Scroll to:
The role of morphofunctional complexes and somnological signs of the pathogenesis of gestational diabetes mellitus in overweight women
https://doi.org/10.21886/2219-8075-2023-14-2-26-35
Abstract
Objective: to identify the diabetogenic type of the lateral behavioral profile of asymmetries, which reveals the highest incidence of GDM and obstetric complications in overweight pregnant women and, on its basis, to study the features of the somnological status in women with diabetogenic lateral constitution at the preconception period.
Materials and methods: the study used special testing (Annette test, sleep quality questionnaire, sleep apnea/hypopnea syndrome questionnaire, sleepiness scale (Epworth)), polysomnographic study.
Results: based on the results of a questionnaire survey and a polysomnographic study, somnological disorders were identified, expressed in a decrease in the efficiency and quality of sleep, fatigue, perceptible drowsiness in wakefulness, breathing disorders during sleep and other sleep disorders, which contributes to an increased risk of gestational diabetes mellitus mainly in women with an ambidextral lateral phenotype.
Conclusion: the identified sleep disorders in overweight women in the pregravid period are, on the one hand, the result of hormonal changes against the background of already existing metabolic and vegetative abnormalities in overweight women planning pregnancy, on the other hand, they act as a “trigger” functional disorders both during the manifestation of GDM and during the formation of obstetric complications due to increasing hypoxemia in the maternal body against the background of the “obstructive sleep apnea” syndrome, which contributes to the development of fetal distress.
Keywords
For citations:
Botasheva T.L., Deriglazova O.I., Lebedenko E.Yu., Zheleznyakova E.V., Zavodnov O.P., Zheltetskaya V.Yu., Ulkina A.A. The role of morphofunctional complexes and somnological signs of the pathogenesis of gestational diabetes mellitus in overweight women. Medical Herald of the South of Russia. 2023;14(2):26-35. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-26-35
Introduction
The incidence of gestational diabetes mellitus (GDM) and associated obstetric pathology continues to grow steadily. This contributes to an increase in the number of studies investigating their pathogenetic mechanisms and aiming at new approaches to prevention, prediction, diagnosis, and treatment [1–16].
Overweight and obesity are recognized as risk factors for GDM; therefore, to develop preventive measures at the preconception stage, researchers show significant interest in this category of women [17][18][12][19][20].
One of the major contributors to overweight and obesity as components of metabolic syndrome in women is problems with quality, timing, and amount of sleep affecting 72% of women [21][22][6][23–25], while overweight patients with metabolic syndrome are more likely to develop or worsen pre-existing sleep disorders. Poor sleep quality has been proven to be associated with weight gain due to higher ghrelin levels synthesized in the gastric fundus and is known to increase the feeling of hunger by activating cells of the arcuate nucleus and limbic dopaminergic system. It also contributes to a decrease in intestinal peptides responsible for the suppression of gastrointestinal motility [26][27]. Chronic sleep deprivation results in decreased production of leptin-mediated orexin, a neuropeptide regulating wakefulness. In addition, the lower synthesis of melanocyte-stimulating hormone can drive increased anabolism [1][28–31]. Sleep disorders that tend to occur during pregnancy are a crucial factor that can determine the pregnancy outcome [1][6]. One of the most severe sleep disorders is obstructive sleep apnea syndrome characterized by intermittent episodes of upper airway obstruction during sleep along with decreased saturation. The incidence of obstructive sleep apnea among pregnant women with metabolic syndrome and obesity is 1.8 and 2.6 times higher, respectively, than in pregnant women with normal body weight [32–34].
It has been established that metabolic processes in females are significantly influenced by functional hemispheric asymmetry: pregnant women with metabolic syndrome and overweight have a predominant activation of metabolism-associated right hemisphere structures [3, 4].
Female morpho-functional asymmetry (MFA) is one of the basic morpho-functional constitutional concepts of living body organization [35][3][36][12]. The significance of MFA for the regulation of visceral, endocrine, and immune processes is determined by the anatomical involvement of the medial prefrontal and insular cortical zones through multiple subcortex components in the forebrain, diencephalon, and brainstem that regulate preganglionic sympathetic and parasympathetic fibers. These include a central cluster of amygdala nuclei, the gray matter of the brainstem, the medullary reticular formation, and the hypothalamus, an autonomic integrative center. Recently, a great deal of convincing experimental and clinical evidence has appeared, suggesting lateralization and functional specialization of the brain systems, which determines individual characteristics of the autonomic and metabolic regulation of functional processes, including in the woman’s body [36–38][12].
