Scroll to:
Serological monitoring of actual natural focal infections in the Rostov Region (2020–2022)
https://doi.org/10.21886/2219-8075-2024-15-1-19-26
Abstract
Objective: to study the level of the immune layer of the population to pathogens of natural focal infectious diseases in order to establish the epidemic activity of natural foci of particularly dangerous infectious diseases in the Rostov region.
Materials and methods: blood serums of healthy donors living in the administrative territories of the region were collected in the period from 2020 to 2022. Specific antibodies in blood sera were determined by the ELISA method. Serum specific IgG class immunoglobulins were used as a serological marker of the transmitted infection.
Results: IgG to the Crimean hemorrhagic fever virus was not detected in 2020. In 2021, the share of seropositive results was 0.7%, in 2022 — 2.0%. The proportion of IgG to West Nile virus in 2020 was 8.7%, in 2021 — 9.9%, in 2022 — 12.4%. Serological testing revealed an immune layer to ixodic tick–borne borreliosis in 2020–2022: 3.1%, 2.6% and 2.0%, respectively. Antibodies to hantaviruses, pathogens of hemorrhagic fever with renal syndrome in the blood of residents of the region in 2020 were found in 7.0% of samples, in 2021 — 4.5%, in 2022 — 7.1%. The share of positive samples in the study for Ku fever in 2022 was 2.3%. Conclusion: Seropositive samples were detected for all studied natural focal infections. The greatest variety of natural focal infections confirmed by serological monitoring data was detected in cities (Rostov-on-Don, Taganrog, Zernograd, Kamensk-Shakhtinsky) and districts of RO (Salsky, Neklinovsky, Remontnensky).
Conclusion. The results of the serological monitoring made it possible to identify the circulation of pathogens of KGL, LZN, ICB, Ku fever, hantaviruses in the territory of two districts of the region. The detection of specific antibodies in the blood sera of healthy donors indicates the epidemic activity of natural foci. Also, a comprehensive study of healthy donors expanded the understanding of the area of the most relevant natural focal infections such as (KGL, LZN, ICB), and less frequently registered at present (HFRS and Ku fever).
Keywords
For citations:
Bereznyak E.A., Trishina A.V., Pichurina N.L., Egiazaryan L.A., Simonova I.R., Gayevskay N.E., Logvin F.V., Batashev V.V., Noskov A.K. Serological monitoring of actual natural focal infections in the Rostov Region (2020–2022). Medical Herald of the South of Russia. 2024;15(1):19-26. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-19-26
Introduction
Epidemiological surveillance on infectious diseases is an important public health tool that gives an opportunity to identify trends in the development of the epidemic process, engaged territories, time characteristics, and risk groups, as well as to detect new pathogens [1]. According to Cherkassky, the investigation of population immunity is included in the system of epidemiological surveillance of infections in order to obtain information about the circulation of infectious disease agents within certain territories and among different population groups [2]. Serological investigations are an essential component of comprehending the prevalence of pathogens circulating within a region. Laboratory-confirmed information on the level of population immunity to current infections contributes to adequate evaluation related to the degree of risk and their epidemiological danger [3]. Currently, serological methods are increasingly used as a tool for generating an array of information and determining public health tactics in relation to infectious diseases. Another advantage of serological monitoring is associated with the fact that a cross-sectional investigation, aimed to analyze the spectrum of pathogens circulating within a region, can be performed at a “single-point” accomplishment [4].
The circulation of different types of pathogens and their uneven distribution within natural foci accounts for the existence of so many unexplored questions on the nature of interspecies interactions [5].
The Rostov Region (RR) is endemic for tularemia, Crimean hemorrhagic fever (CHF), West Nile fever (WNF), ixodid tick-borne borreliosis (ITBB), and Q fever [6][7]. Statistical data attest to a significant proportion of natural focal infections (NFIs) of viral and bacterial etiology in the structure of infectious morbidity. The largest proportion (88.7%) of registered NFIs is associated with three infections, namely Crimean-Congo hemorrhagic fever (CCHF), WNF, and ITBB; the remaining share accrues to sporadic cases of pseudotuberculosis, yersiniosis, leptospirosis, brucellosis, tularemia, rabies, and fever Q1.
