Scroll to:
Age aspects of the effects of the new coronavirus infection (COVID 19) on certain biochemical blood parameters
https://doi.org/10.21886/2219-8075-2023-14-2-90-96
Abstract
Objective: to study the effects of a new coronovirus infection on a number of standard biochemical blood parameters in patients of different ages.
Materials and methods: 514 patients of 3 age groups (group 1st — 25–35 years old, group 2nd — 36–50 years old and group 3rd — over 51 years old) with a diagnosis of community-acquired pneumonia caused by COVID-19 infection were studied. All biochemical parameters of the blood of patients were determined using an Indiko biochemical analyzer.
Results: the study of nitrogen metabolism indicators showed that in patients of all age groups, the content of total protein and urea does not undergo significant changes, however, there is an increase in the concentration of creatinine and uric acid, which is most pronounced in patients of the group 3rd. The study of the activities of AST, ALT in the blood of patients showed a significant increase in all age categories, more significant in persons of the groups 2nd and 3rd. At the same time, the activity of α-amylase, the concentration of bilirubin increase with age, and the levels of iron decrease, reaching pathological values. The content of the inflammation marker — CRP shows the most pronounced dependence on the age of patients: in patients of the group 1st, it increases by 6.9 times, in the 2nd group — by 12.3 times, in patients over 51 years old — by 17.25 once.
Conclusion: with an increase in the age of patients, the deviations of many biochemical parameters from the control levels corresponding to each group become more pronounced and reach values that deviate significantly from the reference.
For citations:
Alimurzaeva M.M., Izudinova S.M., Dzhafarova A.M., Khalilov R.A. Age aspects of the effects of the new coronavirus infection (COVID 19) on certain biochemical blood parameters. Medical Herald of the South of Russia. 2023;14(2):90-96. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-90-96
Introduction
The 2019 novel coronavirus outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) rapidly spread worldwide and led to a global pandemic of coronavirus disease 2019 (COVID-19). Initially, SARS-CoV-2 penetrates target cells that have angiotensin-converting enzyme 2 (ACE-2) receptors [1]. ACE-2 receptors are expressed on various cells in the body, including the respiratory system, esophagus, intestine, heart, adrenals, bladder, brain (hypothalamus), and pituitary, and on endothelial cells and macrophages. However, the earliest primary target of SARS-CoV-2 is alveolar type II (ATII) cells; hence diffuse alveolar damage occurs [2].
The virus can penetrate epithelial cells that make up the lining of the stomach, small and large intestines. There is evidence that SARS-CoV-2 causes specific damage to blood vessels (endothelium), myocardium, kidneys, etc. The SARS-CoV-2 invasion of the brain tissue has been reported to occur through the systemic circulation and/or the cribriform plate. Some studies suggest SARS-CoV-2 can specifically infect lymphocytes and macrophages, so COVID-19 patients generally exhibit leukopenia and hyperactive macrophages [3].
Severe COVID-19 can trigger a cytokine storm, an abnormal immune response characterized by the overproduction of proinflammatory and anti-inflammatory cytokines. In COVID-19, hyperactivated in situ immune responses are often evoked in the lung parenchyma and adjacent bronchial alveolar lymphoid tissue and are associated with acute respiratory distress syndrome. Critically ill patients develop vascular endothelial dysfunction, coagulopathies, and thromboses. COVID-19-associated cytokine storm is often responsible for multiorgan failure and can eventually end in a fatal outcome [4].
High mortality rates and the huge social and economic burden of the COVID-19 pandemic have paved the way to analyze observations and develop effective treatment and prevention of complications. It is known that age and comorbidities may be risk factors for severe COVID-19. Elderly and chronic patients have been identified as the highest-risk group. However, young people without pre-existing chronic health conditions may also experience potentially fatal complications such as fulminant myocarditis and disseminated intravascular coagulation [5]. Thus, people of all age groups are at risk of having COVID-19 infection, developing severe or fatal complications. Hence, there is a need to evaluate the clinical laboratory features of COVID-19 patients of various age groups.
The aim of the study was to investigate basic blood chemistry values in COVID-19 patients of various age groups.