The published literature confirms that the MFA of the female body and reproductive system largely determines the direction pattern of the functional "behavior" programs specific for various aspects of the "mother — placenta — fetus" system depending on the predominance of right-hemispheric or left-hemispheric afferent-efferent regulation. In addition, the nature of potential obstetric complications is also concerned [37][38][3][12]. The spatial consistency of genetically determined pre-gestational and gestational processes becomes feasible only in accordance with the individual lateral constitution (a lateral phenotype). The lateral behavioral asymmetry profile (LBAP) has been identified as its test correlate [36][38][39]. There is evidence that hemispheric asymmetry has its blood chemistry markers characterized by varying contents of proteins and nucleic acids, neurotransmitters, quantity and activity of tropic neuronal receptors, enzymes, and even trace elements. It is directly related to the regulation of metabolic processes [3][37][38]. Unidirectional and multidirectional patterns of pre-existing and gestational asymmetries have been established to be responsible for the normal course of pregnancy. In other cases, they are likely to cause a variety of obstetric complications [38]. There is evidence of the significance of MFA for the pathogenesis of type 2 diabetes mellitus [3][6]. However, a review of the available literature shows that there are no studies investigating the effect of hemispheric asymmetry on the regulation of metabolic processes and sleep status in females before the onset of pregnancy.
The aim of this study was to define the type of LBAP accompanied by the highest incidence of GDM and obstetric complications in overweight pregnant women and accordingly to characterize the sleep features in preconception women with diabetogenic lateral constitution.
Materials and Methods
The LBAP parameters are genetically determined and remain unchanged throughout the whole life. This has become a reason for identifying the most diabetes-associated lateral phenotype using the Annett test in a group of pregnant women. The onset of GDM was an essential condition to switch to the next stage of the study to investigate the sleep status in women at the LBAP-specific preconception risk. The Annett test was performed to determine the LBAP type in 2458 pregnant women with a body mass index (BMI) of 25‒29 at 16‒23 weeks of gestation. Following the Annett test, each result was assigned a weight factor which was used to calculate weighted averages, i.e. right-handed women had at least 90% dominance in their right eyes, ears, hands, feet, and left-handed women had at least 90% dominance favoring the left side. Ambidextrous women were defined as those with at least 60% right dominance and 40% left dominance. Then, 913 pregnant women were randomized to group 0 using the Excel RANDBETWEEN function (Microsoft Office package). Out of them, there were 386 women with right LBAP, 415 with ambidextrous LBAP, and 112 with left LBAP. The objective was to determine the incidence of GDM among them.
For characterizing the sleep status, the screening included overweight women (BMI 25–29) planning their first pregnancy who were diagnosed with metabolic syndrome based on one main and two additional criteria (HOMA-IR ≥ 2.77, fasting blood glucose concentration ≥ 5.1 mmol/L). Among the other criteria, high-density lipoprotein levels < 1.2 mmol/L, low-density lipoprotein levels > 3.0 mmol/L, triglycerides ≥ 1.7 mmol/L, urinary albumin excretion rates > 20 μg/min, blood pressure 140/90 mmHg, and waist-to-hip ratio > 0.85 were considered. The women were assigned to two clinical groups: group I, i.e. main cohort (135 overweight women, BMI 25–29, ambidextrous LBAP), and group II, i.e. controls (156 normal-weight pregnant women, BMI 18–24, ambidextrous LBAP-A).
To assess the sleep status, the Sleep Quality Perception Questionnaire (modified Spiegel Sleep Questionnaire) was used (a total score < 19 signified pathological sleep). Sleep-related breathing disorders were detected using the Sleep Apnea Screening Questionnaire (with a total score of ³ 4 indicating a sleep-related breathing disorder). The Epworth Sleepiness Scale (1990) was used as a measure of daytime sleepiness (5‒9 points interpreted as excessive daytime sleepiness). Night-time polysomnography parameters were measured and interpreted using the Encephalan-EEGR-19/26 EEG Recorder. Electroencephalography (EEG), electrocardiography (ECG), electrooculography (EOG), mylohyoid electromyography (EMG), respiratory rate (RR) recording, pulse oximetry, and actigraphy were performed. Monopolar EEG was recorded with the time constant of 0.3 sec and placing the standard electrodes in symmetrical frontal, temporal, central, parietal, and occipital leads of the 10‒20 EEG system. EEG signals were analyzed for epochs of 20-sec duration; with frequency and spectral response characteristics determined using fast Fourier transform; sleep events were subjected to cluster-based analysis. EEG frequency ranges were categorized as delta (0.5‒2 Hz), delta 2 (2‒4 Hz), theta (4‒8 Hz), alpha (8‒12 Hz), sigma (12‒18 Hz), and beta (18–36 Hz) rhythms; the location for maximum amplitude values of the EEG rhythms were identified for every sleep stage. EEG characteristics were analyzed during wakefulness with eyes closed before sleep onset, in every phase of sleep, and after awakening. Sleep patterns (phases, cycles, stages, and hypnograms) were evaluated using EEG, EMG, ECG, and RR parameters. Sleep efficiency (SE) was calculated in minutes using the following formula: SE = (TST + DST)/(SOL + WH), where: TST is total sleep time, DST is delta sleep time, SOL is sleep onset latency, and WH is waking hours at night. The SE values negatively correlated with more productive sleep. Segmented sleep parameters were evaluated by calculating the number of segments (periods of consistently deep sleep) in sleep stages. The proportion of segments in each stage with a total stage time of 100% was determined. In addition, the number and time of various episodes between the segments during the sleep stages were calculated.
