Scroll to:
Age aspect of the course of new coronavirus infection in the acute and post-Covid in children
https://doi.org/10.21886/2219-8075-2024-15-2-90-100
Abstract
Objective: studying the influence of various factors on the course and outcomes of COVID-19 in children of different ages, to determine patient management tactics.
Materials and methods: the article presents the results of a retrospective assessment of the course of COVID-19 in 89 children observed in clinics of Samara city. A comparative analysis of the course of COVID-19 and post-COVID in children of different ages was carried out. Statistical analysis was carried out by IBM SPSS Statistica 25, p<0.05 was considered the criterion for significance.
Results: the severity of COVID-19 was independent of age. For children from 3 to 6 years of age, a severe course of ARI in anamnesis mattered, and in the group of children from 7 to 11 years old - compliance with the national calendar of prophylactic vaccinations. Post-COVID had different frequency and duration. In children older than 7 years old, asthenovegetative syndrome occurred more often and was quickly eliminated. In children aged 3-6 years asthenovegetative and dyspeptic syndromes disappeared during a longer period. 2 - 3 months after recovery from COVID-19, exacerbation of allergic diseases was observed in children over 3 years old. Decrease in immunoresistance occurred one month after recovery in children under 6 years of age.
Conclusions: it is important to take into account the age-related characteristics of the course of COVID-19 and post-COVID to predict the risks of severe COVID-19 and deterioration of the child’s health.
For citations:
Kiryutkina A.P., Migacheva N.B., Kaganova T.I., Burmistrov V.V., Ginzburg A.S. Age aspect of the course of new coronavirus infection in the acute and post-Covid in children. Medical Herald of the South of Russia. 2024;15(2):90-100. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-2-90-100
Introduction
Three years after the official announcement of the new coronavirus infection (COVID-19) pandemic, WHO reported its termination (spring 2023). In this regard, its degree of importance for society was reduced, yet COVID-19 is still a pressing problem for the healthcare system. According to the Russian Ministry of Health, since the beginning of the coronavirus pandemic, about 560 thousand children contracted the infection in Russia, which comprises about 11% of the total number of cases at that time – 4.96 million (source: Interfax 2021); 350–400 children infected with COVID-19 died (infant mortality is 0.005% of cases (Altshtein, 2021)). At the same time, a severe form of the disease in children, according to Professor A.V. Gorelov, Deputy Director for Research at the Central Research Institute of Epidemiology of Rospotrebnadzor, was recorded extremely rarely – in 0.2% of cases. COVID-19 is a rare case when an etiological study is carried out not only at the stage of specialized care; it has become routine in the provision of primary health care in the Russian Federation. This ensured wide testing coverage, reliable differentiation of COVID-19 from other viral infections in children, and collection of large study material for this dangerous and unpredictable infection. Unlike the course of COVID-19, which is milder in children than in adults, as proven by numerous studies [1][2], the post-COVID period in children does not differ so positively from that in adults [3]. To date, most of the published studies of long-term COVID-19 are mainly focused on the adult population, and information on children is limited. In children and adolescents, the most common symptoms are weakness, fatigue, headache, shortness of breath, abdominal pain, cephalalgia, muscle pain, as well as recurring acute respiratory infections (ARI) [4][5]. Risk factors include age, history of allergic reactions, severe COVID-19, excess weight, neurological diseases, and respiratory pathology [6][7].
Thus, post-COVID syndrome issues in children remain unclear. This is also evidenced by the lack of unified terminology and classification of the post-COVID period. Numerous authors use the following terms interchangeably: long-term COVID-19, post-acute sequelae of COVID-19 (PASC), post-COVID, and COVID syndrome [3][8][9]. The guidelines for the management of children with COVID-19 adopted in the Russian Federation also lack a clear gradation of post-COVID syndrome. The priority is given to the observation of children who had lung damage due to COVID-19, and the main principle of organizing comprehensive rehabilitation of children after COVID-19 is the work of a multidisciplinary rehabilitation team (MRT). Rehabilitation is 2–8 weeks, depending on the severity of the disorders1, but it is already known that the course of the post-COVID period is not limited to these [10]. At the same time, more attention is paid to multisystem inflammatory syndrome in children (MIS-C)2, while other syndromes remain poorly studied, despite their high prevalence [3]. There are no MRTs in children's clinics, but this does not exclude the need for rehabilitation of children after COVID-19, and the role of the MRT is performed by the local pediatrician alone at the initial stage. In the absence of clear clinical guidelines for supporting children with post-COVID syndrome, the pediatrician is forced to manage such patients based only on personal professional experience, in each case individually. Given all of the above, studying the course of COVID-19 and post-COVID syndrome in children is a pressing problem in pediatrics.
The preset research aims to analyze the influence of various factors on the course and outcomes of COVID-19 in children of different age groups in order to clarify the tactics of managing children at the outpatient stage.
Materials and methods
The paper presents the results of a retrospective assessment of the course of COVID-19 in children of different age groups and their health status over the next 3 months after recovery. The study was conducted at the bases of children's clinics in the Kuibyshevsky, Samarsky, and Promyshlenny districts of the city of Samara and included observation and examination of children who had recovered from COVID-19.
Data for the study was collected by questioning parents, studying medical history, and conducting instrumental diagnostic examinations of children. The survey was conducted in 3 stages: parents filled out a questionnaire after the child's recovery, and observations took place 1 and 3 months after recovery. The study included children who met the following criteria: aged 1–17 at the time of illness, a confirmed case of COVID-19 in the medical history, and consent of the legal representative to participate in the study and to process personal data. The final assessment included 89 children who met all the above criteria.