Comprehensive investigations of blood serum make it possible to create a more complete picture of the infectious pathology spectrum. Combined with geographic localization, this approach allows summarizing information for an analysis of the spatiotemporal distribution of the epidemic process [8]. Serological investigations provide additional information on the circulation breadth of infectious disease agents in different areas and among different population groups over long time periods [9].
The purpose of the study is to investigate the level of the population’s immune layer to certain pathogens of natural focal infectious diseases circulating in the territory of the RR over the period from 2020 to 2022.
Materials and methods
The characteristics of morbidity were performed using the method of epidemiological analysis based on the reported data and the statistical forms.
Blood samples were obtained over the period from 2020 to 2022 from healthy donors living in the RR including the city of Rostov-on-Don and the towns of Kamensk-Shakhtinsky, Volgodonsk, Shakhty, Taganrog, Zernograd, and Morozovsk, as well as the Salsky, Remontnensky, Neklinovsky, Azovsky, Veselovsky, Zavetinsky, and Tselinsky Districts. The selection of donors was done considering their permanent residence in different administrative territories of the RR and the absence of a history of previous diseases caused by NFIs. The biomaterial was obtained in accordance with the principles of legality and ethical standards. Informed voluntary consent was obtained from each donor. A total of 1295 blood sera were tested, namely, 419 samples in 2020; 424 in 2021; and 452 in 2022.
Serological monitoring included investigations of a wide range of antibodies to the pathogens in one sample to the studied infections: CCHF, WNF, ITBB, and hemorrhagic fever with renal syndrome (HFRS). Specific antibodies in donor blood samples were determined by enzyme-linked immunosorbent assay (ELISA). Serum-specific IgG antibodies were used as a serological marker of past infection.
Samples were tested with commercial kits produced by the public company Vector-Best (Novosibirsk) for enzyme-linked immunosorbent detection of class G immunoglobulins, including “VectoCrimea-CHL-IgG” to the Crimean-Congo hemorrhagic fever virus, “VectoNil-IgG” to the West Nile virus, “Vector TBEV-IgG" to the tick-borne encephalitis virus (TBE), "LymeBest-IgG" to the pathogens of ITBB or Lyme disease, "VectoHanta-IgG" to hantaviruses in blood serum (plasma), in accordance with the manufacturer's guidelines. To detect class IgG antibodies to Coxiella Burnet antigens, a test system ELISA-anti-Q-G manufactured by St. Petersburg Research Institute of Epidemiology and Microbiology named after Pasteur was used; it was designed for serological current and retrospective in vitro diagnosis of Q fever (coxiellosis). Optical density was measured using an Infinite F50 recording photometer (Tecan, Austria).
Confidence intervals for the proportion of seropositive samples were calculated using Wilson's method at a confidence level of p ≥ 0.95 with software provided by the website https://epitools.ausvet.com.au.
Results
To increase the efficiency of the epidemiological monitoring system of current infections among the population of the RR, since 2020 specialists from the Rostov-on-Don Anti-Plague Institute have been conducting regular investigations of the level of population immunity in relation to CCHF, WNF, and ITBB in order to assess the potential risk of HFRS abundance. The results obtained during monitoring are added to the database “Serological monitoring of natural focal infections in the Rostov Region” (Certificate of state registration No. 2020621999 dated October 22, 2020).
Simultaneous measuring of the level of antibodies to several pathogens provides information on the structure of infectious morbidity in the region, as well as on circulating pathogens [10].