The data were analyzed using descriptive statistics. The M and m values (for normal data distribution), median and interquartile ranges (25%, 75%) (for non-normal data distribution) were evaluated. The statistical significance of the results was tested at a 95% confidence level. The between-group difference was compared using the non-parametric Mann-Whitney test (at an alpha level of 0.05), while the non-parametric Friedman test compared three dependent groups for non-normal data distribution. Statistically significant differences were analyzed post hoc using the Wilcoxon test with Bonferroni correction. Relative parameters (incidence rates, proportions, and percentages) were also compared between groups using chi-square or Fisher's exact test. Statistica version 10.01, Excel 2010, and IBM SPSS 24.0 program packages were used for the statistical analysis.
In accordance with current legislation governing clinical trials, all participating women signed informed consent.
Results
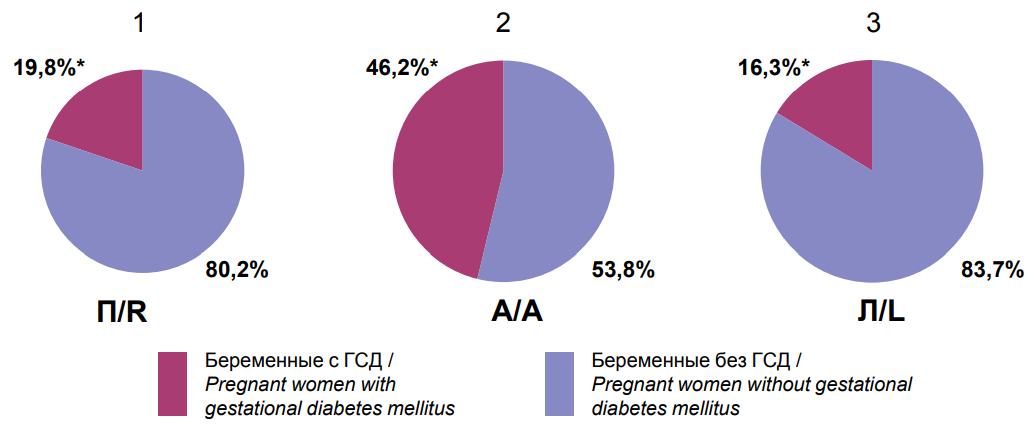
For the incidence of GDM (group 0) in different LBAP subgroups, it was demonstrated that the highest incidence of GDM was reported in pregnant women with ambidextrous LBAP (192/415 (46.2%) vs. 76/386 (19.8%) with right LBAP (p=0.0001) and 18/112 (16.3%) with left LBAP (P = 0.001) (Figure 1).
|
Notes: * — statistical significance of differences between clinical groups; R — right lateral behavioral profile of asymmetries; A — ambidextral lateral behavioral profile of asymmetries; L — left lateral behavioral profile of asymmetries. |
|
Figure 1. The frequency of detection of gestational diabetes mellitus in pregnant women with right (1), ambilateral (2) and left (3) types of lateral behavioral profile of asymmetries. |
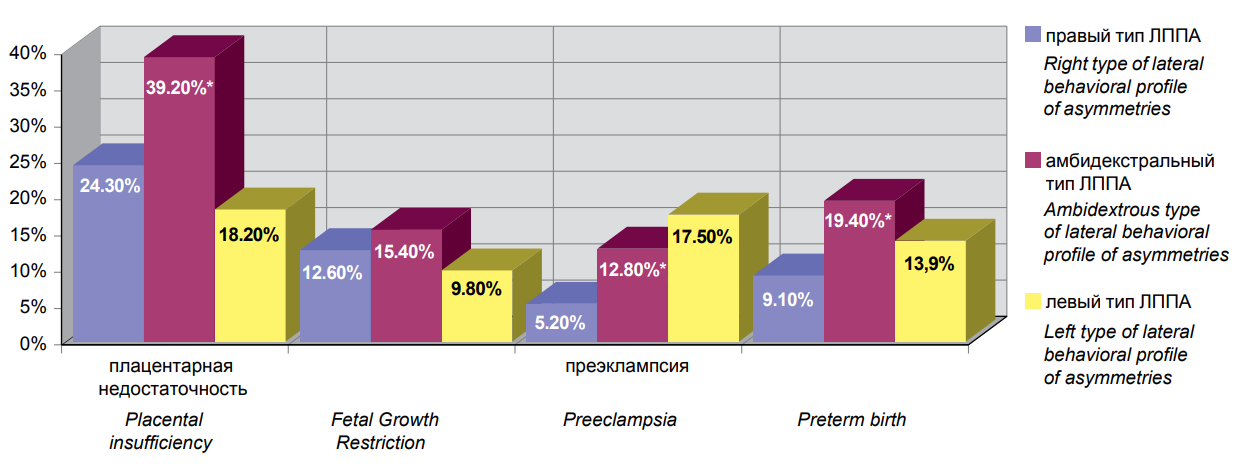
Analysis of the incidence of some obstetric complications in different LBAP subgroups has shown that placental insufficiency was significantly more common in pregnant women with ambidextrous LBAP (163/415 (39.2%) vs. right LBAP 94/386 (24.3%) (p=0.001) and left LBAP (20/112) (18.2%) (P = 0.001) (Figure 2).