The IBM SPSS Statistica 25 software was used to evaluate the study results. Quantitative data are presented as the mean (M) and standard deviation (SD) under the condition of compliance with the normal distribution, and as the median (Me) and the upper (Q1) and lower (Q3) quartiles if they do not correspond to the normal distribution. The Shapiro-Wilk test was used to assess compliance with the normal distribution. The nonparametric Kruskal-Wallis test was used to compare quantitative variables that do not correspond to the normal distribution, after which, if significant differences were detected, pairwise comparison was performed using the Mann-Whitney U test with Bonferroni correction. Binary qualitative variables are presented as absolute frequencies and percentages; the comparison was performed using contingency tables using the Chi-square test or two-sided Fisher's exact test. Differences were considered statistically significant at the significance level of p<0.05.
Results
To achieve the set goal, all the children (n = 89) were divided into 4 groups depending on age. Group 1 included children aged 1–2 (n = 24), Group 2 included children aged 3–6 (n = 22), Group 3 included children aged 7–11 (n = 20), and children aged 12–17 made up Comparison Group 4 (n = 23). No significant differences by gender were found in the four age groups (p>0.05). Table 1 presents the distribution by gender in different groups.
Таблица / Table 1
Распределение по полу в разных группах
Distribution by sex in different groups
|
I группа I group n=24 |
II группа II group n=22 |
III группа III group n=20 |
IV группа IV group n=23 |
Всего Total n=89 |
|
|
Мальчики абс. Boys abs. |
14 |
13 |
12 |
10 |
49 |
|
Мальчики, % Boys, % |
58,3% |
59,1 |
60,0 |
43,5 |
55,1 |
|
Девочки, абс. Girls, abs. |
10 |
9 |
8 |
13 |
40 |
|
Девочки, % Girls, % |
41,7% |
40,9 |
40,0 |
56,5 |
44,9 |
χ² = 1.692 df = 3 p = 0.639
First of all, the medical history of the children was studied. No differences were found in the 4 age groups regarding complicated obstetric history, complicated pregnancy and childbirth. Perinatal CNS damage and its consequences were significantly more common in children aged 2–6 (Group 2) and amounted to 8 people (36.4%) (χ² = 5.150; df = 3; p = 0.023). Table 2 presents the perinatal and neonatal history.
Таблица / Table 2
Перинатальный и неонатальный анамнез
Perinatal and neonatal anamnes
|
I группа I group n=24 |
II группа II group n=22 |
III группа III group n=20 |
IV группа IV group n=23 |
Всего Total n=89 |
Критерий Хи² Criterion χ² |
|
|
Абс. /Abs. (%) |
Абс. /Abs. (%) |
Абс. /Abs. (%) |
Абс. /Abs. (%) |
Абс. /Abs. (%) |
||
|
Отягощенный акушерский анамнез Compromised obstetrical history % |
6 (25,0) |
6 (27,3) |
7 (35,0) |
7 (30,4) |
26 (29,2) |
χ2=0,587 df=3 р=0,900 |
|
Осложнённое течение беременности Complicated pregnancy % |
21 (87,5) |
18 (81,8) |
15 (75,0) |
19 (82,6) |
73 (82,0) |
χ2=1,163 df=3 р=0,762 |
|
Физиологические роды Normal birth % |
15 (62,5) |
15 (68,2) |
14 (70,0) |
14 (60,9) |
58 (65,2) |
χ2=0,556 df=3 р=0,906 |
|
Инфекционно-воспалительные заболевания Infectious and inflammatory diseases % |
2 (8,3) |
7 (31,8) |
4 (20,0) |
5 (21,7) |
18 (20,2) |
χ2=3,969 df=3 р=0,265 |
|
Перинатальное поражение ЦНС Perinatal CNS lesions % |
1 (4,2) |
8 (36,4) |
2 (10,0) |
5 (21,7) |
16 (18,0) |
χ2=9,232 df=3 р=0,026 |
Considering the data on the positive effect of vaccination on the course of COVID-19 [11][12] and on vaccination violation during the pandemic [13], the children’s vaccination history analysis was included in the study.
The vaccination history was determined by one of 3 options: no vaccinations (a refusal was issued) – 1 case (1.1%), vaccinated in violation of the national calendar, according to an individual schedule – 20 cases (22.5%), having all the vaccinations required by age – 68 cases (76.4%). Assessment of the vaccination history showed differences in children of different age groups: the proportion of those vaccinated according to the national calendar is higher with age (χ² = 18.526; df = 6; p = 0.005).
Fig. 1 shows the distribution of vaccination history in different age groups.
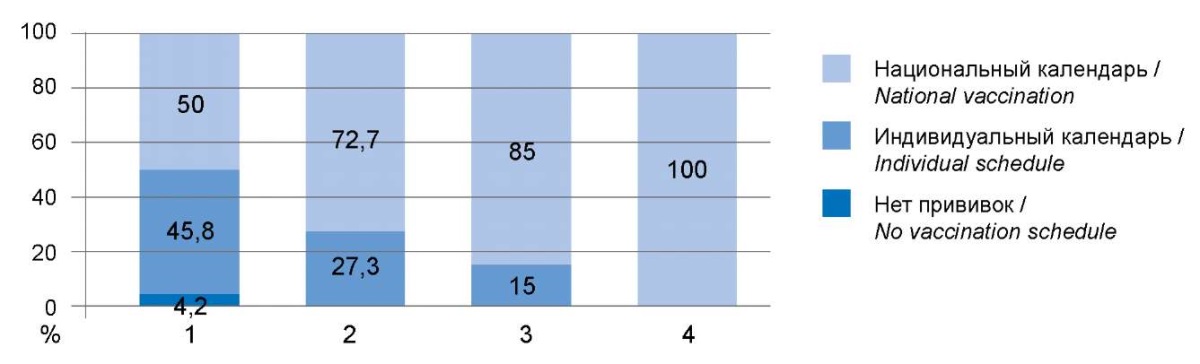
Рисунок 1. Оценка вакцинального анамнеза
в зависимости от возраста обследованных детей.