Crimean hemorrhagic fever. Within recent years, CHF has expanded its nosoarea in the RR. In 2020, patients with CHF were identified in nine administrative districts including the Krasnosulinsky, Morozovsky, Volgodonsky, Proletarsky, Oktyabrsky, Tselinsky, Salsky, Zimovnikovsky, and Orlovsky ones (15 cases in total). In 2021, the incidence was registered in 11 administrative districts including the Oblivsky, Zavetinsky, Salsky, Proletarsky, Zimovnikovsky, Martynovsky, Semikarakorsky, Azovsky, Dubovsky, Tselinsky, and Belokalitvensky ones (16 cases in total) [9]. In 2022, 24 cases of CHF were revealed in the region with a rate of 0.57 per 100 thousand people, which is higher than last year’s rate when 16 cases were registered with a rate of 0.38 per 100 thousand people. Here the incidence has been already registered in 13 administrative districts including the Kagalnitsky, Morozovsky, Tatsinsky, Proletarsky, Aksai, Oktyabrsky, Egorlyksky, Salsky, Tselinsky, Dubovsky, Zimovnikovsky, Orlovsky, Remontnensky, and Novoshakhtinsky ones.
Conducting serological monitoring among the population for the availability of IgG to the CHF pathogen revealed no antibodies in 2020. In 2021, the seropositivity rate was 0.7% [ 0.2–2.0]. Class G immunoglobulins were registered from donors in the towns of Taganrog and Zernograd. In 2022, the proportion of seropositive results in the RR was 2.0% [ 1.1–3.7]. In particular, positive samples were detected in the Salsky District (5.7%), while in the Azovsky, Zavetinsky, and Neklinovsky Districts and the city of Rostov-on-Don, only isolated samples were revealed. Among the seropositive cases, men dominated (58.3%) with an average age of 52.8 years.
The results obtained attest to the activity of natural foci of CHF and contacts of the local population with components of their parasitogenic system. Analyzing the territorial distribution of incidence and positive samplings of serological monitoring revealed the coincidences in the Salsky, Azovsky, and Zavetinsky Districts. In the territory of the Neklinovsky District, the city of Rostov-on-Don, as well as the towns of Zernograd and Taganrog, seropositive samples were found among healthy donors, although no registered cases of morbidity were reported.
West Nile fever. Cases of human disease with WNF have been officially registered in the RR since 1999. The modern nosoarea includes 11 administrative districts, namely, the Chertkovsky, Krasnosulinsky, Kamensky, Veselovsky, Myasnikovsky, Azovsky, Aksaisky, Matveevo-Kurgansky, Neklinovsky, Egorlyksky, and Salsky ones, as well as recreational zones of six towns, namely, Rostov-on-Don, Bataysk, Kamensk-Shakhtinsky, Novoshakhtinsk, Taganrog, and Shakhty. In 2020, the incidence of WNF was not registered, while in 2021 two laboratory-confirmed cases were detected in two administrative territories in the town of Bataysk and the Aksaisky District [11]. In 2022, two verified cases were revealed in Rostov-on-Don and Volgodonsk.
Despite the lack of registration of cases of the disease in 2020, antibodies to WNF were detected in blood serum in almost all surveyed areas of the region. The total proportion of IgG with high titers was 8.7% [ 6.2–12.1]. In detail, these rates were as follows: 2.8% in Rostov-on-Don, 8.3% in Kamensk-Shakhtinsky, and 8.1% in Volgodonsk. By districts of the region, these rates were as follows: 25.0% in the Neklinovsky, 16.6% in the Salsky, and 9.1% in the Remontnensky Districts. In the Neklinovsky District, three positive results were found among 12 tested samples.
In 2021, positive samples were detected in 9.9% [ 7.4–13.1] samples. Antibodies to WNF were recorded in Morozovsk (20.6%), Volgodonsk (10.0%), Shakhty (8.9%), Taganrog (8.2%), Rostov-on-Don (5.9%), Kamensk-Shakhtinsky (5.6%), Zernograd (5.0%), and two administrative districts of the region, namely, in the Neklinovsky (17.1%) and Salsky (8.6%) ones.
In 2022, antibodies to WNF were revealed in 12.4% [ 9.8–15.6] of donors. The distribution of rates of the immune layer of the population was as follows: 22.2% in the Veselovsky District; 18.0% in the city of Rostov-on-Don, 12.2% in the Neklinovsky District, 11.9% in the Salsky District, 9.7% in the Azovsky District, and 7.5% in the Zavetinsky District.