 |
|||||
|
Note: * — statistical significance of differences between clinical groups. Figure 2. The frequency of detection of certain types of obstetric complications in pregnant women with right (1), ambilateral (2) and left (3) types of lateral behavioral profile of asymmetries. |
There was no statistically significant difference in the incidence of fetal growth retardation between the lateral subgroups: (ambidextrous LBAP 64/415 (15.4%) vs. right LBAP (94/386 (12.6%) (P = 0.25)) and left LBAP (11/112 (9.8%) (P = 0.13)). The incidence of pre-eclampsia was significantly higher in women with ambidextrous LBAP (53/415 (12.8%) vs. right LBAP (20/386 (5.2%) (P = 0.002)). No significant difference was found in the left LBAP group ((20/112 (17.5) (P = 0.21)). Preterm labor was also significantly more common among pregnant women with ambidextrous LBAP, i.e. 81/415 (19.4%), but only when compared to right LBAP (35/386 (9.1%) (P = 0.0001). No significant differences were found in the left LBAP subgroup (16/112 (13.9%) (P = 0.18)).
Thus, as a result of lateral typing of overweight pregnant women, an ambidextrous (mixed) lateral asymmetry profile was identified, with the highest incidence of GDM and some obstetric complications. This phenotype has further provided a basis for sampling high-risk overweight patients for preconception polysomnography testing.
Analysis of the questionnaire scores for the purpose of sleep pattern evaluation has shown that all women had sleep time ranging within 7.1–7.7 hours. In clinical group I, women reported more severe fatigue, poor sleep quality, and drowsiness episodes (Table 1).
Table 1
Indicators of tests for studying the features of somnological status in women with excess weight and normal body weight with an ambilateral behavioral profile of asymmetries (preconception period (M ± m))
|
Tests to study the immunological status |
Groups of surveyed women |
P |
|
|
Group I (n=135) |
Group II (n=156) |
||
|
Sleep Quality Questionnaire |
17.1±2.1 |
24.5±1.9 |
0.04129 |
|
Sleep apnea/hypopnea questionnaire |
7.9±1.8 |
2.0±1.3 |
0.0453 |
|
Sleepiness scale (Epworth) |
6.4±1.2 |
3.7±0.3 |
0.0306 |
Note: p<0.05 — statistically valid differences between groups.
Higher scores of the Sleep Apnea Scale indicate that sleep disorders were more frequent among the women of group I.
Polysomnographic characteristics reflecting a true picture of sleep, including cardiorespiratory parameters, demonstrate lower breathing rate and depth with lower HR in the slow-wave phase in group I compared to group II. In addition, paradoxical sleep and the first and second stages of slow-wave sleep were accompanied by episodes of highly variable heart rhythm. However, at the second and fourth stages of slow-wave sleep, overweight patients experienced episodes of relative HR stability.
In group I, statistically significant differences were seen in the maximum and minimum values of sleep heart rate, which, along with sleep-related breathing disorders, would inevitably contribute to obstetric complications at the earliest stages of the maternal-fetal system development (Table 2).