Figure 1. Evaluation of vaccination according to the age of children.
To assess the level of immunological resistance, ARI history during the year before the case of COVID-19 was analyzed according to the following features: the number of viral infections (including complicated ones) and criteria that allow classifying the child as frequently ill. Fig. 2 shows the number of ARI episodes.
The frequency of ARI before the case of COVID-19 was significantly higher in Group II (Me 6.0 Q1 4.0; Q3 7.5). Moreover, children in this group more often had severe or complicated ARIs – 17 cases (77.3%) (χ² = 7.66; df = 3; p = 0.005), and 17 children were classified as frequently ill, which is higher than in other age groups (χ² = 9.81; df = 3; p = 0.002).
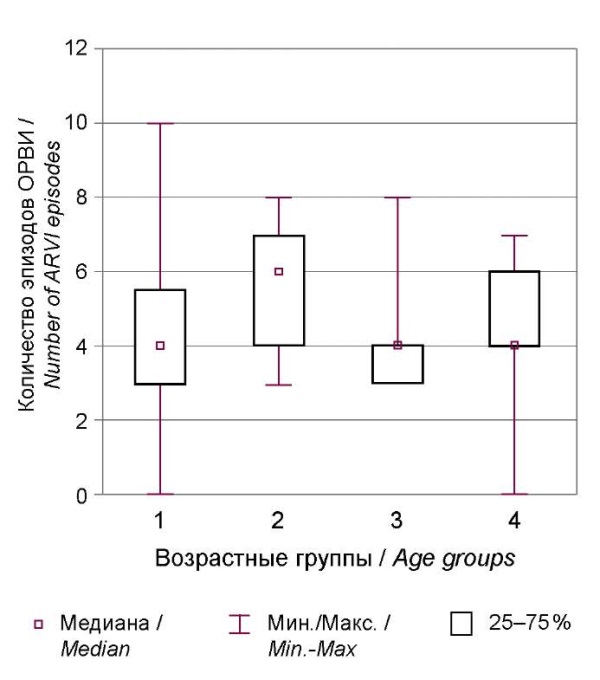
Рисунок 2. Количество ОРИ, перенесённых в течение года до развития COVID-19
в зависимости от возраста детей.
Figure 2. Number of ARI suffered during the year before COVID-19
depending on age.
Patients with chronic diseases are at risk of unfavorable course of COVID-19; various publications confirm the hypothesis in relation to adults [14]. Therefore, the authors of the present study analyzed the frequency of various somatic and neurological diseases in children who had COVID-19 (Table 3). Allergic diseases were detected somewhat more often in Group 2 (50%), but the differences were not significant (p = 0.933). Chronic pathology of ENT organs was more common in Group 2, namely in 9 cases (40.9%), in Group 1, only in 1 case (4.2%), in Group 3, in 4 children (20%) and in Group 4, in 2 children (8.7%) (χ² = 12.349; df = 3; p = 0.006). Diseases of the nervous system occurred with approximately the same frequency in Groups 1 and 2: in 6 cases in both groups (25% and 27.3%, respectively). In Group 3, 10% of children were observed due to a nervous system disease. Children in Group 4 had no changes in their neurological status, which statistically significantly distinguished them from younger children (χ² = 8.556; df = 3; p = 0.036). Moreover, no significant differences were found in the frequency of drug intolerance and anemia in different groups, as shown in Table 3.
Таблица / Table 3
Сопутствующая патология у обследованного контингента детей в возрастном аспекте
Comorbidities in the examined group of children in the age aspect
|
I группа I group n=24 |
II группа II group n=22 |
III группа III group n=20 |
IV группа IV group n=23 |
Всего Total n=89 |
Критерий Хи² Criterion χ2 |
|
|
Абс. /Abs. (%) |
Абс. /Abs. (%) |
Абс. /Abs. (%) |
Абс. /Abs. (%) |
Абс. /Abs. (%) |
||
|
Аллергические заболевания Allergic diseases |
10 (41,7) |
9 (40,9) |
10 (50,0) |
10 (43,5) |
39 (43,8) |
χ2=0,432 df=3 р=0,9033 |
|
Заболевания ЛОР-органов Diseases of the ENT organs |
1 (4,2) |
9 (40,9) |
4 (20,0) |
2 (8,7) |
16 (18,0) |
χ2=12,349 df=3 р=0,006 |
|
Заболевания органов дыхания Respiratory diseases |
1 (4,2) |
3 (13,6) |
4 (20,0) |
0 (0) |
8 (9,0) |
χ2=6,499 df=3 р=0,090 |
|
Неврологическая патология Neurological disorders |
6 (25) |
6 (27,3) |
2 (10,0) |
0 (0) |
14 (15,7) |
χ2=8,556 df=3 р=0,036 |
|
Анемия Anemia |
4 (16,7) |
0 (0) |
1 (5,0) |
1 (4,3) |
6 (6,7) |
χ2=5,657 df=3 р=0,130 |
|
Заболевания ЖКТ Digestive system diseases |
2 (8,3) |
2 (9,1) |
1 (5,0) |
4 (17,4) |
9 (10,1) |
χ2=2,025 df=3 р=0,567 |
|
Лекарственная непереносимость Drug allergy |
1 (4,2) |
0 (0) |
2 (10,0) |
3 (13,0) |
6 (6,7) |
χ2=3,634 df=3 р=0,304 |
Course of coronavirus infection in children of different age groups
The vast majority of children with both mild and moderate severity in the present study received outpatient treatment, despite the fact that, according to guidelines for the treatment of COVID-19 in children, patients with moderate and severe disease are put in a specialized infectious diseases hospital (for the treatment of patients with COVID-19)3. This is explained by the fact that the authors assessed the severity of COVID-19 retrospectively, based on the maximum temperature during the illness, severity of intoxication, respiratory tract damage, and gastrointestinal disorders. During direct observation of children, the maximum temperature was recorded in the first days of the disease, which corresponded to the first visit and collection of material for analysis; when a diagnosis of a new coronavirus infection was established (ICD-10 U07.1), children showed positive dynamics in their condition and required no hospitalization.
