Scroll to:
Prediction injury of the central nervous system in the neonatal period in preterm newborns
https://doi.org/10.21886/2219-8075-2022-13-2-122-133
Abstract
Objective: to develop a system for predicting the probability of developing damage of the central nervous
system (CNS) in the neonatal period in children who was born prematurely.
Materials and methods: the main group of the study is premature newborns with gestational age (GA) less than 36 weeks inclusive (n = 60), the control group – fullterm newborns (n = 35). In the process of dynamic observation, the main group was divided into subgroups depending on the diagnosed CNS injury in the neonatal period, according to the neurosonography (intraventricular hemorrhages (IVH), periventricular leukomalacia (PVL): subgroup 1 (prematurity babies with CNS injury) – 25 people, subgroup 2 (premature babies without CNS injury) – 35 people. we estimated clinical and anamnestic, laboratory and instrumental data of children at birth and in dynamics, indicators of antioxidant protection (manganese-containing superoxide dismutase (Mn-SOD), copper, zinc-containing superoxide dismutase (Cu, Zn-SOD), reduced glutathione (GSH), oxidized glutathione (GSSG), total antioxidant capacity of blood (TAS/TAC), oxidative stress (TOS/TOC), 4 polymorphisms of Mn-SOD gene.
Results: we designed two prognostic models which can determine the probability of developing IVH and PVL in the neonatal period in premature newborns. This models are included GA, Apgar scores at 1 and 5 minutes of life, presence of respiratory failure at birth, invasive mechanical ventilation in the neonatal period, presence of intrauterine infection in the neonatal period and indicators of antioxidant protection (GSH, TAS/TAC; model 2).
Conclusions: a comprehensive assessment of the clinical and anamnestic data of premature newborns at birth, as well as the determination of indicators that take into account the level of antioxidant protection, will make it possible to identify a premature baby at the risk for organic injury of the CNS and to correct the therapeutic strategies in the neonatal period.
Keywords
For citations:
Pavlinova E.B., Gubich A.A., Savchenko O.A. Prediction injury of the central nervous system in the neonatal period in preterm newborns. Medical Herald of the South of Russia. 2022;13(2):122-133. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-122-133
Introduction
To date, significant progress has been made in the care of premature infants. Methods of providing resuscitation care at birth are being advanced to automatism, and methods of nursing in intensive care units (ICUs) and wards of premature newborns are being improved. Therapeutic strategies for respiratory distress syndrome (RDS), bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, diseases mainly associated with prematurity, are advancing. However, it is worth noting that, despite the accumulated experience, preventing or minimizing organic brain damage is still not completely solved.
Intraventricular hemorrhage (IVH) is a group of intracranial hemorrhages of different etiologies occurring in the subependymal caudothalamic zone with possible spread to the ventricles and brain parenchyma.
Periventricular leukomalacia (PVL) is perinatally induced necrosis and/or gliosis of the white matter of the brain, localized primarily in areas adjacent to the dorsal and lateral margins of the lateral ventricles, with minor lesions in the white matter farther away from these foci [1].
These conditions are mostly typical for premature newborns because of the anatomical and physiological characteristics, which include a germinal matrix, peculiarities of blood supply in the periventricular area (terminal type of blood supply), venous outflow and autoregulation processes in the brain, the effect of free radicals, non-specific inflammatory factors on brain cells, etc.1 [1–8]. Failure of cerebral blood flow in a sick newborn, which develops for several reasons, and insufficient supply of brain tissue with oxygen can lead to both hypoxic and hemorrhagic structural changes in the brain. It is not uncommon to find during neurosonography (NSG) that the same child has both IVH and PVL at the same time. It is also worth noting that despite anatomical and physiological prerequisites, not all preterm infants end up developing IVH or PVL. It can be assumed that there are certain risk factors and individual characteristics, which in some cases, may contribute to brain tissue damage. Along with risk factors, it is possible to talk about protective features in a particular premature infant that prevent the implementation of structural changes in the brain.
In neonatal practice, there is no standard way to predict the development of severe organic lesions of the central nervous system (CNS) in preterm infants. There is evidence of the role of an infectious process in the realization of IVH and PVL if it was present in the mother or in the child during pregnancy [9–14]. Some studies are devoted to the evaluation of the somatic status of the pregnant woman, the course of the present pregnancy [15][16], resuscitation and stabilization of the child after birth, clinical symptomatology, and the significance of these factors in the development of IVH and PVL [17–19]. The search for early predictors of organic CNS lesions based on the list of laboratory and instrumental parameters and various neurochemical markers is a relevant issue [20–22]. The search for prognostic indicators that can be used to determine the likelihood of developing IVH and PVL in preterm infants is crucial, because it can contribute to the development of new approaches to further reduce the occurrence of adverse neurological outcomes through early correction of the ongoing treatment.