To exclude antigen cross-reaction that exists between WNF and tick-borne encephalitis virus [12], all WNF-positive blood serum samples from donors were tested for cross-reactivity to TBE. In 2020 and 2021, no cross-reactions were found in any case. In 2022, antibodies with a titer of 1:100 to the causative agent of tick-borne viral encephalitis were revealed in two samples obtained in the Neklinovsky District from women over 80 years old.
Despite the low incidence rate during the 2020–2022 period, the results of serological monitoring showed a significant level of WNF seroprevalence among residents of large cities and districts of the RR; territorial coincidences with the incidence were identified in Rostov-on-Don, Kamensk-Shakhtinsky, Shakhty, and Taganrog, as well as in the Neklinovsky, Azovsky, Salsky, and Veselovsky Districts. Moreover, despite the fact that no registered case of WNF disease was revealed, antibodies were detected among residents of the towns of Morozovsk, Volgodonsk, Zernograd, and the Zavetinsky and Remontnensky Districts during the monitoring. The detection of antibodies to WNF in a healthy population in different territories of the RR indicates a nosoareal breadth and, presumably, an immune layer in the population after a mild or subclinical form of the disease. Overall, within the three years of investigation, the main population characteristics were the following: the average age of seropositive individuals was 55 years, and positive results were more common in women (54.1%).
The annual detection of high proportions of seropositive samples can be explained by the fact that the majority (up to 80%) of people infected with WNF do not have severe symptoms of the disease, and the fact of the disease often remains unknown [13][14].
Ixodid tick-borne borreliosis. Currently, in the territory of the RR, there are natural and anthropourgic foci of ITBB. The nosoarea covers 24 administrative territories and includes 11 cities/towns, such as Rostov-on-Don, Azov, Bataysk, Volgodonsk, Donetsk, Kamensk-Shakhtinsky, Novocherkassk, Salsk, Taganrog, Shakhty, Gukovo, and 13 administrative districts, in particular, the Tarasovsky, Kamensky, Belokalitvinsky, Bagaevsky, Matveevo-Kurgansky, Kuibyshevsky, Myasnikovsky, Azovsky, Aksaisky, Rodionovo-Nesvetaysky, Zernogradsky, Salsky, and Orlovsky ones [15].
In 2020, no case of ITBB was registered, however, during a serological examination, positive samples (IgG) were found in donors living in Rostov-on-Don (8.3%) and Taganrog (5.1%). In addition, isolated positive samples were revealed in the town of Volgodonsk, the Salsky, Remontnensky, and Neklinovsky Districts.
In 2021, five cases of ITBB were registered in the Orlovsky District (1), the town of Kamensk-Shakhtinsky (1), and the city of Rostov-on-Don (3). During seromonitoring, the largest number of positive samplings was obtained in the Neklinovsky District (8.6%), Rostov-on-Don (5.9%), and Morozovsk (5.9%). Besides, isolated positive samples were detected in the towns of Kamensk-Shakhtinsky and Shakhty, as well as in the Salsky District.
In 2022, 13 cases of ITBB were also identified. They included isolated cases in the Salsky, Kuibyshevsky, and Kamensky Districts, as well as in the towns of Azov, Gukovo, Taganrog, and Shakhty. In addition, six cases of the disease were revealed in Rostov-on-Don. Positive samples from donors were found in the Neklinovsky District (6.1%), as well as in the city of Rostov-on-Don (3.6%); isolated cases were found in the Azovsky District.
Serological blood testing among healthy donors, performed in 2020–2022, showed the contacts of the local population with pathogens of ITBB. In this respect, the proportion of seropositive samples was 3.1% [ 1.7–5.5], 2.6% [ 1.5–4.6], and 2.0% [ 1.1–3.7], related to the year of examination. Positive results in 70.0% of cases were obtained when examining material collected from men aged from 43 to 88 years.