No statistically significant differences were found in the severity of COVID-19 in different age groups.
Table 4 presents the characteristics of the course of COVID-19. In Groups 1, 3, and 4, most children had mild COVID-19 (58.3; 60.0; 69.6%, respectively), in Group 2, only 50% of cases had mild COVID-19 (p>0.05). The authors analyzed the dependence of the severity of COVID-19 on the frequency of severe ARIs in the anamnesis and obtained a reliable significant relationship in children in Group 2: children with moderate COVID-19 more often had a history of severe ARIs. Out of 11 with moderate COVID-19, 9 children (81.8%) had a history of severe ARIs (χ² = 8.054; df = 3; p = 0.045). In addition, the authors assessed the effect of vaccination status on the course of COVID-19. The proportion of vaccinated children with mild COVID-19 was higher than that of children with moderate severity in all age groups. However, a reliable significant relationship between vaccination and severity of the disease was found only in Group 3: 12 children had a mild form of the disease, all of them were fully vaccinated (100%), and among 8 children with moderate severity of COVID-19, only 5 children (62.5%) were fully vaccinated (χ² = 5.294; df = 1; p = 0.021).
Таблица / Table 4
Характеристика течения COVID-19 у детей в зависимости от возраста
Features of the clinical course of COVID-19 in children depending on age
|
Признак |
I группа I group n=24 |
II группа II group n=22 |
III группа III group n=20 |
IV группа IV group n=23 |
Всего Total n=89 |
Критерий Хи² Criterion χ2 |
|
Абс./Abs. (%) |
Абс./Abs. (%) |
Абс./Abs. (%) |
Абс./Abs. (%) |
Абс./Abs (%) |
||
|
Лёгкая степень тяжести течения COVID-19 Mild course |
14 (58,3) |
11 (50,0) |
12 (60,0) |
7 (30,4) |
53 (59,6) |
χ2=1,807 df=6 р=0,613 |
|
Средняя степень тяжести течения COVID-19 Moderate course |
10 (41,7) |
11 (50,0) |
8 (40,0) |
16 (69,6) |
36 (40,4) |
|
|
Итоксикационный синдром Intoxication |
19 (79,2) |
20 (90,9) |
19 (95,.0) |
20 (87,0) |
78 (87,6) |
χ2=2,88 df=3 р=0,421 |
|
Катаральный синдром Catarrhal syndrome |
21 (87,5) |
20 (90,9) |
18 (90,0) |
23 (100,0) |
82 (92,1) |
χ2=2,846 df=3 р=0,416 |
|
Ларинготрахеит Laryngotracheitis |
4 (16,7) |
7 (31,8) |
4 (20,0) |
10 (43,5) |
25 (28,1) |
χ2=5,046 df=3 р=0,168 |
|
Диспепсия Dyspepsia |
3 (12,5) |
6 (27,3) |
3 (15,0) |
3 (13,0) |
15 (16,9) |
χ2=2,316 df=3 р=0,509 |
|
Синдром бронхообструкции Bronchial obstruction |
1 (4,2) |
0 (0) |
1 (5,0) |
1 (4,3) |
3 (3,4) |
χ2=1,045 df=3 р=0,790 |
|
Дизосмия /дисгевзия Dysomnia /dysgeusia |
0 (0,0) |
4 (18,2) |
2 (10,0) |
2 (8,7) |
8 (9,0) |
χ2=4,671 df=3 р=0,198 |
|
Антибиотикотерапия Antibiotic therapy |
1 (4,2) |
5 (22,7) |
3 (15,0) |
3 (13,0) |
12 (13,5) |
χ2=3,441 df=3 р=0,329 |
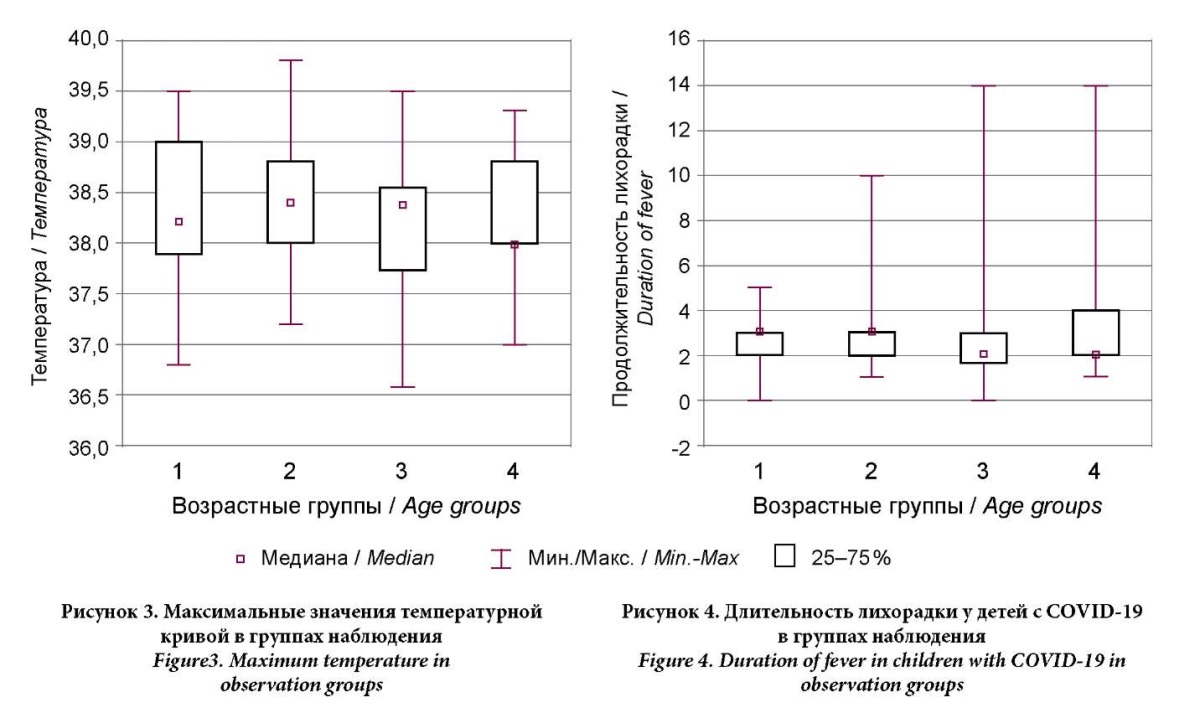