Table 2
Features of the functional activity of the cardio-respiratory system during sleep in women with excess weight and normal body weight with an ambilateral lateral behavioral profile of asymmetries
(preconception period) (Me (Q1-Q3))
|
Parameters of the cardiorespiratory system of women in the process of polysomnographic study |
Group I (n=135) |
Group II (n=156) |
P |
|
Average heart rate in wakefulness, beats/min |
82.0 [ 76.1–87.3] |
75.2 [ 65.8–76.4] |
0.0531 |
|
Average heart rate during sleep, bpm |
73.7 [ 68.3–79.1] |
65.1 [ 59.4–70.6] |
0.0632 |
|
Minimum heart rate, bpm |
64.9 [ 59.3–84.6] |
50.2 [ 65.4–75.3] |
0.0458 |
|
Maximum heart rate, bpm |
139.0 [ 97.0–38.5] |
92.3 [ 116.4–125.2] |
0.0315 |
|
Average heart rate in superficial sleep (1st + 2st), bpm |
58.3 [ 52.7–69.8] |
64.9 [ 59.5–76.3] |
0.2371 |
|
Average heart rate in delta sleep (3st + 4st), bpm |
67.2 [ 60.7–73.8] |
60.4 [ 58.2–69.9] |
0.2421 |
|
Average heart rate in the paradoxical sleep phase, bpm |
81.6 [ 75.3–87.4] |
72.2 [ 69.7–77.5] |
0.4257 |
|
Apnea index |
2.8 [ 0.9–3.5] |
0.7 [ 0.4–0.8] |
0.0444 |
|
Apnea/hypopnea index |
10.6 [ 8.5–11.4] |
3.9 [ 1.6–4.3] |
0.0263 |
|
Minimum blood oxygen saturation, % |
87.1 [ 86.4–89.7] |
95.6 [ 92.5–99.1] |
0.0252 |
|
Duration of desaturations, sec |
136.4 [ 108.2–144.8] |
30.1 [ 25.9–46.3] |
0.0069 |
|
Number of episodes of snoring |
514.7 [ 349.1–583.5] |
85.6 [ 68.3–97.9] |
0.0072 |
Note: p<0.05 — statistically valid differences between groups.
In clinical group I, respiratory rates were characterized by a high incidence of snoring episodes, sleep apnea/hypopnea, incidence and duration of sleep desaturation suggestive of overweight-related hypoxemia. Desaturation episodes reported in clinical group I were considered a direct result of obstructive sleep apnea symptoms. These processes are associated with damage to nocturnal sleep patterns going with deprivation of deep phases. Among other things, this is attributed to the dysfunction of hormone secretion in overweight women.
In addition, they experienced a statistically significant decrease in HR during superficial sleep, which indicates the predominance of the parasympathetic division of the autonomic nervous system.
The polysomnographic EEG data analysis has shown a prolongation of the first and second stages with less total time for slow and REM sleep phases, and poorer sleep quality in clinical group I.
In addition, clinical group I had a lower representation of REM phases and prolonged episodes of night-time waking hours. No significant differences were reported in the number of sleep cycles between the main cohort and controls (5 cycles on average, P = 0.07). However, there were differences in cycle time: the second cycle was the longest in group I (P = 0.03), and the third cycle — in group II (P = 0.04). Proportions of sleep stages within each cycle were assessed. Analysis of these proportions has shown that clinical group I had the predominance of the slow-wave phase over all sleep cycles; however, its presentation was greater due to superficial sleep in the fourth and fifth cycles, while the paradoxical phase was less evident than in controls. In clinical group II, the first three cycles were characterized by the predominating slow-wave phase, while in the fourth and fifth cycles, the paradoxical phase prevailed, i.e. in the first three sleep cycles in the slow-wave phase, sleep was largely deep, while the fourth and fifth cycles were described by superficial stages.
Overweight women with ambidextrous LBAP were characterized by the lowest segmentation for the slow-wave phase and the highest — for the fast-wave phase compared to women with normal body weight, who had the highest segmentation values of both sleep phases.
Discussion
The results offer a broader insight into metabolic disorders in overweight females. Apparently, the more significant involvement of right hemispheric structures associated with the body metabolism in the mixed (ambidextrous) LBAP into the regulatory processes determines the predominance of anabolic processes, preconception overweight, and the higher incidence of GDM in women with this lateral constitution. Similar results were obtained by Palieva (2018) [3], who investigated mechanisms of central metabolism regulation in pregnant women and reported the highest incidence of metabolic disorders in the ambidextrous and left-sided placenta of right-handed women, which paved the way for female ambidextrism resulting in the activation of the metabolism-associated right hemisphere. It contributes to a significant increase in metabolic "breakdown" episodes, which includes GDM. There is a higher activity of stress release subsystems in the female body and the sympathetic division of the autonomic nervous system. Endothelial dysfunction becomes aggravated, and various obstetric complications develop.
The diabetes-associated lateral phenotype in pregnant women has significantly narrowed down the researched cohort of women (overweight) at risk for GDM, since this group may include a category who do not manifest GDM, as well as those who develop GDM with normal body weight.