During three years of observation, seropositive samples were detected in the Neklinovsky District without registering cases of the disease. In Rostov-on-Don, IgG to the pathogens of ITBB was detected in residents during three years of investigation with registration of the disease in 2021 and 2022. Territorial coincidence of incidence with detection of antibodies to Borrelia antigens in the blood sera of local residents was revealed in the Salsky and Azovsky Districts, the city of Rostov-on-Don, as well as the towns of Shakhty, Taganrog, Volgodonsk, and Kamensk-Shakhtinsky.
Hemorrhagic fever with renal syndrome. The first case of HFRS confirmed in the laboratory by ELISA in the RR was recorded in 2018 in the Peschanokopsky District. In 2019, two cases of the disease were detected in the city of Rostov-on-Don and the Myasnikovsky District; the rate of incidence was 0.05 per 100 thousand people [8]. In the period of 2020–2021, there were no official data on cases of HFRS registration in the RR. In 2022, two cases of the disease were registered in Rostov-on-Don and Taganrog. The Reference Center for Monitoring the Incidence included the RR in the second group with a low incidence rate of HFRS; the range of intensive incidence rates was from 0.05 to 0.91 per 100 thousand people [16].
In 2020, virus-specific antibodies of class G in healthy donors were detected in 7.0% [ 4.1–11.6] cases. Specifically, the rates were distributed as follows: the towns of Taganrog (10.8%) and Volgodonsk (4.1%), the city of Rostov-on-Don (2.8%), the Remontnensky District (13.5%), and Salsky (12.2%) District.
In 2021, according to the results obtained, the availability of specific class G antibodies to HFRS pathogen was confirmed in various territories with a frequency from 1.9% to 14.3%; on average, the proportion of seropositive results was 4.5% [ 2.9–6.9]. Antibodies were detected in Taganrog (14.3%), Morozovsk (11.8%), Shakhty (5.4%), and Zernograd (5.0). Isolated cases were revealed in Rostov-on-Don, Kamensk-Shakhtinsky, and Volgodonsk.
In 2022, positive results were revealed in 7.1% [ 5.1–9.8] donors. Antibodies were found in residents of a number of districts, namely, 15.7% in the Remontnensky District, 14.3% in the Neklinovsky District, and 13.0% in the Salsky District, as well as 4.3% in Rostov-on-Don. Isolated positive samples were recorded in the Veselovsky and Tselinsky Districts.
In recent years, an increase in cases of this infection among the urban population was noted throughout the country due to the expansion of the sector of kitchen-garden communities, improving the accessibility of recreational areas for urban residents and trips to enzootic areas. In the natural foci of HFRS, where the pathogen Puumala hantavirus circulates, a high percentage (64.6%) of infection among urban residents was found [17].
The results of monitoring revealed that antibodies to hantaviruses were detected in residents of 12 surveyed territories. Among those who were seropositive, residents of small towns with a predominance of private households and household plots dominated. Women made up the majority (53.8%) against men (46.2%), and their average age corresponded to 50.6 years.
Thus, the results obtained during complex serological monitoring indicate the presence of an immune layer in the population of the RR to the examined spectrum of NFIs. A change in the proportion of positive samples over the examined period from 2020 to 2022 is presented in Figure 1. Seropositive samples were detected for all examined NFIs, which attested to their active circulation in the biocenoses of the region. In the figure, one can see that the confidence intervals for the data obtained for the 2020, 2021, and 2022 periods are overlapped for each of the examined infections. That indicates the absence of significant differences in their levels over time.