Materials and Methods
The retrospective study was conducted at the Dagestan Ministry of Interior Hospital (Makhachkala). At the beginning of the coronavirus pandemic (April‒May 2020), the hospital created a coronavirus-only hospital unit to manage patients diagnosed with community-acquired pneumonia caused by COVID-19 infection (confirmed by SARS-CoV-2 RNA RT-qPCR or CT images in rare cases). At presentation and on day 6.5±2.5 of the disease, blood samples were taken for blood chemistry profiles. The comparative analysis included males on hospital day 1 with moderate disease, i.e. lung involvement of 20–50% in chest CT scans. The main reasons for exclusion were a history of myocardial infarction and stroke, unstable angina pectoris, NYHA class II–IV heart failure, or endocrine disorders.
The patients were assigned to one of the following age groups: 109 patients aged 25‒35 years (group I), 310 patients aged 36‒50 years (group II), and 95 patients aged 51‒75 years (group III). The control group included age-matched males without COVID-19 infection or other significant cardiovascular, digestive, endocrine, and excretory system diseases. On admission day 1, fasting venous blood samples were collected in the morning using clot activator vacuum tubes. All serum chemistry values were measured using the Indiko Clinical Chemistry Analyzer (Thermal Fisher, USA).
Statistical data processing was performed using a one-way analysis of variance (ANOVA) in the Statistica 8.0 software package (StatSoft, Inc., USA). The normality of distribution was tested using the Shapiro-Wilk test. The statistical significance of the normally distributed variable was determined with the F-test at the significance level of 0.05 (P ≤0.05). The tabulated data are presented as mean (M) ± standard error (m).
Results
The variables related to hepatic nitrogen metabolism and catabolism of proteins and nucleic acids to non-protein nitrogen compounds were measured in patients of various age groups. The study demonstrated that total protein and urea remained essentially unchanged across all age groups (Table 1).
Table 1
The content of markers of nitrogen metabolism in the blood of patients with COVID-19
|
Age of patients |
Total protein (g/l) |
Urea (µmol/l) |
Creatinine (µmol/l) |
Uric acid (µmol/l) |
|
|
25–35 |
Control (n=32) |
73.68±0.84 |
4.73±0.23 |
75.04±0.93 |
259.50±15.04 |
|
Sick (n =83) |
71.12±0.74 |
4.89±0.14 |
96.16±2.05* |
358.0±23.67* |
|
|
36–50 |
Control (n =36) |
71.85±0.83 |
5.17±0.29 |
90.41±2.13 |
259.0±15.04 |
|
Sick (n=288) |
69.83±0.65 |
4.91±0.15 |
100.82±3.89* |
371.14±21.53* |
|
|
51–75 |
Control (n =33) |
75.66-±1.22 |
5.47±0.28 |
79.57±1.05 |
255.60±22.20 |
|
Sick (n =66) |
68.85±1.36 |
6.80±0.95 |
115.14±6.59*+ |
410.60±31.17* |
|
|
Reference intervals |
65–85 |
3.2–7.3 |
74–110 |
210–420 |
|
Note: p<0.05 compared to * — the control group, + — group 1st, # — group 2nd.
However, there was an age-related increase in creatinine and uric acid. Thus, the nitrogen metabolism assays were remarkable for 28.2% and 38.2% increases in group I (P <0.05), 50% and 43.3% increases in group II (P <0.05), and 44.3% and 61% increases in group III (P <0.05) relative to their age matches. Interestingly, the comparative analysis of creatinine and uric acid in age groups II and III did not reveal any statistically significant differences. It should be noted that although creatinine and uric acid increased significantly in all age groups, the values still remained within the reference ranges.
All age groups showed a dramatic elevation in the biochemical markers of cytolysis, particularly of hepatocytes (AST, ALT) and pancreatic cells (α-amylase) (Table 2).