Conclusion
The obtained data suggest that lateral phenotyping identifies the category of patients at the highest risk of GDM among overweight women. For this purpose, diabetes-associated phenotype (ambidextrous LBAP) should be essentially identified. These women are 2.7 times more likely to develop GDM at later pregnancy. The results are crucial for understanding the pathogenetic mechanisms of GDM. Sleep disorders in overweight women with preconception ambidextrous LBAP are attributed to hormonal shifts with pre-existing metabolic and vegetative abnormalities in women planning pregnancy. Sleep disorders may be used as triggers of functional failures in GDM and obstetric complications (placental dysfunction, fetal growth retardation, fetal distress, pre-eclampsia, etc.) due to increasing maternal hypoxemia associated with obstructive sleep apnea, which will inevitably contribute to fetal distress and unfavorable perinatal morbidity and mortality.
The obtained data show a clear need for further development of methods to treat sleep disorders in overweight women planning a pregnancy to prevent GDM and associated obstetric complications.
References
1. Ajlamazyan E. K., ed. Caharnyj diabet i reproduktivnaya sistema zhenshchiny: rukovodstvo dlya vrachej. Moscow: GEOTAR-Media; 2017. (In Russ.).
2. Aulamazyan E. K., Evsyukova I. I., Yarmolinskaya M. I. The role of melatonin in development of gestational diabetes mellitus. Journal of obstetrics and women's diseases. 2018; 67 (1): 85-91. doi: 10.17816/JOwD67185-91
3. Palieva N. V., Botasheva T. L., Khloponina A. V., Zavodnov O. P., Zheleznyakova E. V., Ganikovskaya Yu. V. Effect of morpho-functional asymmetries of the mother - placenta - fetus system on metabolic homeostasis during pregnancy. The Bulletin of Adyghe State University: Internet Scientific Journal. 2018; (4): 63-70. (In Russ.). eLIBRARY ID: 37024359
4. Botasheva T. L., Palieva N. V., Khloponina A. V., Vasiljeva V. V., Zheleznyakova E. V., et al. Fetal sex in the development of gestational diabetes mellitus and endothelial dysfunction. Obstetrics and gynecology. 2020; (9): 56-64 (In Russ.). doi: 10.18565/aig.2020.9.56-64
5. Orazmuradov A. A., Akhmatova A. N., Arakelyan G. A., Savenkova I. V., Minaeva A. V. Obesity and gestational weight gain in the development of gestational diabetes mellitus and its complications. Akusherstvo i ginekologiya. Novosti. Mneniya. Obuchenie. 2020; 8 (3 (29)): 86-89. (In Russ.). URL: https://cyberleninka.ru/article/n/ozhirenie-i-gestatsionnoe-uvelichenie-massy-tela-v-razvitii-gestatsionnogo-saharnogo-diabeta-i-ego-oslozhneniy
6. Radzinskij V. E., Botasheva T. L., Koytash G. A., eds. Ozhirenie. Diabet. Beremennost'. Versii i kontraversii. Klinicheskie praktiki. Perspektivy. Moscow: GEOTAR-Media; 2020. (In Russ.).
7. Khodzhaeva Z. S., Snetkova N. V., Muminova K. T., Gorina K. A., Abramova M. Ye., Esayan R. M. Clinical characteristics of pregnancy in women with gestational diabetes mellitus. Obstetrics and Gynecology. 2020; 7: 47-52. (In Russ.). doi: 10.18565/aig.2020.7.47-52
8. Abramova M. E., Khodzhaeva Z. S., Gorina K. A., Muminova K. T., Goryunov K. V., et al. Gestational diabetes mellitus: screening and diagnostic criteria in early pregnancy. Obstetrics and Gynecology. 2021 (5): 25-32. (In Russ.). doi: 10.18565/aig.2021.5.25-32
9. Kapustin R. V., Kopteeva E. V., Alexeenkova E. N., Tsybuk E. M., Arzhanova O. N. Analysis of risk factors and perinatal mortality structure in pregnant patients with diabetes mellitus. doctor.ru. 2021; 20 (6): 46-52. (In Russ.). doi: 10.31550/1727-2378-2021-20-6-46-52
10. Mateykovich E. A. Аdverse pregnancy outcomes and gestational diabetes: from the hapo study to current data. Obstetrics and Gynecology. 2021; 2: 13-20. (In Russ.). doi: 10.18565/aig.2021.2.13-20