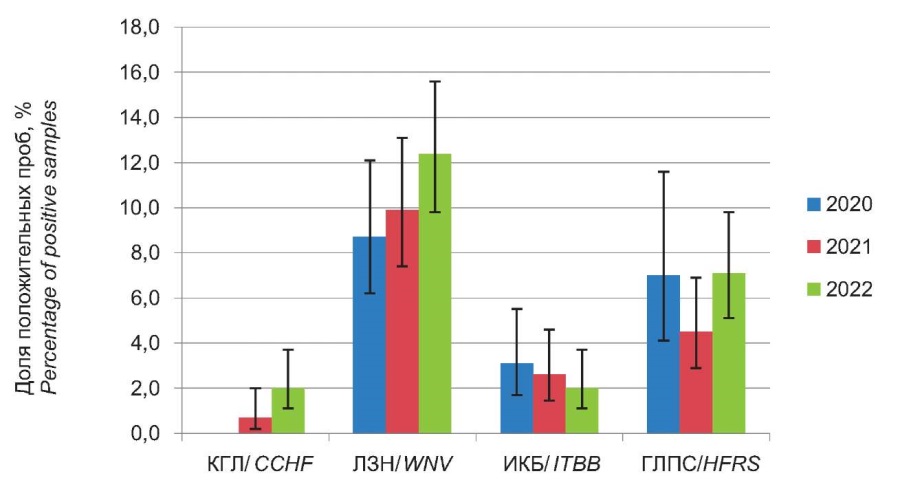
Figure 1. The level of the immune layer to NFI in RR in 2020–2022.
Рисунок 1. Уровень иммунной прослойки к ПОИ в РО в 2020–2022 гг.
Q fever. Q fever has been known in the RR since the early 1950s. Examining the level of the immune layer to Coxiella burnetii in risk groups of the population revealed seropositive results ranging from 15.3% to 22.0% [18]. Later, cases of the disease were detected in the steppe territories of the region southeast including in the Salsky (1994 and 2002) and Peschanokopsky (2002) administrative districts, as well as in the city of Rostov-on-Don (1994, 2001).
In 2022, after a long period of epidemiological well-being, 27 cases of Q fever were revealed in three districts of the RR including the Remontnensky, Salsky, and Tselinsky ones2,3. In order to clarify possible contacts of the population with the pathogen C. burnetii in areas with registered cases of the disease, serological screening was carried out among 308 local residents. The proportion of seropositive samples was 2.3%. Specific antibodies were found in the blood sera of residents of the Salsky (3.0%) and Remontnensky (1.3%) Districts. No antibodies were detected in the Tselinsky District. Considering the great epidemiological significance of this infectious disease, an urgent task is the further examination of seroprevalence among residents of the region for Q fever.
Conclusion
In the course of the conducted examination, high proportions of WNF and HFRS in seropositive donors were revealed, while the incidence of these infections in the RR was low. Detection of antibodies to WNF during monitoring among residents in the towns of Morozovsk, Volgodonsk, and Zernograd, as well as in the Zavetinsky and Remontnensky Districts, simultaneously with the absence of incidence registration, indicates the ongoing circulation of WNF in these areas and intensive contact of the population with the pathogen. Antibodies to hantaviruses have been detected over the years among residents of 12 surveyed territories, although cases of the disease were revealed only in 2022 in Rostov-on-Don and Taganrog.
The conducted examinations showed the circulation of pathogens of CCHF, WNF, ITBB, Q fever, and hantaviruses in the region. This information expands the concept on the areal of NFIs, both the most common and sufficiently examined ones such as CHF, WNF, and ITBB, and less common infections such as HFRS and Q fever.
The results obtained during serological monitoring are included in the educational materials of the Department of Epidemiology of the Rostov State Medical University and are used in the training of epidemiologists and medical workers with secondary vocational education in additional professional education programs.
For well-timed and effective prevention of the incidence of NFIs among the population of the RR, further research in this direction should continue on an ongoing basis.
1. Report on the state of sanitary and epidemiological well-being of the population of the Rostov Region in 2021, dated May 31, 2022. Available at: http://www.61.rospotrebnadzor.ru/index.php?option=com_content&view=category&layout=blog&id=96&Itemid=116. Link active on September 11, 2023
2. On the state of sanitary and epidemiological well-being of the population of the Rostov Region in 2021. Moscow: Federal Service for Supervision of Consumer Rights Protection and Human Welfare. 2022. 340 p. http://61.rospotrebnadzor.ru/index.php?option=com_content&view=article&id=11464:2022-08-30-11-16-10&catid=38:2009-09-16-04-45-57&Itemid=57 (accessed December 26, 2022).