The authors analyzed the characteristics of fever in children of different ages. The average temperature in all four groups ranged from 38.2 °C to 38.4 °C. The maximum body temperature was higher in Group 2 (39.8 °C); in Groups 2 and 3, it was equal (39.5 °C), and in Group 4, it did not exceed 39.3 °C (Figs. 3, 4). The differences in the maximum temperature and duration of fever in COVID-19 were not significant (H(3,N = 89) = 1.311; p = 0.726 and H(3,N = 89) = 0.856; p = 0.836), but the duration and maximum values of fever are expected to correlate with each other. Thus, with higher values of the temperature curve, a longer duration of fever can be expected in children with COVID-19, regardless of the child's age (r = 0.25; p = 0.017).
Intoxication, catarrhal and dyspeptic syndrome, and respiratory damage were recorded with equal frequency in different age groups. Notably, when analyzing the entire sample of cases, antibacterial therapy within a year before the disease is significantly associated with intoxication syndrome in the development of COVID-19 clinical symptoms (Fig. 5). Intoxication was more often observed in those children who received repeated courses of antibiotics. This can be explained by a decrease in the body's reactivity against the background of diseases for which antibacterial drugs were prescribed and by the immunosuppressive effect of the antibiotics themselves.
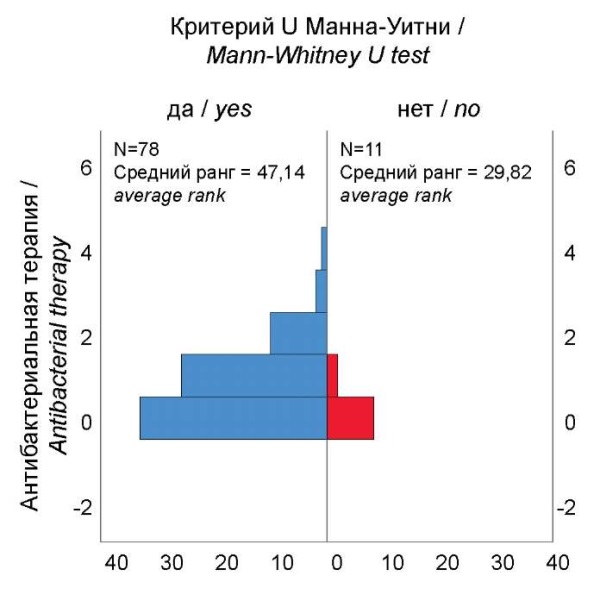
Рисунок 5. Частота интоксикационного синдрома при COVID-19
в зависимости от количества курсов антибактериальной терапии в течение года.
Figure. 5. Frequency of intoxication syndrome of the COVID-19
depending on the number of courses of antibiotic therapy during the year.
Etiotropic treatment of COVID-19 was carried out according to the guidelines for the management of COVID-19 in children and depended on age. Therefore, the use of regimens differs significantly in different age groups. Thus, in Groups 3 and 4, interferon inducers were used significantly more often, which corresponds to 45% and 74% (p = 0.000), and in Groups 1 and 2, interferon monotherapy was used significantly more often (67% and 41% of cases, p = 0.000).
Characteristics of the post-COVID period in children of different ages
The post-COVID period is characterized by symptoms of post-COVID syndrome for 3 months after recovery, the development of exacerbations of chronic diseases, and the deterioration of the immune reactivity of the body.
The most common manifestation of post-COVID syndrome is asthenic vegetative syndrome. Asthenic vegetative syndrome was significantly less common in Group 1 (χ² = 16.05; df = 3; p = 0.001); in Groups 3 and 4, the frequency was the same. In a significant number of cases, it was stopped 3 months after recovery, and in children aged 3–6, asthenic vegetative syndrome persisted longer (Fig. 6).
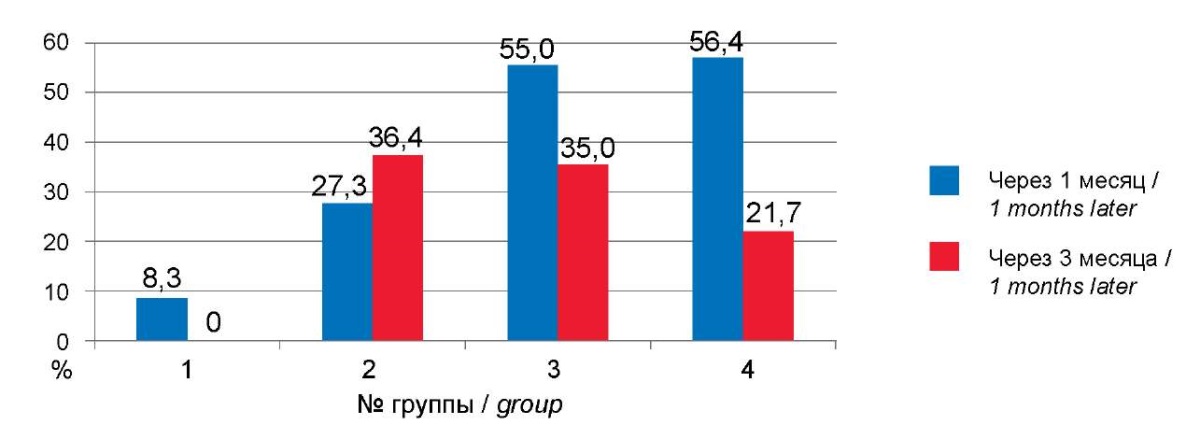
Рисунок 6. Динамика астеновегетативного синдрома в постковидном периоде.
