequence of the 16S RNA gene fragment allowed detecting the Borrelia species identity in 7 pools of ixodid ticks and 24 SM liver samples. In particular, the genospecies of Borrelia afzelii was determined in 27 isolates (87%), and Borrelia miyamotoi was determined in four isolates (13%). B. afzelii was widespread in the surveyed areas of the Zaporozhye and Kherson Regions; B. miyamotoi was found only in the Zaporozhye Region.
The results of fragmentary sequencing of the ospA gene of B. afzellii made it possible to determine the genetic proximity of the pathogens identified in the Antratsitovsky District of the LPR in 2023 and the pathogens found in 2022 in the territories of the LPR and the Rostov Region.
The species identity of rickettsiae, which was revealed in 31 pools of ticks collected in the Zaporozhye and Kherson Regions, was determined from the nucleotide sequence analysis of fragments of the gltA (552 bp) and OmpB (720 bp) genes. Finally, rickettsia of four genotypes were identified in these territories: Rickettsia aeschlimannii was found in 12 isolates (38.7%), Rickettsia slovaca was in 9 isolates (29.0%), Rickettsia heilongjiangensis was revealed in 6 isolates (19.4%), and Rickettsia conorii was in 4 isolates (12.9%).
The strains of pathogens of natural focal infections identified in new territories in 2023 are genetically close to the strains circulating in the regions of the southern European part of Russia, including the Rostov Region and the Republic of Crimea, which may attest to the existence of common natural foci of infections. Nevertheless, it is necessary to continue planned genetic monitoring in order to determine the characteristics of the territorial distribution of genovariants and establish the boundaries of natural foci of infections. An in-depth genetic characterization of a number of pathogens, including R. conorii, is required to establish the subspecies of the pathogen.
Epidemiological risks
The biogeocenotic systems existing in the new subjects of Russia can maintain epidemic and epizootic processes for all natural focal infections that are relevant for these territories. In addition, the current social conditions contribute to the deterioration of the epidemiological situation with some diseases.
According to scientific sources, in the territory of the DPR, the natural focus of tularemia is located in the coastal, central, and western parts of the region; in the LPR, it is located in the southern and western areas of the region; in the Kherson and Zaporozhye Regions, it is located in the coastal zones. The circulation of F. tularensis was confirmed in populations of carriers and transmitters collected in the territories of all four new regions indicating the high activity of the natural focus.
For the first time, the circulation of the CCHF virus was established using the PCR method in the territories of the Berdyansky and Melitopolsky Districts of the Zaporozhye Region. In addition, the causative agent of CCHF was identified in field material from the Amvrosievsky and Starobeshevsky Districts of the DPR. The index of abundance of H. marginatum tick species on cattle was assessed as epidemiologically significant in all four new subjects.
During an epidemiological examination in relation to the causative agent of ITBB, the species B. afzelii, which is pathogenic for humans, was found in almost all specimens of mouse-like rodents, as well as in a specimen of insectivores and their ectoparasites. Markers of rickettsiae were detected in ticks, collected from different species of SMs, and in mosquitoes. An epizootic of high intensity was observed from April to August. Detecting pathogen markers of ITBB, rickettsiosis, and HGA in a tick sample from the combing of a white-breasted hedgehog, as well as ITBB and rickettsiosis in a tick sample from the combing of a Kurganchik mouse, indicated the existence of not only combined but also coupled foci of infections.
Owing to the conducted work, the time and risk groups for natural focal infections were determined, which allows making certain generalizations.
- Its natural focus is active year-round; the greatest risk of human disease accrues to the autumn-winter period; risk groups include military personnel, agricultural workers, rural residents, fishermen, and hunters in the case of resumption of hunting permits;
- Its natural focus is active, as a rule, from April to October; risk groups are rural residents associated with the breeding of cattle and small cattle and medical workers;
- ITBB and rickettsiosis. The active functioning of combined and coupled foci can continue year-round at positive average daily temperatures; the greatest risk accrues to the period from July to October; risk groups are military personnel, rural residents, and summer residents;
- WNF and leptospirosis. Their natural foci, as a rule, are inactive when the average daily water temperature passes below 13 °C, that is, from October to April; the greatest risk accrues to the period from May to September; risk groups are military personnel, rural residents, fishermen, and hunters in the case of the resumption of hunting permits.
In the future, the work aimed at clarifying the spatial boundaries of natural foci and risk areas for infections in new regions of Russia should be continued.
Thus, the main endangerments to the local population, military personnel, and civilians temporarily located in the regions are associated with the following factors:
- circulation of pathogens of natural focal infections confirmed during a laboratory examination of field material including tularemia, CCHF, WNF, ITBB, rickettsial infections, HGA, leptospirosis, HFRS, Q fever, intestinal yersiniosis, Bataille, Inco, Sindbis, and Tyagin fevers;
- a large number of susceptible individuals who are in close contact with carriers and transmitters of infections in the field environment, in particular, military personnel;
- possibility of an increase in the numbers of infection carriers (SMs) due to favorable conditions and the availability of food supply in areas of military contingent deployment, and an increase in the numbers of lagomorphs due to the hunting ban.