Table 2
The activity of cellular enzymes and the content of bilirubin in the blood of patients with COVID-19
|
Age of patients |
AST (U/l) |
ALT (U/l) |
Total bilirubin (µmol/l) |
Direct bilirubin (µmol/l) |
α-amylase (U/l) |
|
|
25–35 |
Control (n=32) |
24.83 ±1.83 |
24.72 ±9.50 |
10.31 ±4.08 |
4.90 ±0.39 |
46.40 ±3.69 |
|
Sick (n =83) |
39.72 ±2.95 * |
52.43 ±5.98 * |
11.13 ±5.37 |
5.32 ±0.32 |
69. 00 ±6.80 * |
|
|
36–50 |
Control (n =36) |
26.58 ±1.63 |
29.72 ±1.62 |
10.52 ±0.65 |
5.26 ±0.47 |
57.71 ±5.67 |
|
Sick (n=288) |
71.65 ±5.03 *+ |
77.58 ±4.30 *+ |
31.82 ±2.24 *+ |
12.61 ±0.84 *+ |
86.26 ±5.78 |
|
|
51–75 |
Control (n =33) |
28.85 ±2.61 |
35.23 ±3.49 |
9.21 ±0.61 |
4.61 ±0.40 |
60.20 ±3.05 |
|
Sick (n =66) |
75.21 ±8.86 *+ |
89.05 ±13.09 *+ |
23.33 ±1.56 *+ |
12.46 ±2.06 *+ |
111.75 ±4.09 *+ # |
|
|
Reference intervals |
< 40 E/l |
< 40 E/l |
3.4–20.5 |
< 9.2 |
< 100 |
|
Note: p<0.05 compared to * — the control group, + — group 1st, # — group 2nd.
In group I, AST and ALT levels were 1.9 and 2.1 times the reference values reported in healthy matches, respectively (P <0.05). The patients of older age groups had an even higher increase: AST and ALT levels were 2.7 and 2.60 times higher in group II, and 2.6 and 2.5 times higher in group III (P <0.05). ALT and AST activity values in older patients suggest a significant disruption of cytoplasmic (ALT) and mitochondrial (AST) membrane permeability in hepatocytes. Interestingly, bilirubin remained unchanged in group I, suggesting the preserved functional activity of hepatocytes and biliary epithelium. However, total and conjugated bilirubin levels increased by 3 and 2.4 times in group II (P <0.05), and 2.52 and 2.7 times in group III, respectively (P <0.05). Interestingly, the comparative analysis of all the above variables (AST, ALT, bilirubin) across the study groups showed that statistically significant differences were observed only between group I and each of groups II and III, while the differences between groups II and III were insignificant.
Groups I and II of COVID-19 patients exhibited an increase (by 48.7% and 49.5% (P <0.05)) in α-amylase activity, which was however within the reference range. In age group III, the evaluation was significant (by 85.6%) and beyond the reference values (P <0.05).
The coronavirus infection induces a marked inflammatory response in patients of all ages. This is evidenced by an increase in C-reactive protein (CRP), a classical marker of inflammation. Figure 1 shows a 6.9-fold increase in CRP in group I (P <0.05), a 12.3-fold increase in group II (P <0.05), and a 17.25-fold increase in individuals older than 51 years (P <0.05). High CRP values in the infected study patients, especially in the elderly, are indicative of the progression of acute inflammation.