Figure 6. Dynamics of asthenovegetative syndrome of the post-COVID.
The frequency of arthralgia did not differ significantly and amounted to 9.1% in Group 2, to 5% in Group 3, and to 13% in Group 4; in Group 1, cases of arthralgia were not recorded at all (χ² = 3.477; df = 3; p = 0.324). Notably, arthralgia was relieved in all cases in Groups 3 and 4 and only in half of the cases in Group 2. Long-term persistence of COVID-19 symptoms did not differ significantly in age groups and was completely relieved within the next 2 months in children of all ages. The largest number of children with digestive disorders after suffering from COVID-19 were aged 1–2: 4 (16.7%) cases were recorded at 1 month and 2 cases after 3 months, but complaints were noted in other children (McNemar = 0.167; p = 0.684). In Group 2, there were 2 cases, 1 during the next observation (p = 1.0); in Groups 3 and 4, complaints were noted in 3 and 2 children, respectively, only in the first month after recovery (p = 0.084, p = 0.480). In Groups 1 and 2, dyspepsia was longer lasting, but the described differences were not statistically significant.
Anemia after recovery was noted in children in all 4 groups: two cases in Group 1 and one case in each of the other groups. The differences are not significant, yet in Groups 2 and 4, anemia was no longer detected during the follow-up examination, and in Groups 1 and 3, the number of children with anemia remained the same after 3 months.
According to the authors’ data, exacerbations of allergic diseases were significantly higher 3 months after COVID-19 and were 15.7% compared to the first month of the post-COVID period, 10.1% (χ² = 24.1; p = 0.000); notably, they increased due to new cases (McNemar p = 0.18). No significant differences were found in the frequency of allergic diseases in different age groups (p > 0.05). Fig. 7 presents exacerbations of allergic diseases at different ages and shows that an increase in allergic diseases is to be expected at a later date at the age of 3-17.
The immunosuppressive effect of COVID-19 is discussed in the literature [15], so the authors of the present research analyzed the course and frequency of severe viral infections in children after recovery from COVID-19. No significant differences were found in the number of cases or the frequency of ARI in children of different age groups after COVID-19 in the first month. When assessing the entire statistical sample in all age groups, the number of ARI episodes was significantly higher after 3 months than in the first month after COVID-19 (Fig. 8, 9). When analyzing the severity of ARI, clinical manifestations of complicated ARI were significantly more common after 3 months (McNemar = 4.0; p = 0.046) compared to 1 month of observation after COVID-19. In the first month of the post-COVID period, in most cases (more than 50%), no relapses of ARI were observed (Fig. 8). Over the next 2 months, the number of children who suffered from severe ARIs increased, most pronounced in Group 2 and amounting to 9 (40.9%) (p<0.05), and the average number of ARIs increased to 1. In Group 1, the average number of ARI episodes also increased and amounted to 1.4 episodes, but severe forms of ARI were observed only in 2 children. In Groups 3 and 4, the average number of ARI cases remained approximately the same as in the previous observation and did not exceed 0.6 episodes, and severe forms did not exceed 2 (8.3%) cases. Thus, a decrease in the immunological resistance of the body is to be expected a month after recovery at the age of 1-6; at the age of 1-2 in the form of more frequent viral infections, and at the age of 3-6 in the form of a more severe course of respiratory infections, which is prognostically less favorable.
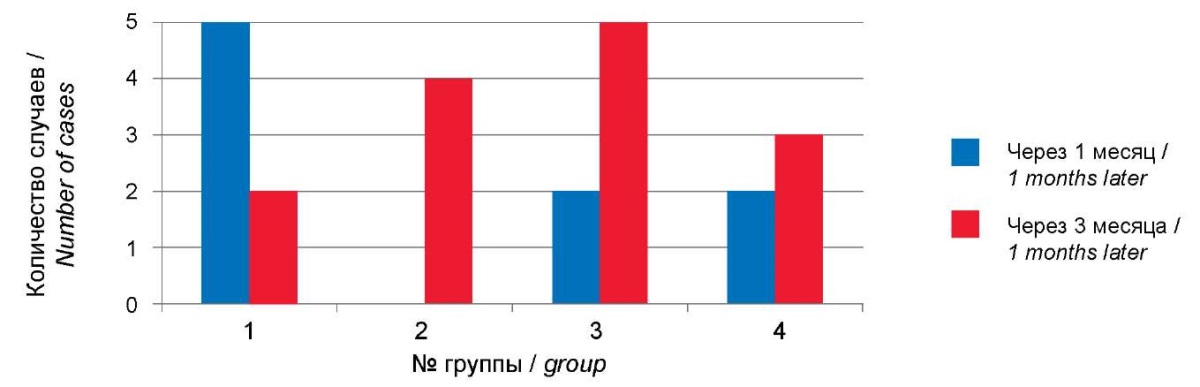
Рисунок 7. Количество случаев обострений аллергических заболеваний
в постковидном периоде.
Figure 7. Number of cases of exacerbations of allergic diseases in the post-COVID.