Conclusion
Within the frame of the implementation of the Comprehensive Plan in 2023, data on carriers and transmitters, circulating variants of pathogens of natural focal infections, and their genetic characteristics were obtained. This information served as the basis for determining the areas, times, and populations at risk in relation to infections.
The main epidemiological endangerments to the local population and those temporarily located in the territories of the new subjects of Russia are represented by the existing natural foci of tularemia, CCHF, WNF, leptospirosis, combined and coupled foci of ITBB and rickettsiosis. New information was obtained during the conducted work:
- for the first time, the circulation of the CCHF virus was detected in the Zaporozhye Region, namely in the Berdyansky and Melitopolsky Districts, with the PCR method; the results of whole-genome sequencing made it possible to detect belonging of the RNA isolate of the CCHF virus from the Berdyansky District to the genetic line Europe-1, the genovariant VaVaVa, which is widespread in the territory of the natural focus of CCHF in the Russian Federation;
- for the first time in the territories of the DPR and LPR, the circulation of the Inko, Sindbis, Tyagin, and Batai viruses was established in populations of SMs, namely in rodents and insectivores;
- in the territories of the Kherson and Zaporozhye Regions, the presence of combined and coupled natural foci of ITBB, rickettsiosis, and HGA was revealed;
- the molecular genetic analysis determined that the causative agents of natural focal infections revealed in 2023, including tularemia, CCHF, WNF, and ITBB, are genetically close to the strains circulating in the regions of the south of the European part of Russia.
These data should be used when planning and arranging surveillance and preventive activities in 2024.
References
1. Putintseva E.V., Udovichenko S.K., Nikitin D.N., Borodai N.V., Shpak I.M., et al. West Nile Fever: Results of Monitoring over the Causative Agent in the Russian Federation in 2021, the Incidence Forecast for 2022. Problems of Particularly Dangerous Infections. 2022;(1):43-53. (In Russ.) https://doi.org/10.21055/0370-1069-2022-1-43-53
2. Hightower J, Kracalik IT, Vydayko N, Goodin D, Glass G, Blackburn JK. Historical distribution and host-vector diversity of Francisella tularensis, the causative agent of tularemia, in Ukraine. Parasit Vectors. 2014;7:453. https://doi.org/10.1186/s13071-014-0453-2
3. Ukhovskyi V, Pyskun A, Korniienko L, Aliekseieva H, Moroz O, et al. Serological prevalence of Leptospira serovars among pigs in Ukraine during the period of 2001–2019. Vet Med-Czech. 2022;67(1):13-27. https://doi.org/10.17221/50/2021-VETMED
4. Kovryha N, Tsyhankova A, Zelenuchina O, Mashchak O, Terekhov R, Rogovskyy AS. Prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum in Ixodid Ticks from Southeastern Ukraine. Vector Borne Zoonotic Dis. 2021;21(4):242-246. https://doi.org/10.1089/vbz.2020.2716
5. Yurchenko OO, Dubina DO, Vynograd NO, Gonzalez JP. Partial Characterization of Tick-Borne Encephalitis Virus Isolates from Ticks of Southern Ukraine. Vector Borne Zoonotic Dis. 2017;17(8):550-557. https://doi.org/10.1089/vbz.2016.2094
6. Lozynskyi I, Shulgan A, Zarichna O, Ben I, Kessler W, et al. Seroprevalence of Old World Hantaviruses and Crimean Congo Hemorrhagic Fever Viruses in Human Populations in Northwestern Ukraine. Front Cell Infect Microbiol. 2020;10:589464. https://doi.org/10.3389/fcimb.2020.589464
7. Nebogatkin I, Onishchuk O, Hnatiuk O, Erber W, Vuković- Janković T. TBE in Ukraine. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. https://doi.org/10.33442/26613980_12b34-6
8. Romanenko T.A., Skripka L.V. The analysis of the incidence of tularemia in the population of the Donetsk region. University Clinic. 2021;4(41):100–107. (In Russ.). https://doi.org/10.26435/uc.v0i4(41).750
9. Efremenko D.V., Kuznetsova I.V., Orobey V.G., Efremenkо А.А., Dubyanskiy V.M. et al. Risk-oriented approach application at planning and orginizing antiepidemic provision of mass events. Health Risk Analysis. 2017;1:4–12. (In Russ.). https://doi.org/10.21668/health.risk/2017.1.01 10.