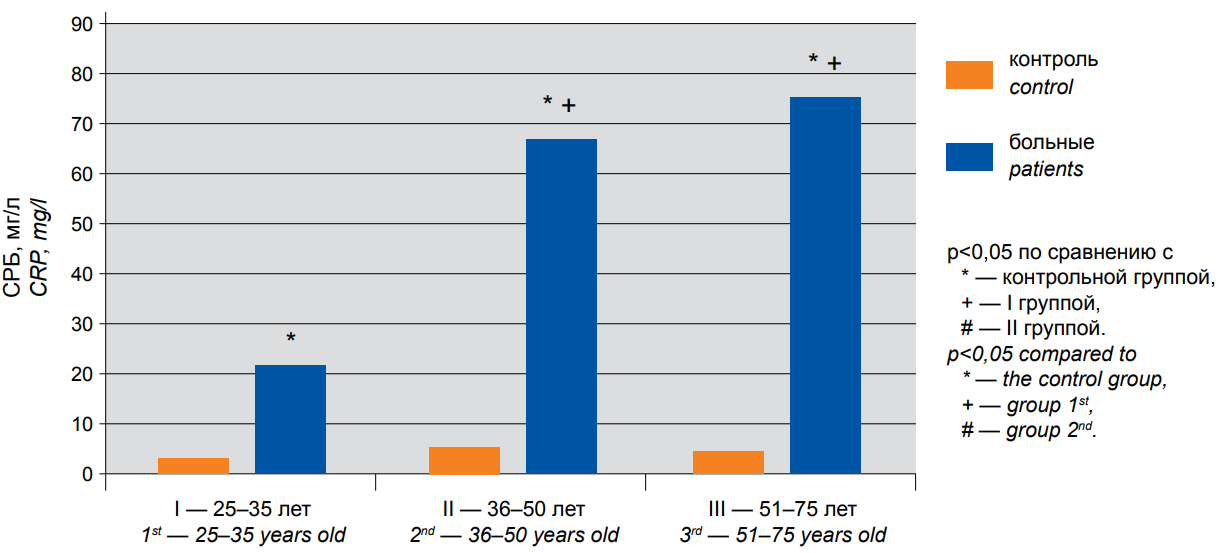
Figure 1. The content of CRP in the blood serum of patients with COVID-19 of different age groups: 1st — 25–35 years old, 2nd — 36–50 years old, 3rd — 51–75 years old. — control, — patients. * — p<0,05 compared to * — the control group, + — group I, # — group II.
In addition to classical inflammatory markers, infections may be generally associated with changes in iron metabolism. Figure 2 shows that serum iron reduction reached 54.2% in group I (P <0.05) and 74.2% in group II (P <0.05), coming up to abnormally low values. A similar dramatic decrease in serum iron, i.e. 66.7%, was observed in group III. Thus, in older age groups, concentrations of the essential trace element become significantly lower than reference limits.
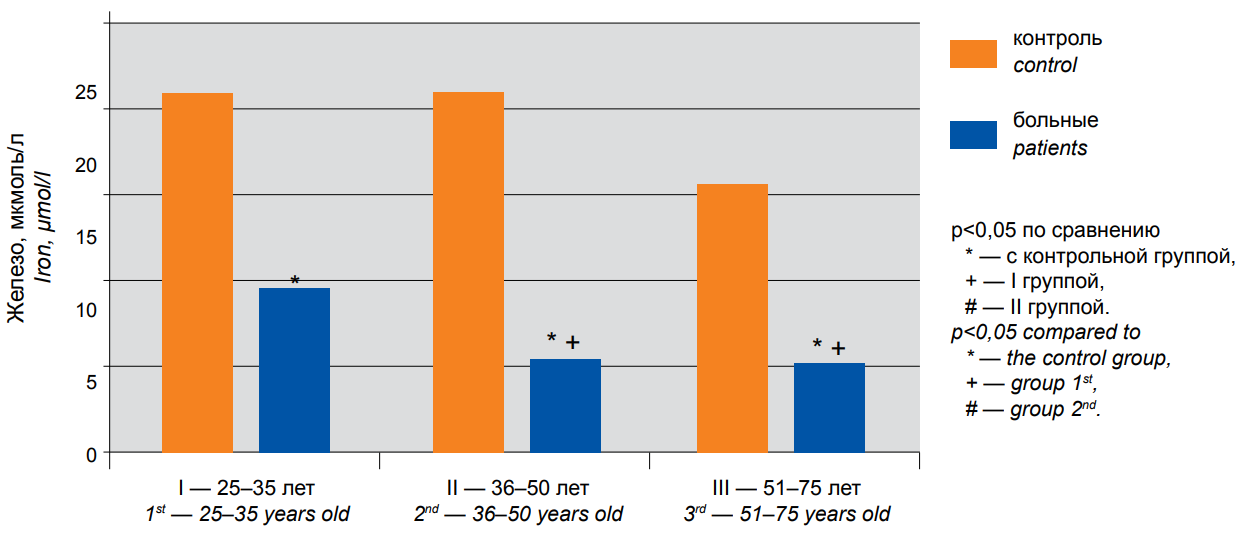
Figure 2. The content of iron in the blood serum of patients with COVID-19 of different age groups: 1st — 25–35 years old, 2nd — 36–50 years old, 3rd — 51–75 years old. — control, — patients. * — p<0,05 compared to * — the control group, + — group I, # — group II.