3. Pichurina N.L., Sokirkina E.N., Simakova D.I. et al. “Clinical and epidemiological characteristics of Q fever cases in the Rostov Region.” Collection XVI Interstate Scientific and Practical Conference on Sanitary Protection of the Territory and Reducing the Risk of the Spread of Plague. Saint Petersburg: 2022: 144–145.
References
1. World Health Organization. Global Strategy on Comprehensive Vaccine-Preventable Disease Surveillance. WHO, Geneva, Switzerland, 2020
2. Cherkasskii B.L. Guide to general Epidemiology. Moscow. Medicine; 2001. (In Russ.)
3. Negodenkoa A.О., Luchinin D.N., Konovalov P.S., Pavlyukova O.A., Skrynnikova E.A., et al. A screening for serum markers of arbovirus infections in healthy blood donors from the Volgograd Region. Russian Journal of Infection and Immunity. 2020;9(5-6):743-749. https://doi.org/10.15789/2220-7619-2019-5-6-743-749
4. Haselbeck AH, Im J, Prifti K, Marks F, Holm M, Zellweger RM. Serology as a Tool to Assess Infectious Disease Landscapes and Guide Public Health Policy. Pathogens. 2022;11(7):732. https://doi.org/10.3390/pathogens11070732
5. Vynograd N. Natural foci diseases as a stable biological threat. Arch Immunol Ther Exp (Warsz). 2014;62(6):445-447. https://doi.org/10.1007/s00005-014-0316-8
6. Kovalev E., Titova S., Tverdokhlebova T., Shchipeleva I., Markovskaya E. Topical issues of epidemiology, microbiology and diagnostics of infectious and parasitic diseases in the Rostov region. Glavnyi vrach Uga Russia. 2018;59(1):8-9. (In Russ.) eLIBRARY ID: 32322140 EDN: PCHTBG
7. Lukshina E.Y., Batashev V.V., Kovalev E.V., Karpushchenko G.V., Balakhnova V.V., et al. Results of epizootic monitoring of natural foci of particularly dangerous infections common to humans and animals in Rostov Oblast. Medical Herald of the South of Russia. 2021;12(4):83-90. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-83-90
8. Metcalf CJ, Farrar J, Cutts FT, Basta NE, Graham AL, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;388(10045):728-730. https://doi.org/10.1016/S0140-6736(16)30164-7
9. Konshina O.S., Eropkin M.Yu., Nikonorov I.Yu., Pozdnyakova M.G. Role of biobanks in the study of population immunity. Journal Infectology. 2018;10(2):39-47. (In Russ.) https://doi.org/10.22625/2072-6732-2018-10-2-39-47
10. Arnold BF, Scobie HM, Priest JW, Lammie PJ. Integrated Serologic Surveillance of Population Immunity and Disease Transmission. Emerg Infect Dis. 2018;24(7):1188-1194. https://doi.org/10.3201/eid2407.171928
11. Putintseva E.V., Udovichenko S.K., Nikitin D.N., Borodai N.V., Shpak I.M., et al. West Nile Fever: Results of Monitoring over the Causative Agent in the Russian Federation in 2021, the Incidence Forecast for 2022. Problems of Particularly Dangerous Infections. 2022;(1):43-53. (In Russ.) https://doi.org/10.21055/0370-1069-2022-1-43-53
12. Sanchini A, Donoso-Mantke O, Papa A, Sambri V, Teichmann A, Niedrig M. Second international diagnostic accuracy study for the serological detection of West Nile virus infection. PLoS Negl Trop Dis. 2013;7(4):e2184. https://doi.org/10.1371/journal.pntd.0002184
13. Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6(2):606-623. https://doi.org/10.3390/v6020606