The study aimed to develop a system for predicting the probability of developing damage of the CNS in the neonatal period in children who were born prematurely to improve medical care for neonates.
Materials and Methods
A clinical observational, analytical, combined study was conducted (part of the study was conducted by the longitudinal type and part by the case-control type). The study was approved by the ethical committee of the Omsk State Medical University, conducted in accordance with international standards Guideline for Good Clinical Practice.
The study included 95 newborns treated at the first and second stages of nursing at the Clinical Maternity Hospital No.1 (Omsk) and the Regional Children's Clinical Hospital (Omsk) during the period from November 2019 to November 2020. The main group consisted of premature infants with gestational age (GA) less than 36 weeks inclusive (n=60), who met the following inclusion criteria: a) GA up to 36 weeks (inclusive); b) availability of informed voluntary consent of the child's legal representatives to participate in the study. Exclusion criteria were the following: a) congenital malformations of the CNS; b) the presence of hereditary diseases; c) the absence of informed voluntary consent of the child's legal representatives to participate in the study. During the regular follow-up of the children from the main group in the inpatient settings, the group was divided into two subgroups depending on the presence or absence of structural changes of the brain (IVH, PVL) according to the NSG data (subgroup I and subgroup II, respectively).
The control group included full-term newborns (n=35) who were recruited to control specific biochemical and genetic parameters of the antioxidant system. Criteria for inclusion of children in the control group: a) GA over 37 weeks (inclusive); b) relatively healthy full-term newborns without organic lesions of the CNS in the neonatal period and congenital malformations of the CNS, according to neuroimaging methods; c) informed voluntary consent of the child's legal representatives to participate in the study. Exclusion criteria: a) the presence of organic lesions of the CNS, congenital malformations of the CNS, according to methods of neuroimaging; b) the presence of hereditary diseases; c) the absence of informed voluntary consent of the child's legal representatives to participate in the study.
During the study, the authors evaluated the condition of premature newborns after birth (according to the Apgar scale), the leading syndromes at birth, and the volume of resuscitation measures at birth. The authors dynamically monitored the baby's condition after transfer from the delivery room to the wards of premature newborns or to the ICU, as well as whether further respiratory support was required.
The laboratory part of the study included the evaluation of the following parameters: total antioxidant status/antioxidant capacity of blood plasma and total oxidative status/oxidative stress evaluated by blood plasma samples using the ImAnOx (TAS/TAC) Kit (Immundiagnostik, Germany) and PerOx (TOS/TOC) Kit (Immundiagnostik, Germany) reagent kits, respectively. The blood copper, zinc containing superoxide dismutase (Cu,Zn-SOD; Human Cu/ZnSOD reagent kit, Elisa Kit, eBioscience, USA), manganese superoxide dismutase (mitochondrial, manganin superoxide dismutase, Mn-SOD, SOD2; Human Superoxide Dismutase 2 reagent kit, ELISA, AbFrontier, Korea) and glutathione in reduced (GSH) and oxidized (GSSG) states (Glutathione Assay Kit, Cayman Chemical, USA) were assessed. The polymorphisms of 4 genes were determined during the study: SOD2 gene rs4880 (47C>T, Ala16Val), SOD2 gene rs1141718 (58T>C, Thr58Ile), SOD2 gene rs11575993 (60C>T, Leu60Phe), and GCLC gene rs17883901 (-129C>T). Blood samples were taken from the peripheral vein in a volume of 2 ml once in the early neonatal period (first 7 days of life) before manifestation of structural changes in the brain, according to NSG.
Statistical analysis of the obtained data was performed using the Statistica software (version 6.1), IBM SPSS Statistics 24. The type of data distribution (normal or non-normal) was determined using histograms and the Shapiro-Wilk test. The distribution of all quantitative characteristics in the study was different from normal, therefore, quantitative data were presented as Me [QL; QU], where Me is the median, QL is the lower quartile, and QU is the upper quartile. Statistical hypothesis testing was performed using the Mann-Whitney U-test (when comparing two independent variables) and Kruskal-Wallis H-test (when comparing three or more independent variables). Fisher's exact test was used to compare groups by qualitative characteristics; data were presented as absolute numbers with percentages (%) and relative risk (RR) and 95% confidence interval (CI), the thresholds of which were presented as upper and lower quartiles. The Spearman correlation coefficient was used to determine the direction and strength of the relationship between the events. In statistical calculations, the critical level of error p was taken to be 0.05. The adjusted statistical significance level p<0.017 (considering the three pair-wise comparisons) was applied taking into account the Bonferroni correction. To build mathematical models, which allow calculating the probability of CNS lesions in preterm infants during the neonatal period, the authors used the method of logistic regression analysis in the NCSS2021 program with further ROC analysis and the study of the Area Under Curve in order to evaluate the quality of the developed models.