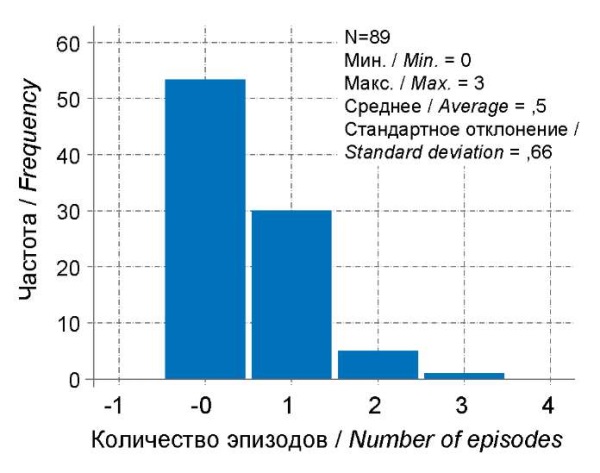
Рисунок 8. Частота ОРИ на первом месяце постковидного периода.
Figure 8. Incidence of ARI in the first month of the post-COVID.
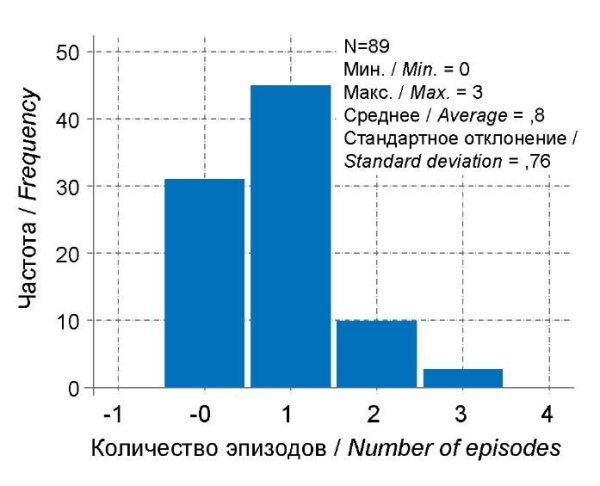
Рисунок 9. Частота ОРИ на третьем месяце постковидного периода.
Figure 9. Incidence of ARI in the third month of the post-COVID.
Discussion
An assessment of the medical history of children of different ages who had COVID-19 showed that the age groups were homogeneous in terms of gender, perinatal and neonatal history, and the frequency of allergic and gastrointestinal diseases. Statistical differences found in the proportion of frequent illnesses, vaccination history, and ENT and CNS diseases are explained by the anatomical and physiological characteristics corresponding to each period of childhood. The revealed clear decrease in the frequency of nervous diseases with increasing age, even taking into account the reliability of the differences, can be explained by the functional nature of the pathology of the CNS in children under 2 years of age and compensation for impaired functions in most children after 3 years. According to the authors’ study, the existing diseases in the child's history did not have a statistically significant effect on the severity of COVID-19, which is apparently due to the fact that chronic processes in children are diagnosed less often than in adults, and their effect on the child's body is relieved by the dynamic development of the body. The authors of the present research did not find any significant differences in the leading COVID-19 syndrome in children of different ages. Intoxication and upper respiratory tract catarrh were equally common in children, and a small number of cases of dyspepsia and laryngotracheitis were recorded. The characteristics of fever did not depend on age, but the duration and maximum values of fever correlate with each other. At higher values of the temperature curve, a longer duration of fever in children with COVID-19 can be expected, regardless of the child's age. Intoxication was more often observed in those children who received repeated courses of antibiotics before COVID-19, which can be explained by a decrease in the body's reactivity against the background of diseases and the immunosuppressive effect of the antibiotics themselves. Post-COVID syndrome can be relieved at different times in children of different ages. For example, arthralgia and long-term persistence of symptoms do not differ and are equally well relieved, and dyspepsia is equally common but is relieved worse in children under 6 years of age. In children aged 3 to 6 years, asthenic vegetative syndrome manifested itself as more prolonged complaints due to age-related features of the autonomic nervous system formation in children. The age from 3 to 6 years is a transitional period in the formation of autonomic reactions, during which the autonomic nervous system is most vulnerable.
No reliable differences were found in the frequency of anemia due to the small number of studies conducted, which indicates the need to study this issue and more widely use CBC in children who have had COVID-19. Notably, according to the authors’ data, manifestations of allergic diseases occur in the second to third month after recovery at the age of 3–17. Clinical symptoms of allergic diseases were poorly relieved in all age groups of observation, which is apparently associated with the dysfunction of the immune response against the background of the hyperactivation of the immune system by the SARS-CoV-2 virus. These changes do not completely normalize in all children in the first 3 months of the post-COVID period. A decrease in the immunological resistance of the body is to be expected a month after recovery from COVID-19 at the age of 1–6; in the form of more frequent viral infections at the age of 1–2, and in the form of a more severe course of respiratory infections at the age of 3–6, which is prognostically less favorable.
Conclusions
The severity of COVID-19 does not depend on age, yet for children aged 3–6, a severe course of ARI in the anamnesis is important, and for children aged 7–11, compliance with the vaccination schedule has an impact (children with a mild course are usually fully vaccinated), so it is necessary to carefully collect anamnesis regarding the frequency and severity of past viral infections and vaccination status to predict the risks of a more severe course of COVID-19. In all age groups, intoxication was more often observed in those children who received repeated courses of antibiotics before the onset of COVID-19, which should be taken into account by the local pediatrician when choosing the tactics of minimally optimal antibacterial therapy.
The post-COVID period has differences in the course of asthenic vegetative syndrome: the older the children, the greater the likelihood of said syndrome, yet it is better stopped, and children aged 3–6 are less likely to seek help for it, even though the complaints are more persistent.