The comparative analysis of serum CRP and iron levels in patients of different ages demonstrated the statistically significant differences only between group I and each of groups II and III, while the differences between groups II and III were insignificant.
Discussion
The basic blood chemistry panel evaluating the proper functions of major organs and systems suggests that the Sars-Cov-2 infection leads to various structural and functional disorders. The following trends may be observed: pathological effects of virus propagation increase with the patient’s age.
The analysis of nitrogen metabolism products showed an elevation of serum creatinine and uric acid in COVID-19 patients. This may be a marker of the impaired renal clearance of these low-molecular-weight substances due to structural and functional changes in nephrons. Since ACE-2, an entry point for SARS-CoV-2, is widely expressed in the human body, including the renal tissue, kidneys are known to be one of the primary target organs of COVID-19. ACE-2 has been found in glomerular podocytes, mesangial cells, parietal epithelium of Bowman's capsule, proximal tubular epithelial cells, and collecting tubular cells [6].
The study showed varying dramatic elevations in AST and ALT levels in all age groups of patients with the coronavirus infection. Both enzymes may be found in all body tissues. Since ALT is located primarily in the liver, this enzyme is a specific marker of hepatic damage. AST is found in various human tissues, including the heart, liver, kidneys, nerve tissue, skeletal muscles, and other organs. Elevations in blood serum AST levels may be associated with its release accompanied by the cytolysis of myocardial, hepatic, and other cells. Since COVID-19 equally contributes to increases in both AST and ALT, hepatocyte necrosis may be suggested.
Mechanisms of liver damage may vary from the direct cytopathic effect of SARS-CoV-2 to associated medication toxicities. In addition, COVID-19 severity essentially correlates with various chronic hepatitises, fatty hepatosis, chemical-driven and autoimmune damage, and cirrhosis. Signs of virus-associated hepatocyte damage (apoptosis and regeneration) and degeneration (adipose and ballooning) may be found [7]. Moreover, cases of the more severe disease were more often remarkable for the signs of liver damage.
Also, the elevated AST activity may be a marker of cardiomyocyte damage. According to global statistics, signs of heart damage (chest pain, hypotension, cardiac arrhythmia, signs of heart failure) are observed in 19% of patients hospitalized for COVID-19; the infection may also trigger various mechanisms responsible for bradyarrhythmia and acute coronary syndrome [8].
Higher serum α-amylase in COVID-19 patients may be due to disrupted membrane permeability of pancreatic cells. ACE-2 is known to be extensively expressed in the pancreas, with its mRNA levels higher than those in the lungs. ACE2 is expressed in both cells of the exocrine pancreas and endocrine islet cells [9]. Damage to the exocrine pancreas is manifested by elevated serum amylase and/or lipase levels in 1‒2 and 17% of patients with non-severe and severe disease, respectively. However, any serious disease may be associated with stress-related hyperglycemia. Yang et al. have described that patients with SARS pneumonia (caused by SARS-CoV, a "cousin" of SARS-CoV-2) who had never received glucocorticoids had significantly higher fasting plasma glucose levels compared to patients with non-SARS pneumonia. In another study, SARS-CoV-mediated damage to pancreatic β-cells was suggested as a probable mechanism of acute diabetes in SARS patients [10].
The results of this study demonstrate that the coronavirus infection was associated with significant increases in CRP levels and decreases in serum iron. CRP, an acute-phase protein, shows dramatic elevations in acute respiratory diseases and is used as a marker for their diagnosis. Monitoring the markers of inflammation, including CRP, as part of blood chemistry is recommended as per COVID-19 national guidelines in many countries, including Russia. UpToDate considers CRP levels higher than 100 mg/L to be associated with severe COVID-19 [11]. Thus, older patients with critical CRP levels are at the highest risk of severe COVID-19.
The study has shown a significant decrease in serum iron levels, especially in older patients. The decrease in iron levels is thought to be a result of its absorption and deposition by activated histiocytes (macrophages). Iron is accumulated mainly in macrophages ferritin, and iron transfer from ferritin to transferrin is disturbed; hence, blood ferritin increases by 3 times or more, which can be seen in COVID-19 patients [3].