Results
Subgroup I consisted of 25 preterm infants with structural brain changes in the form of IVH and PVL. In this subgroup, 15 children were found to have IVH of varying severity (unilateral/bilateral (60%)), 5 children were diagnosed with PVL (20%), and 5 children had concurrent PVL and IVH of varying severity (unilateral/bilateral (20%)).
Subgroup II included premature infants who never had structural changes of the brain, according to the NSG data, during the regular follow-up. There were 35 such patients in total.
The authors compared subgroup I and subgroup II, according to different clinical and anamnestic findings. The GA of children in subgroup I was 29 weeks [ 26;33], and in subgroup II, it was 34 weeks [ 31;35]. Thus, the GA of preterm infants with identified CNS lesions was statistically lower than that of preterm infants without CNS lesions (M-U, p=0.0013).
Further, the structure of the leading syndromes in premature newborns at birth was assessed. The obtained data are presented in Table 1.
Таблица / Table 1
Характеристика ведущих синдромов при рождении в подгруппах I и II
Characteristics of leading syndromes at birth in subgroups 1 and 2
|
Показатель Indicator |
Недоношенные новорожёенные с поражением ЦНС (I подгруппа, n=25) Preterm infants with injury of the CNS (subgroup 1, n=25) |
Недоношенные новорождённые без поражения ЦНС (II подгруппа, n=35) Preterm infants without injury of the CNS (subgroup 2, n=35) |
Уровень значимости различий, p Significance level of differences, p |
||
|
Абс. Abs. |
% |
Абс. Abs. |
% |
||
|
Угнетение Depression |
14 |
56,0 |
23 |
65,71 |
0,5910 |
|
Возбуждение Arousal |
0 |
0 |
2 |
5,71 |
0,5056 |
|
Асфиксия Asphyxia |
2 |
8,0 |
5 |
14,29 |
0,6882 |
|
Период адаптации Period of adaptation |
1 |
4,0 |
6 |
17,14 |
0,2216 |
|
Дыхательная недостаточность/ дыхательные нарушения Respiratory failure/ respiratory disorders |
21 |
84,0 |
18 |
51,43 |
0,0132* |
Примечание: *различия между группами статистически значимы (p<0,05); сравнение с помощью критерия Фишера — двустороннее.
Note: *differences between groups are statistically significant (p<0,05); comparison using the Fisher test two-sided.
Respiratory failure syndrome at birth prevailed in subgroup I, which was statistically significant (Fisher's test, two-sided, p=0.0132, OR 1.63; 95% CI 1.13; 2.35).
Apgar scores at the 1st and 5th minutes of life in children with structural brain changes were statistically lower than those in children without IVH and PVL. Apgar scores at 1 minute of life were 5 [ 3;5] in the first subgroup and 6 [ 4;7] in the second subgroup (M-U, p=0.0052). The Apgar score at 5 minutes of life in the first subgroup was 6 points [ 5;7] and 7 points in the second subgroup [ 7;7] (M-U, p=0.0057).
Some patients in both subgroups underwent resuscitation. Their volume is presented in Table 2.
Таблица / Table 2
Объём реанимационных мероприятий при рождении в подгруппах I и II
Resuscitation at birth in subgroups 1 and 2
|
Показатель Indicator |
Недоношенные новорождённые с поражением ЦНС (I подгруппа, n=25) Preterm infants with injury of the CNS (subgroup 1, n=25) |
Недоношенные новорождённые без поражения ЦНС (II подгруппа, n=35) Preterm infants without injury of the CNS (subgroup 2, n=35) |
Уровень значимости различий, p Significance level of differences, p |
||
|
Абс. Abs. |
% |
Абс. Abs. |
% |
||
|
Искусственная вентиляция лёгких маской при рождении Artificial ventilation of the lungs with a mask at birth |
12 |
48,0 |
16 |
45,71 |
1,0000 |
|
Методика Constant positive airway pressure при рождении Constant positive airway pressure technique at birth |
11 |
44,0 |
16 |
45,71 |
1,0000 |
|
Интубация при рождении Intubation at birth |
9 |
36,0 |
4 |
11,43 |
0,0297* |
|
Непрямой массаж сердца Indirect cardiac massage |
0 |
0 |
0 |
0 |
1,0000 |
|
Введение лекарственных препаратов The administration of medicines |
0 |
0 |
0 |
0 |
1,0000 |
Примечание: *различия между группами статистически значимыы (p<0,05); сравнение с помощью критерия Фишера — двустороннее.
Note: *differences between groups are statistically significant (p<0,05); comparison using the Fisher test two-sided.
The subgroups differed significantly in the rate of tracheal intubation at birth (Fisher's test, two-sided, p=0.0297, OR 3.15; 95% CI 1.09; 9.09).