11. Denisov A. A., Bashmakova N. V., Tretyakova T. B., Davydenko N. B. Pathogenetic approaches to predicting preeclampsia from the perspective of lipid metabolism. Lechenie i profilaktika. 2022; 12 (2): 33-38. (In Russ.). eLIBRARY ID: 49300693
12. Botasheva T. L., Rymashevsky A. N., Fabrikant A. D., Petrov Yu. A., Palieva N. V., et al. Features of the glycemic status, pro- and contrinsular factors in pregnant women with gestational diabetes mellitus, depending on the gender of the fetus. Glavnyj vrach Yuga Rossii. 2022; 1 (82): 6-9. (In Russ.). eLIBRARY ID: 47918117
13. Billionnet C., Mitanchez D., Weill A., Nizard J., Alla F., et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017; 60 (4): 636-644. doi: 10.1007/s00125-017-4206-6
14. Akkurt M. O., Turan O. M., Crimmins S., Harman C. R., Turan S. Increased fetal epicardial fat thickness: A novel ultrasound marker for altered fetal metabolism in diabetic pregnancies. J Clin Ultrasound. 2018; 46 (6): 397-402. doi: 10.1002/jcu.22602
15. Chu A. H. Y., Yuan W. L., Loy S. L., Soh S. E., Bernard J. Y., et al. Maternal height, gestational diabetes mellitus and pregnancy complications. Diabetes Res Clin Pract. 2021; 178: 108978. doi: 10.1016/j.diabres.2021.108978
16. Shi P., Liu A., Yin X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2021; 21 (1): 508. doi: 10.1186/s12884-021-03982-4
17. Tazhetdinov E. Kh., Kostin I. N., Li K. I., Arshinova O. V., Cheporeva O. N., et al. Prospects for early screening for gestational diabetes. Akusherstvo i ginekologiya. Novosti. Mneniya. Obuchenie. 2020; 8 (3 (29)): 90-94. (In Russ.). URL: https://cyberleninka.ru/article/n/perspektivy-rannego-skrininga-gestatsionnogo-saharnogo-diabeta
18. Glavnova O. B., Shelygin M. S., Saluhova A. V. Gestacionnyj saharnyj diabet: profilaktika reproduktivnyh poter'. Farmateka. 2021; 28 (4): 34-37. (In Russ.). doi: 10.18565/pharmateca.2021.4.34-37
19. Boriboonhirunsarn D. Second trimester weight gain > 7 kg increases the risk of gestational diabetes after normal first trimester screening. J Obstet Gynaecol Res. 2017; 43 (3): 462-467. doi: 10.1111/jog.13231
20. Phelan S., Jelalian E., Coustan D., Caughey A. B., Castorino K., et al. Protocol for a randomized controlled trial of pre-pregnancy lifestyle intervention to reduce recurrence of gestational diabetes: Gestational Diabetes Prevention/Prevención de la Diabetes Gestacional. Trials. 2021; 22 (1): 256. doi: 10.1186/s13063-021-05204-w
21. Koval’zon V. M., Dolgikh V. V. Regulation of sleepwakefulness cycle. Nevrologicheskiy Zhurnal (Neurological Journal). 2016; 21 (6): 316–322. (in Russ.). URL: https://www.koob.ru/kovalzon/bodrstvovaniye-son
22. Poluektova M. G., ed. Somnology and Sleep Medicine. National Manual in Memory of A. M. Vein and Y. I. Levin. Moscow: Medforum; 2016. (in Russ.).
23. Burchakov D. I., Poluektov M. G., Kuznetsova I. V. Sleep Disorders in Pregnancy: Current Options for Management. Lechebnoe delo. 2022; 1: 57-65. (in Russ.). doi: 10.24412/2071-5315-2022-12486
24. Stone P. R., Burgess W., McIntyre J., Gunn A. J., Lear C. A., et al. An investigation of fetal behavioural states during maternal sleep in healthy late gestation pregnancy: an observational study. J Physiol. 2017; 595 (24): 7441-7450. doi: 10.1113/JP275084
25. Saadati F., Sehhatiei Shafaei F., Mirghafourvand M. Sleep quality and its relationship with quality of life among high-risk pregnant women (gestational diabetes and hypertension). J Matern Fetal Neonatal Med. 2018; 31 (2): 150-157. doi: 10.1080/14767058.2016.1277704
26. Tsvetkova E. S., Romantsova T. I., Poluektov M. G., Runova G. E., Glinkina I. V., Fadeev V. V. The importance of melatonin in the regulation of metabolism, eating behavior, sleep, and the prospects for the use of melatonin drugs for obesity treatment. Obesity and metabolism. 2021; 18 (2): 112-124. (In Russ.) doi: 10.14341/omet12279
27. Kryger M. H., Roth T., Dement W. C., eds. Principles and Practice of Sleep Medicine, 6th ed. Philadelphia. Elsevier; 2017.