14. Visentin A, Nasillo V, Marchetti M, Ferrarini I, Paolini R, et al. Clinical Characteristics and Outcome of West Nile Virus Infection in Patients with Lymphoid Neoplasms: An Italian Multicentre Study. Hemasphere. 2020;4(3):e395. https://doi.org/10.1097/HS9.0000000000000395
15. Khametova A.P., Pichurina N.L., Karpushchenko G.V., Polonskiy A.V., Zabashta M.V., et al. Epidemiological analysis of the lyme borreliosis incidence in Rostov region. Medical Herald of the South of Russia. 2019;10(4):92-97. https://doi.org/10.21886/2219-8075-2019-10-4-92-97
16. Savitskaya T.A., Ivanova A.V., Isaeva G.Sh., Reshetnikova I.D., Trifonov V.A., et al. Analysis of the Epidemiological Situation of Hemorrhagic Fever with Renal Syndrome in the Russian Federation in 2022 and Forecast of its Development for 2023. Problems of Particularly Dangerous Infections. 2023;(1):85-95. (In Russ.) https://doi.org/10.21055/0370-1069-2023-1-85-95
17. Demidova T.N., Sharapova N.E., Gorshenko V.V., Mikhailova T.V., Semihin A.S., Ivanova A.E. Epidemiological Manifestation of Combined Natural Foci of Tularemia, Leptospirosis and Hemorrhagic Fever with Renal Syndrome: Mixed Infections. Epidemiology and Vaccinal Prevention. 2022;21(2):38-45. (In Russ.) https://doi.org/10.31631/2073-3046-2022-21-2-38-45
18. Zdrodovskii P.F., Golinevich E.M. The doctrine of rickettsiae and rickettsiosis. Moscow: Medicine, 1972. (In Russ.)
19. Pichurina N.L., Sokirkina E.N., Simakova D.I. et al. Clinical and epidemiological characteristics of cases of Q fever in the Rostov region. Collection of the XVI Interstate scientific and practical conference on sanitary protection of the territory and reducing the risk of the spread of plague. Saint Petersburg. 2022:144-145. (In Russ.)
About the Authors
E. A. BereznyakRussian Federation
Elena A. Bereznyak, Cand. Sci. (Bio.), senior scientist researcher of laboratories of natural focal and zoonotic infections
Rostov-on-Don
A. V. Trishina
Russian Federation
Alena V. Trishina, Cand. Sci. (Bio.), senior scientist researcher of laboratories of natural focal and zoonotic infections
Rostov-on-Don
N. L. Pichurina
Russian Federation
Natalya L. Pichurina, Cand. Sci. (Med.), the Head of laboratory of epidemiology of especially dangerous infections
Rostov-on-Don
L. A. Egiazaryan
Russian Federation
Liana A. Egiazaryan, junior researcher of department of microbiology of cholera and other acute intestinal infections
Rostov-on-Don
I. R. Simonova
Russian Federation
Irina R. Simonova, researcher at the laboratory of diagnostic preparations
Rostov-on-Don
N. E. Gayevskay
Russian Federation
Natalya E. Gayevskaya, Cand. Sci. (Med.), leading researcher, department of diagnostic preparations
Rostov-on-Don
F. V. Logvin
Russian Federation
Fedor V. Logvin, Cand. Sci. (Med.), Head of the Department of Epidemiology
Rostov-on-Don
V. V. Batashev
Russian Federation
Viktor V. Batashev, Cand. Sci. (Med.), Associate Professor of the Department of Epidemiology
Rostov-on-Don
A. K. Noskov
Russian Federation
Alexey K. Noskov, Cand. Sci. (Med.), Head
Rostov-on-Don
Review
For citations:
Bereznyak E.A., Trishina A.V., Pichurina N.L., Egiazaryan L.A., Simonova I.R., Gayevskay N.E., Logvin F.V., Batashev V.V., Noskov A.K. Serological monitoring of actual natural focal infections in the Rostov Region (2020–2022). Medical Herald of the South of Russia. 2024;15(1):19-26. (In Russ.) https://doi.org/10.21886/2219-8075-2024-15-1-19-26







