The dynamic follow-up of children in the main group showed that premature infants with IVH and PVL required respiratory support after transfer from the delivery room in a higher percentage of cases (22 children in subgroup I (88.0%) versus 17 children in subgroup II (48.57%) (Fisher's test, two-sided, p=0.0022, OR 1.81; 95% CI 1.25; 2.62). Invasive respiratory support in the ICU was required significantly more often (14 children in subgroup I (56.0%) vs 6 children in subgroup II (17.14%), Fisher's test two-sided, p=0.0024, OR 3.27; 95% CI 1.46; 7.32). Noninvasive respiratory support in the ICU was provided to 13 children in subgroup I (52.0%) and 11 children in subgroup II (31.43%). There were no significant differences revealed in this parameter (Fisher's test, two-sided, p=0.1204).
The structure of diagnoses in children from the main group in the early neonatal period was represented by the following conditions: seizure syndrome, RDS, transient tachypnea, congenital pneumonia, pulmonary hypertension, jaundice associated with shortened gestation, conjugation hyperbilirubinemia, hemolytic disease of the newborn, open oval window, open arterial duct, abnormal left ventricular chords, congenital heart defects, concentric left ventricular hypertrophy, pyelectasis, congenital malformations, anemia of various genesis, hypoglycemia, electrolyte disorders, polycythemia, hemorrhagic syndrome, disseminated intravascular coagulation syndrome, hepatic hematoma, torticollis, umbilical hernia, diabetic fetopathy, cold trauma, small by GA, small weight by GA, large by GA. Seven children in the first subgroup (28.0%) and one child in the second subgroup (2.86%) had clinical and laboratory findings suggestive of intrauterine infection (IUI). Statistical differences between subgroups were found for this parameter (Fisher's test, two-sided, p=0.007, OR 9.8; 95% CI 1.29; 74.73).
The correlation analysis (Spearman correlation) revealed the following significant associations: there was a negative association of average strength between the presence of IVH and PVL and GA of the baby (r=-0.41, p<0.05), body weight at birth (r=-0.38, p<0.05), Apgar scores at 1 and 5 minutes of life (r=-0.35, p<0.05 and r=-0.34, p<0.05 respectively). The direct correlation of average strength between the presence of organic lesions of the CNS in premature infants and respiratory failure syndrome at birth (r=0.34, p<0.05), invasive continuous mandatory ventilation in the ICU (r=0.41, p<0.05), clinical and laboratory data indicating IUI during the neonatal period (r=0.39, p<0.05) was established.
The authors evaluated some parameters of the antioxidant system. The obtained data are presented in Table 3.
Таблица / Table 3
Показатели антиоксидантной системы у новорождённых основной и контрольной групп, Ме [QL; QU]
Indicators of the antioxidant system in newborns in the main and control groups, Me [QL; QU]
|
Показатель Indicator |
Основная группа (n=60) Main group (n=60) |
Контрольная группа (n=35) Control group (n=35) |
Уровень значимости различий между I подгруппой, II подгруппой и контрольной группами, p Significance level of differences between subgroup 1, subgroup 2 and control groups, p |
Уровень значимости различий между I подгруппой и II подгруппой, p Significance level of differences between subgroup 1 and subgroup 2, p |
Уровень значимости различий между контрольной группой и II подгруппой, p Significance level of differences between the control group and subgroup 2, p |
|
|
Недоношенные новорождённые с поражением ЦНС (I подгруппа, n=25) Preterm infants with injury of the CNS (subgroup 1, n=25) |
Недоношенные новорождённые без поражения ЦНС (II подгруппа, n=35) Preterm infants without injury of the CNS (subgroup 2, n=35) |
Доношенные новорождённые Full-term newborns |
||||
|
Cu,Zn-СОД, нг/мл Cu,Zn-SOD, ng/ml |
136 [ 86;250] |
264 [ 170;432] |
235 [ 176;356] |
0,0045* |
0,0021** |
0,5049 |
|
Mn-СОД, нг/мл Mn-SOD, ng/ml |
48,56 [ 42,20; 63,36] |
50,73 [ 35,06; 70,48] |
59,19 [ 42,80; 72,51] |
0,5559 |
0,8396 |
0,3104 |
|
GSH, мкмоль GSH, µmol |
3,88 [ 2,28;7,26] |
14,78 [ 9,32;17,82] |
14,37 [ 9,30;17,39] |
<0,0001* |
0,0001** |
0,9700 |
|
GSSG, мкмоль GSSG, µmol |
1,94 [ 1,14;3,63] |
7,39 [ 4,66;8,91] |
7,19 [ 4,65;8,70] |
<0,0001* |
0,0001** |
0,9700 |
|
TAS/TAC, мкмоль/л TAS/TAC, µmol/l |
320,65 [ 253,81;384,09] |
391,65 [ 381,52;393,94] |
370,28 [ 348,34;390,82] |
0,0002* |
0,0001** |
0,0806 |
Примечания: *различия между группами статистически значимы (p<0,05); сравнение нескольких групп переменных с помощью критерия Краскела-Уоллиса; **различия между группами статистически значимы, сравнение двух независимых переменных с помощью критерия Манна-Уитни (р<0,017, скорректированный уровень статистической значимости с учётом поправки Бонферрони).