28. Misnikova I. V., Kovaleva Yu. A. Son i narusheniya metabolizma. Russkijkij medicinskij zhurnal. 2017; 22: 1641-1645. (In Russ.) eLIBRARY ID: 32244016
29. Khabarov S. V., Sterlikova N. A. Мelatonin and its role in circadian regulation of reproductive function (Literature Review). Journal of new Medical Technologies. 2022; 29 (3): 17-31. (In Russ.) doi: 10.24412/1609-2163-2022-3-17-31
30. Ding F., O'Donnell J., Xu Q., Kang N., Goldman N., Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016; 352 (6285): 550-5. doi: 10.1126/science.aad4821
31. Román-Gálvez R. M., Amezcua-Prieto C., Salcedo-Bellido I., Martínez-Galiano J. M., Khan K. S., Bueno-Cavanillas A. Factors associated with insomnia in pregnancy: A prospective Cohort Study. Eur J Obstet Gynecol Reprod Biol. 2018; 221: 70-75. doi: 10.1016/j.ejogrb.2017.12.007
32. Golokov V. A., Schnider N. A., Nikolaeva T. Ya., Golokova E. A., Moskaleva P. V., Nasyrova R. F. Sleep disorders and pregnancy (review of literature). Vestnik Severo-Vostochnogo federal'nogo universiteta im. M. K. Ammosova. Seriya: Medicinskie nauki. 2019; 2 (15): 81-93. (In Russ.) ГКД: https://cyberleninka.ru/article/n/narushenie-sna-i-beremennost-analiz-literatury
33. Madaeva I. M., Protopopova N. V., Sakhyanova N. L., Berdina O. N., Semenova N. V., et al. Sleep apnea syndrome, pregnancy and fetal condition. Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova. 2021; 121 (4- 2): 103- 109. (In Russ.) doi: 10.17116/jnevro2021121402103
34. Bublitz M. H., Monteiro J. F., Caraganis A., Martin S., Parker J., et al. Obstructive Sleep Apnea in Gestational Diabetes: A Pilot Study of the Role of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Sleep Med. 2018; 14 (1): 87-93. doi: 10.5664/jcsm.6888
35. Lobanov S. A. Morfofunkcional'naya asimmetriya mozga. Vestnik Bashkirskogo gosudarstvennogo pedagogicheskogo universiteta im. M. Akmully. 2011; 2 (25): 73-88. (In Russ.) eLIBRARY ID: 21236307
36. Bragina I. I., Dobrohotova T. A. Funkcional'nye asimmetrii cheloveka. M.: «Medicina»; 1988. (In Russ.)
37. Chernositov A. V., Orlov V. I., Vasil'eva V. V. Funkcional'naya mezhpolusharnaya asimmetriya mozga – kak ob"ekt reproduktivnogo sistemogeneza. In: Rukovodstvo po funkcional'noj mezhpolusharnoj asimmetrii. Moscow: Nauchnyj mir; 2009. (In Russ.) eLIBRARY ID: 35080017
38. Chernositov A. V. Funkcional'naya asimmetiriya mozga: mediko-biologicheskie, psihologicheskie i social'no-pedagogicheskie aspekty. Izdanie 2-e dopoln. Rostov-na-Donu: Izdatel'stvo IPO PI YUFU; 2011. (In Russ.)
39. Panteleeva A. M., Berdichevskaya E. M. Osobennosti proyavlenij simmetrii-asimmetrii pri staticheskoj nagruzke u futbolistov-pravshej. Resursy konkurentosposobnosti sportsmenov: teoriya i praktika realizacii. 2020; 1: 205-207. (In Russ.) eLIBRARY ID: 44407152
About the Authors
T. L. BotashevaRussian Federation
Tatyana Leonidovna Botasheva, Dr. Sci. (Med.), Professor, Principal Research Scientist
Department of Obstetrics and Gynecology
Rostov-on-Don
O. I. Deriglazova
Russian Federation
Olga. I. Deriglazova, doctor endocrinologist
Rostov region
E. Yu. Lebedenko
Russian Federation
Elizaveta Yu. Lebedenko, MD, PhD, Professor
Chair of obstetrics and gynecology № 3
Rostov-on-Don
E. V. Zheleznyakova
Russian Federation
Elena V. Zheleznyakova, PhD, Research
Obstetrics and Gynecology Department
Rostov-on-Don
O. P. Zavodnov
Russian Federation
Oleg P. Zavodnov, PhD in Biology, Researcher
Obstetrics and Gynecology Department
Rostov-on-Don
V. Yu. Zheltetskaya
Russian Federation
Victoria Yu. Zheltetskaya, Doctor of medicine
Rostov-on-Don
A. A. Ulkina
Russian Federation
Anastasia A. Ulkina, student of the 6th year of the 11b group
medical faculty
Rostov-on-Don
Review
For citations:
Botasheva T.L., Deriglazova O.I., Lebedenko E.Yu., Zheleznyakova E.V., Zavodnov O.P., Zheltetskaya V.Yu., Ulkina A.A. The role of morphofunctional complexes and somnological signs of the pathogenesis of gestational diabetes mellitus in overweight women. Medical Herald of the South of Russia. 2023;14(2):26-35. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-26-35