10. Vodop’yanov A.S., Pisanov R.V., Vodop’yanov S.O., Tsimbalistova M.V., Pichurina N.L., et al. Comparative Molecular-Genetic Analysis of Francisella tularensis Strains Isolated in the Rostov Region in 2020 and Genome Sequences of the Strains Collected in Various Regions of the World. Problems of Particularly Dangerous Infections. 2023;(3):59-65. (In Russ.) https://doi.org/10.21055/0370-1069-2023-3-59-65
11. Kudryavtseva T.Yu., Popov V.P., Mokrievich A.N., Kulikalova E.S., Kholin A.V., et al. Multidrug Resistance of F. tularensis subsp. holarctica, Epizootiological and Epidemiological Analysis of the Situation on Tularemia in the Russian Federation in 2022 and Forecast for 2023. Problems of Particularly Dangerous Infections. 2023;(1):37-47. (In Russ.) https://doi.org/10.21055/0370-1069-2023-1-37-47
About the Authors
A. Yu. PopovaРоссия
Anna Yu. Popova, Dr. Sci. (Med.), Professor, Head; Chief State Sanitary Physician of the Russian Federation, Head of Department of Organization of the Sanitary and Epidemiological Service
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
A. N. Kulichenko
Россия
lexander N. Kulichenko, Academician of the Russian Academy of Sciences, Dr. Sci. (Med.), Professor, Head
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
A. K. Noskov
Россия
Alexey K. Noskov, Cand. Sci. (Med.), Head
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
D. V. Efremenko
Россия
Dmitriy V. Efremenko, Cand. Sci. (Med.), Leading Researcher, Laboratory of Epidemiology
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
A. S. Volynkina
Россия
Anna S. Volynkina, Cand. Sci. (Bio.), Head of Laboratory for Diagnostics of Viral Infections
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
N. V. Tsapko
Россия
Nikolay V. Tsapko, Cand. Sci. (Bio.), Biologist, Laboratory of Medical Parasitology
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
E. S. Kotenev
Россия
Egor S. Kotenev, Cand. Sci. (Bio.), Head of Laboratory of Microbiology of Plague
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
O. V. Maletskaya
Россия
Olga V. Maletskaya, Dr. Sci. (Med.), Professor, Deputy Director for Anti-Epidemic Work
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
S. A. Kurcheva
Россия
Svetlana A. Kurcheva, Cand. Sci. (Bio.), Senior Researcher, Laboratory of Diagnostics of Viral Infections
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
O. V. Vasilyeva
Россия
Oksana V. Vasilyeva, Cand. Sci. (Bio.), Head of Laboratory of Diagnostics of Bacterial Infections
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
A. Yu. Gazieva
Россия
Alina Yu. Gazieva, Cand. Sci. (Bio.), Head of Laboratory of Medical Zoology
Moscow
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
O. P. Dobrovolsky
Россия
Oleg P. Dobrovolsky, Cand. Sci. (Med.), Acting Head of the Zoological and Parasitological Research Group
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
M. V. Zabashta
Россия
Marina V. Zabashta, Cand. Sci. (Med.), Senior Researcher of Zoological and Parasitological Research Group
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
A. P. Khametova
Россия
Anna P. Khametova, Junior Researcher of Laboratory of Experimental Biological Models and Biological Safety
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
N. V. Panasyuk
Россия
Nikita V. Panasyuk, Cand. Sci. (Med.), Zoologist of Zoological and Parasitological Research Group
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
O. S. Chemisova
Россия
Olga S. Chemisova, Cand. Sci. (Bio.), Acting Deputy Director for Scientific and Experimental Work, Head of the Laboratory «Collection of Pathogenic Microorganisms»
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
A. V. Tsai
Россия
Alexander V. Tsai, Junior Researcher of Epidemiology Department
Rostov-on-Don
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
N. Ye. Ananyeva
Россия
Natalya E. Ananyeva, Head
Donetsk
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
D. A. Dokashenko
Россия
Dmitriy A. Dokashenko, Head
Lugansk
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
N. V. Khattatova
Россия
Natalya V. Khattatova, Head
Melitopol
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
V. M. Turov
Россия
Vladimir M. Turov, Head
Genichesk
Competing Interests:
Авторы заявляют об отсутствии конфликта интересов.
Review
For citations:
Popova A.Yu., Kulichenko A.N., Noskov A.K., Efremenko D.V., Volynkina A.S., Tsapko N.V., Kotenev E.S., Maletskaya O.V., Kurcheva S.A., Vasilyeva O.V., Gazieva A.Yu., Dobrovolsky O.P., Zabashta M.V., Khametova A.P., Panasyuk N.V., Chemisova O.S., Tsai A.V., Ananyeva N.Ye., Dokashenko D.A., Khattatova N.V., Turov V.M. Epizootological situation and epidemiological risks for natural focal infections in the territory of new subjects of the Russian Federation (Donetsk People's Republic, Lugansk People's Republic, Zaporozhye and Kherson regions). Medical Herald of the South of Russia. 2024;15(1):7-18. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-7-18
JATS XML







