Notes: *differences between groups are statistically significant (p<0,05); comparison of several groups of variables using the Kruskal-Wallis test; **differences between groups are statistically significant, comparison of two independent variables using the Mann-Whitney test (p<0,017, adjusted level of statistical significance, taking into account the Bonferroni correction).
In the subgroup of premature infants with IVH and PVL, the levels of Cu, Zn-SOD, GSH, GSSG, and total antioxidant capacity (TAS/TAC) were lower than in the group without organic CNS lesions (M-U, p<0.017). The control group and premature infants without structural CNS changes did not differ significantly in the above parameters (M-U, p>0.017).
In subgroup I, an analysis of the TOS/TOC parameter revealed that 18 preterm infants had low oxidative stress (72.0%) and 7 infants had moderate to high oxidative stress (28.0%). All premature infants without IVH and PVL (n=35) had low levels of oxidative stress (100%). Twenty-four preterm infants had low levels of oxidative stress (96.0%) and only 1 infant had a moderate level (4.0%). Premature infants with CNS lesions had greater oxidative stress than infants without CNS lesions in the neonatal period (Fisher's exact test, two-sided, p=0.0012, 95% CI Infinity; NaN; Infinity). Preterm infants and premature infants without CNS lesions did not differ by this parameter (Fisher's exact test, two-sided, p=0.4167).
Further, the authors compared antioxidant system parameters in pairs in the formed groups depending on GA: preterm infants with GA up to and including 31 weeks and preterm infants with GA 32 weeks or more. The results are presented in Table 4.
Таблица / Table 4
Показатели антиоксидантной системы у детей основной группы в зависимости от гестационного возраста, Ме [QL; QU]
Indicators of the antioxidant system depending on gestational age in children in the main group, Me [QL; QU]
|
Показатель Indicator |
Недоношенные новорожденные с поражением ЦНС, ГВ менее 31 недели включительно (n=14) Preterm infants with injury of the CNS, |
Недоношенные новорожденные без поражения ЦНС, ГВ менее 31 недели включительно (n=9) Preterm infants without injury of the CNS, GA less than 31 weeks inclusive (n=9) |
Уровень значимости различий, p Significance level of differences, p |
Недоношенные новорожденные с поражением ЦНС, ГВ 32 недели и более (n=11) Preterm infants with injury of the CNS, GA 32 weeks or more (n=11) |
Недоношенные новорожденные без поражения ЦНС, ГВ 32 недели и более (n=26) Preterm infants without injury of the CNS |
Уровень значимости различий, p Significance level of differences, p |
|
Cu,Zn-СОД, нг/мл Cu,Zn-SOD, ng/ml |
137,00 [ 92,00;168,00] |
180 [ 164;336] |
0,1386 |
136,00 [ 84,00;326,00] |
296,00 [ 210,00;440,00] |
0,0249* |
|
GSH, мкмоль GSH, µmol |
2,9 [ 2,06;4,84] |
12,58 [ 2,6;14,78] |
0,1227 |
6,32 [ 2,94;10,14] |
15,84 [ 10,7;17,95] |
0,0055* |
|
GSSG, мкмоль GSSG, µmol |
1,45 [ 1,03;2,42] |
6,29 [ 1,3;7,39] |
0,1227 |
3,16 [ 1,47;5,07] |
7,92 [ 5,35;8,97] |
0,0055* |
|
TAS/TAC, мкмоль/л TAS/TAC, µmol/l |
294,17 [ 246,80; 340,71] |
372,01 [ 296,98;391,65] |
0,1388 |
371,59 [ 253,81;391,17] |
392,62 [ 386,17;395,19] |
0,0019* |
Примечание: *различия между группами статистически значимы (p<0,05); сравнение двух независимых переменных с помощью критерия Манна-Уитни.
Note: *differences between groups are statistically significant (p<0,05); comparison of two independent variables using the Mann-Whitney test.
Discussion
The studied parameters in children with and without CNS lesions with GA less than 31 weeks (inclusive) did not differ significantly (M-U, p>0.05 for all studied parameters). However, they were lower in the group of children with IVH and PVL. The comparison of the same groups, but with GA of 32 weeks or more, revealed significant differences. The studied parameters were lower (M-U, p<0.05 for all studied parameters) in the group of children with IVH and PVL.
The correlation analysis (Spearman correlation) revealed the following significant associations: there was a negative association of medium strength between the probability of IVH and PVL and the level of GSSG and GSH (r=-0.40, p<0.05 and r=-0.44, p<0.05, respectively), total blood antioxidant capacity, and organic lesion of the CNS (r=-0.50, p<0.05). The relationship between the oxidative stress index and an organic CNS lesion was direct and medium strength (r=0.40, p<0.05). The relationship was negative weak (r=-0.20, p<0.05) for Cu, Zn-SOD levels.