A decrease in the immunological resistance of the child's body is to be expected in the second or third month at the age of 1–6; in the form of more frequent viral infections at the age of 1–2, and in the form of a more severe course of respiratory infections at the age of 3–6, which is prognostically less favorable. When managing a child in outpatient practice, it is necessary to take into account the age-related characteristics of the COVID-19 clinic and the course of the post-COVID period in different age groups.
There are no differences in exacerbations of allergic diseases, yet these are to be expected at a later date at the age of 3–17 years. The pediatrician is to treat with greater care children with allergic diseases and with a predisposition to atopy, and actively conduct medical examinations in the later stages after recovery.
1. Methodological recommendations of the Ministry of Health of the Russian Federation “Features of clinical manifestations and treatment of the disease caused by the new coronavirus infection (COVID-19) in children” Version 2 (03.07.2020).
2. Ibid.
3. Ibid.
References
1. Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, et al. Screening and Severity of Coronavirus Disease 2019 (COVID-19) in Children in Madrid, Spain. JAMA Pediatr. 2020:e201346. Epub ahead of print. Erratum in: JAMA Pediatr. 2020;174(10):1009. https://doi.org/10.1001/jamapediatrics.2020.1346.
2. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088-1095. https://doi.org/10.1111/apa.15270
3. Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, Perelman C, Sepulveda R, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12(1):9950. https://doi.org/10.1038/s41598-022-13495-5
4. Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6(4):230-239. Erratum in: Lancet Child Adolesc Health. 2022;6(7):e21. https://doi.org/10.1016/S2352-4642(22)00022-0
5. Buonsenso D, Espuny Pujol F, Munblit D, Pata D, McFarland S, Simpson FK. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol. 2022;17(8):577-588. https://doi.org/10.2217/fmb-2021-0285
6. Uskov A.N., Lobzin Yu.V., Rychkova S.V., Babachenko I.V., Fedorov V.V., et al. Course of a new coronavirus infection in children: some aspects of monitoring and analysis of mortality. Journal Infectology. 2020;12(3):12-20. (In Russ.) https://doi.org/10.22625/2072-6732-2020-12-3-12-20
7. Mazankova L.N., Samitova E.R., Osmanov I.M., Afukov I.I., Akimkin V.G., et al. COVID-19 and comorbidities in children. Vopr. prakt. pediatr. (Clinical Practice in Pediatrics). 2022;17(1):16–23. (In Russ.). https://doi.org/10.20953/1817-7646-2022-1-16-23
8. Stephenson T, Allin B, Nugawela MD, Rojas N, Dalrymple E, et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022;107(7):674-680. https://doi.org/10.1136/archdischild-2021-323624
9. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. Erratum in: BMJ. 2022;376:o126. https://doi.org/10.1136/bmj.n136
10. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. https://doi.org/10.1038/s41591-021-01283-z
11. Kostinov A.M., Kostinov M.P., Mashilov C.V. Antagonism between pneumococcal vaccines and COVID-19. Meditsinskiy sovet = Medical Council. 2020;(17):66-73. (In Russ.) https://doi.org/10.21518/2079-701X-2020-17-66-73
12. Ashford JW, Gold JE, Huenergardt MA, Katz RBA, Strand SE, et al. MMR Vaccination: A Potential Strategy to Reduce Severity and Mortality of COVID-19 Illness. Am J Med. 2021;134(2):153-155. https://doi.org/10.1016/j.amjmed.2020.10.003
13. Girina A.A., Zaplatnikov A.L., Petrovskiy F.I., Tandalova L.P. Childhood vaccination as a part of the National Immunization Schedule during the COVID-19: problems and potential solutions. Russian Journal of Woman and Child Health. 2021;4(1):85–89. (In Russ.) https://doi.org/10.32364/2618-8430-2021-4-1-85-89
14. Ng WH, Tipih T, Makoah NA, Vermeulen JG, Goedhals D, et al. Comorbidities in SARS-CoV-2 Patients: a Systematic Review and Meta-Analysis. mBio. 2021;12(1):e03647-20. https://doi.org/10.1128/mBio.03647-20
15. Moskaleva E.V., Petrova A.G., Rychkova L.V., Novikova E.A., Vanyarkinа A.S. Indicators of the Immune Status in Children after a New Coronavirus Infection. Acta Biomedica Scientifica. 2021;6(2):58-62. (In Russ.) https://doi.org/10.29413/ABS.2021-6.2.6
About the Authors
A. P. KiryutkinaРоссия
Anastasia P. Kiryutkina - pediatricians precinct of the Children's polyclinic Department.
Samara
Competing Interests:
Authors declares no conflict of interest
N. B. Migacheva
Россия
Natalia B. Migacheva - Dr. Sci. (Med.), Associate Professor, Head of the Department of Pediatrics.
Samara
Competing Interests:
Authors declares no conflict of interest
T. I. Kaganova
Россия
Tatiana I. Kaganova - Dr. Sci. (Med.), Professor of the Department of Pediatrics.
Samara
Competing Interests:
Authors declares no conflict of interest
V. V. Burmistrov
Россия
Victor V. Burmistrov - Cand. Sci. (Med.), Associate Professor of the Department of Pediatrics.
Samara
Competing Interests:
Authors declares no conflict of interest
A. S. Ginzburg
Россия
Anna S. Ginzburg - assistant of the Department of Pediatrics.
Samara
Competing Interests:
Authors declares no conflict of interest
Review
For citations:
Kiryutkina A.P., Migacheva N.B., Kaganova T.I., Burmistrov V.V., Ginzburg A.S. Age aspect of the course of new coronavirus infection in the acute and post-Covid in children. Medical Herald of the South of Russia. 2024;15(2):90-100. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-2-90-100
JATS XML







