Conclusion
SARS-CoV2 infection leads to significant changes in the basic blood chemistry panel (creatinine, uric acid, AST, ALT, α-amylase) even in young people (25–35 years old). With the increasing age of patients, many abnormal values differ more severely — in some cases significantly — from the age reference ranges. The comparisons across the study groups have suggested that the differences in various blood chemistry variables in groups II (36–50 years) and III (51–75 years) are not statistically significant. Thus, the statistical analysis of laboratory blood values in various age groups has shown that age is a strong predictor of severe COVID-19; however, it is not absolute, since people of different ages may be at risk of disease progression.
References
1. Lan J., Ge J., Yu J., Shan S., Zhou H., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581 (7807): 215-220. doi: 10.1038/s41586-020-2180-5
2. Perrella A., Trama U., Bernardi F. F., Russo G., Monastra L., et al. Editorial - COVID-19, more than a viral pneumonia. Eur Rev Med Pharmacol Sci. 2020; 24 (9): 5183-5185. doi: 10.26355/eurrev_202005_21216
3. Polushin Yu. S., Shlyk I. V., Gavrilova E. G., Parshin E. V., Ginzburg A. M. The Role of Ferritin in Assessing COVID-19 Severity. Messenger of anesthesiology and resuscitation. 2021; 18 (4): 20-28. (In Russ.) doi: 10.21292/2078-5658-2021-18-4-20-28
4. Chan J. F., Zhang A. J., Yuan S., Poon V. K., Chan C. C., et al. Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin Infect Dis. 2020; 71 (9): 2428-2446. doi: 10.1093/cid/ciaa325
5. Madjid M., Safavi-Naeini P., Solomon S. D., Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020; 5 (7): 831-840. doi: 10.1001/jamacardio.2020.1286
6. Aragão D. S., Cunha T. S., Arita D. Y., Andrade M. C., Fernandes AB, et al. Purification and characterization of angiotensin converting enzyme 2 (ACE2) from murine model of mesangial cell in culture. Int J Biol Macromol. 2011; 49 (1): 79-84. doi: 10.1016/j.ijbiomac.2011.03.018
7. Zheng K. I., Gao F., wang X. B., Sun Q. F., Pan K. H., et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020; 108: 154244. doi: 10.1016/j.metabol.2020.154244
8. Liu P. P., Blet A., Smyth D., Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020; 142 (1): 68-78. doi: 10.1161/CIRCULATIONAHA.120.047549
9. Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020; 18 (9): 2128-2130.e2. doi: 10.1016/j.cgh.2020.04.040
10. Yang J. K., Feng Y., Yuan M. Y., Yuan S. Y., Fu H. J., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006; 23 (6): 623-8. doi: 10.1111/j.1464-5491.2006.01861.x
11. Andreeva E. A. C-reactive protein in the assessment of patients with respiratory symptoms before and during the COVID-19 pandemic. RMJ. 2021; 6: 14–17. (in Russ.). eLIBRARY ID: 46433896
About the Authors
M. M. AlimurzaevaRussian Federation
Maryam M. Alimurzaeva, master of the 2nd year of study, medical assistant-laboratory assistant
Dagestan
Makhachkala
S. M. Izudinova
Russian Federation
Sagibat M. Izudinova, laboratory assistant
Dagestan
Makhachkala
A. M. Dzhafarova
Russian Federation
Albina M. Dzhafarova, Associate Professor, laboratory assistant
Department of Biochemistry and Biophysics
Dagestan
Makhachkala
R. A. Khalilov
Russian Federation
Rustam A. Khalilov, Head of the Department
Department of Biochemistry and Biophysics
Dagestan
Makhachkala
Review
For citations:
Alimurzaeva M.M., Izudinova S.M., Dzhafarova A.M., Khalilov R.A. Age aspects of the effects of the new coronavirus infection (COVID 19) on certain biochemical blood parameters. Medical Herald of the South of Russia. 2023;14(2):90-96. (In Russ.) https://doi.org/10.21886/2219-8075-2023-14-2-90-96







