The study showed that there were no significant differences in the occurrence of the studied genotypes and alleles among premature infants with IVH and PVL and without CNS lesions, as well as between full-term and pre-term infants.
The result of this study allowed the authors to develop two prognostic models to determine the likelihood of developing IVH and PVL in the neonatal period in premature infants (model 1 and model 2). These models include such parameters as GA, Apgar score at 1 and 5 minutes of life, presence of RDS at birth, invasive continuous mandatory ventilation in the neonatal period in the ICU, and the presence of clinical and laboratory data indicating IUI in the neonatal period (Figure 1).
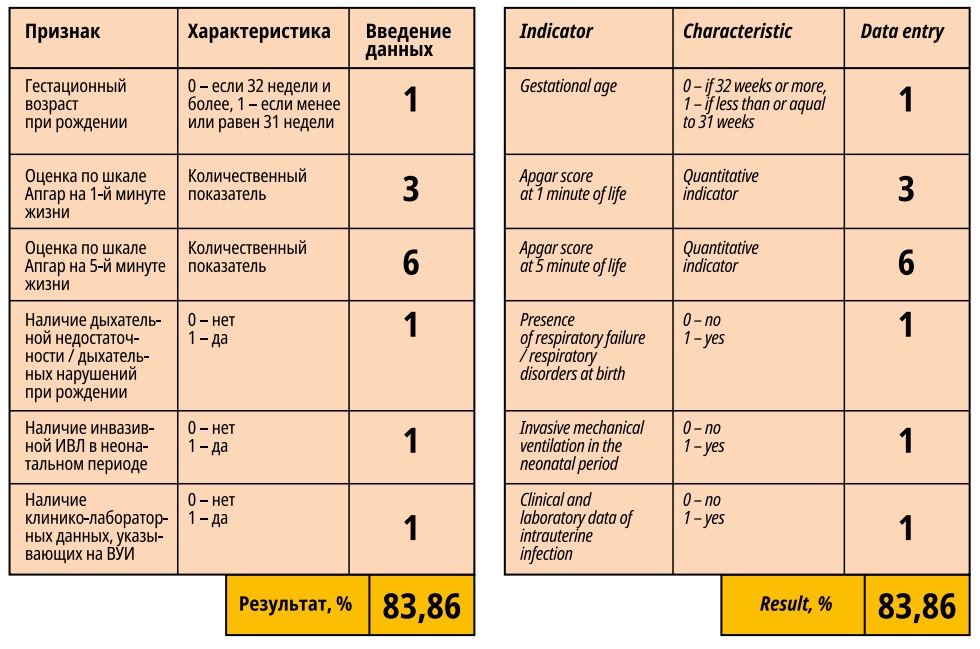
Figure 1. Program for assessing the probability of developing an organic injury of the central nervous system in the neonatal period in premature newborns. Model 1.
An extended version of the model (model 2) takes into account reduced glutathione levels (GSH) and total blood antioxidant capacity (TAS/TAC) determined in the early neonatal period (Figure 2).

Figure 2. Program for assessing the probability of developing an organic injury of the central nervous system in the neonatal period in premature newborns. Model 2.
The assessment of the quality of the developed models showed that the area under the ROC curve was 0.84345 for model 1 and 0.87202 – for model 2, i.e. the quality of both models is "very good".
Discussion
As a rule, the incidence of organic CNS damage in preterm infants is inversely proportional to GA. Morpho-functional maturation of the brain structures that are important in the implementation of organic CNS damage occurs up to the end of the third trimester of pregnancy, so children born prematurely are a particularly vulnerable category of patients.
Apgar's score is a routine method of examination in the practice of a neonatologist. A low score indicates failure of adaptation to extrauterine life and the need for a certain amount of resuscitation measures in this category of patients, which may also contribute to the development of IVH and PVL in the neonatal period.
The etiology of RDS in preterm infants during the neonatal period can be associated with numerous conditions, but the most relevant are RDS and pneumonia. Respiratory failure leads to the stasis in the superior vena cava resulting in slowed blood flow, venous stasis in the vena Galeni (periventricular areas of the lateral ventricles of the brain), and aggregation of blood form elements. The forming sludges occlude capillaries and neglected capillaries appear, which subsequently collapse. All these lead to impeded blood flow in the microcirculatory channels and can contribute to the state of cerebral hypoperfusion, which plays a role in the occurrence of PVL. It is noted that during continuous mandatory ventilation, there may be fluctuation of cerebral blood flow and increased cerebral venous pressure, which can lead to the realization of IVH in the conditions of imperfect autoregulation of cerebral blood flow in preterm infants.
The association between the formation of IVH and PVL in patients and the "presence of clinical and laboratory data indicating IUI" was also established. This parameter was included in the models. Infectious agents can affect the vascular wall and cerebral blood flow autoregulation processes in newborns, activate microglia, and influence oligodendrocyte precursors [7][23][24].
Oxidative stress is a condition that results from a physiological imbalance between antioxidant and oxidant levels in favor of oxidants.
In this study, the total blood antioxidant capacity (TAS/TAC) was lower in the group of premature infants with different variants of organic CNS lesions. At the same time, this category of patients experienced greater oxidative stress than infants without structural brain changes. That is, there was an imbalance between protective factors, on the one hand, and damaging factors, on the other hand, which could result in the realization of IVH and PVL.
Glutathione is a tripeptide (L-γ-glutamyl-L-cysteinylglycine, L-γ-glutamyl-L-cysteinylglycine) that performs multiple functions in living organisms: it acts as an antioxidant, either directly interacting with reactive oxygen or nitrogen forms and electrophiles or acting as a cofactor for various enzymes. The role of glutathione was described for ischemia-reperfusion processes-based diseases, which is also relevant in the context of organic CNS damage in premature infants [25].
Thus, analysis of the features of clinical and anamnestic and laboratory parameters of premature infants with IVH and PVL allows establishing the most significant risk factors for the development of the latter and their prognostic significance. Premature infants with low values of GSH, GSSG, total blood antioxidant capacity, and exposed to moderate or high oxidative stress in the early neonatal period have a significant risk of developing structural changes in the brain. Comprehensive assessment of the clinical and anamnestic data of premature newborns at birth, as well as the determination of parameters considering the level of antioxidant protection, such as GSH and total blood antioxidant capacity, will identify the premature neonate risk group for organic lesions of the CNS and adjust therapeutic management tactics in the neonatal period.
Conclusions
- Premature infants with structural changes of the brain had lower GA, birth weight, Apgar scores at 1 and 5 minutes of life, more often developed RDS (respiratory impairments) in the delivery room, primarily required tracheal intubation as part of resuscitation measures, and needed respiratory support after transfer from the delivery room and invasive respiratory support in the ICU. They also had signs of IUI in the neonatal period more frequently. The identified correlations indicate a significant role of the above data in the genesis of organic lesions of the CNS.
- The distribution of allele and genotype prevalence by the studied polymorphisms of antioxidant system enzyme genes did not differ significantly in the comparison of children with and without organic CNS lesions in the neonatal period, as well as in their comparison with the pre-term neonatal group.
- Premature infants with organic lesions of the CNS had lower values of Cu,Zn-SOD, GSH, GSSG, total blood antioxidant capacity, and experienced greater oxidative stress than children without CNS lesions in the neonatal period. The indices of Cu,Zn-SOD, GSH, GSSG, and total blood antioxidant capacity in preterm infants with IVH and PVL in the group of children with GA less than 31 weeks (inclusive) and 32 weeks or more were lower than those in the corresponding GA groups of children but without organic lesion of the CNS. Still, significant differences were confirmed only for the group of preterm children with GA of 32 weeks or more. The identified correlations indicate a significant role of the levels of GSSG and GSH, the index of total blood antioxidant capacity, and oxidative stress in the genesis of structural changes in the brain of preterm infants.
- Prognostic models have been developed to determine the probability of the development of IVH and PVL in the neonatal period in premature infants based on clinical and anamnestic data and indicators that include the state of antioxidant protection.
References
1. Guzeva V.I., Ivanov D.O., Aleksandrovich YU.S. [i dr.]. Neotlozhnaya nevrologiya novorozhdennyh i detej rannego vozrasta. Sankt-Peterburg : SpecLit; 2017. (In Russ.)
2. Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524-551.DOI: 10.1016/j.redox.2015.08.020.
3. Gano D. white matter injury in premature newborns. Neonatal Netw. 2016;35(2):73-77.DOI: 10.1891/0730-0832.35.2.73.
4. Bennet L., Dhillon S., Lear C.A., van den Heuij L., King V. [et al.] Chronic inflammation and impaired development of the preterm brain. J Reprod Immunol. 2018;125:45-55.DOI: 10.1016/j.jri.2017.11.003.
5. Novak C.M., Ozen M., Burd I. Perinatal brain injury: mechanisms, prevention, and outcomes. Clin Perinatol. 2018;45(2):357-375.DOI: 10.1016/j.clp.2018.01.015.
6. Perrone S., Santacroce A., Longini M., Proietti F., Bazzini F. [et al.] The free radical diseases of prematurity: from cellular mechanisms to bedside. Oxid Med Cel Long.2018.Vol.2018:1-15.
7. van Tilborg E., de Theije C.G.M., van Hal M., Wagenaar N., de Vries L.S. [et al.] Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia. 2018;66(2):221-238.DOI: 10.1002/glia.23256.
8. Volpe J.J. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr Neurol. 2019 Jun;95:42-66.DOI: 10.1016/j.pediatrneurol.2019.02.016.
9. Boyle A.K., Rinaldi S.F., Norman J.E., Stock S.J. Preterm birth: Inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62-66.DOI: 10.1016/j.jri.2016.11.008.
10. Elders P.N.D., In 't Veld J., Termote J., de Vries L.S., Hemels M.A.C. [et al.] Congenital cytomegalovirus infection and the occurrence of cystic periventricular leukomalacia. Pediatr Neurol. 2018;79:59-60. DOI:10.1016/j.pediatrneurol.2017.10.016.
11. Lawrence S.M., Wynn J.L. Chorioamnionitis, IL-17A, and fetal origins of neurologic disease. Am J Reprod Immunol. 2018;79(5):e12803.DOI: 10.1111/aji.12803.
12. Poryo M., Boeckh J.C., Gortner L., Zemlin M., Duppré P. [et al.] Ante-, peri- and postnatal factors associated with intraventricular hemorrhage in very premature infants. Early Hum Dev. 2018;116:1-8.DOI: 10.1016/j.earlhumdev.2017.08.010.
13. Huang J., Meng J., Choonara I., Xiong T., Wang Y. [et al.] Antenatal infection and intraventricular hemorrhage in preterm infants: A meta-analysis. Medicine (Baltimore). 2019;98(31):e16665.DOI: 10.1097/MD.0000000000016665.
14. Schneider J., Miller S.P. Preterm brain Injury: White matter injury. Handb Clin Neurol. 2019;162:155-172.DOI: 10.1016/B978-0-444-64029-1.00007-2.
15. Pai V.V., Carmichael S.L., Kan P., Leonard S.A., Lee H.C. Maternal body mass index and risk of intraventricular hemorrhage in preterm infants. Pediatr Res. 2018;83(6):1146-1151.DOI: 10.1038/pr.2018.47.
16. Polavarapu S.R., Fitzgerald G.D., Contag S., Hoffman S.B. Utility of prenatal Doppler ultrasound to predict neonatal impaired cerebral autoregulation. J Perinatol. 2018;38(5):474-481.DOI: 10.1038/s41372-018-0050-x.
17. Vesoulis Z.A., Ters N.E., Foster A., Trivedi S.B., Liao S.M. [et al.] Response to dopamine in prematurity: a biomarker for brain injury? J Perinatol. 2016;36(6):453-458.DOI: 10.1038/jp.2016.5.
18. Handley S.C., Passarella M., Lee H.C., Lorch S.A. Incidence trends and risk factor variation in severe intraventricular hemorrhage across a population based Cohort. J Pediatr. 2018;200:24-29.e3.DOI: 10.1016/j.jpeds.2018.04.020.
19. He L., Zhou W., Zhao X., Liu X., Rong X. [et al.] Development and validation of a novel scoring system to predict severe intraventricular hemorrhage in very low birth weight infants. Brain Dev. 2019;41(8):671-677.DOI: 10.1016/j.braindev.2019.04.013.
20. Nejrobiologicheskie osnovy vozniknoveniya i vosstanovitel'nogo lecheniya perinatal'nogo porazheniya central'noj nervnoj sistemy u detej. FGAU «Nauch. centr zdorov'ya detej» Minzdrava Rossii, Soyuz pediatrov Rossii; pod red. Namazovoj-Baranovoj L. S. M.: Pediatr", 2016. (In Russ.)
21. Lee J., Hong M., Yum S.K., Lee J.H. Perinatal prediction model for severe intraventricular hemorrhage and the effect of early postnatal acidosis. Childs Nerv Syst. 2018;34(11):2215-2222.DOI: 10.1007/s00381-018-3868-9.
22. Glover Williams A., Odd D., Bates S., Russell G., Heep A. Elevated international normalized ratio (INR) is associated with an increased risk of intraventricular hemorrhage in extremely preterm infants. J Pediatr Hematol Oncol. 2019;41(5):355-360.DOI: 10.1097/MPH.0000000000001509.
23. Stark M.J., Hodyl N.A., Belegar V.K.K., Andersen C.C. Intrauterine inflammation, cerebral oxygen consumption and susceptibility to early brain injury in very preterm newborns. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F137-142. DOI: 10.1136/archdischild-2014-306945.
24. Sofronova L. N., Fedorova L. A. Nedonoshennyj rebenok. Spravochnik. M.: Redakciya zhurnala StatusPraesens; 2020. (In Russ.)
25. Ighodaro O. M. First line defence antioxidants superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.2018;54(4):287-293. DOI: 10.1016/j.ajme.2017.09.001.
About the Authors
E. B. PavlinovaRussian Federation
Elena B. Pavlinova, Dr. Sci. (Med.), Associate Professor, Head of the Department of Hospital Pediatrics with Postgraduate Course, Vice-Rector for Academic Affairs
Omsk
A. A. Gubich
Russian Federation
Anastasiya A. Gubich, Ph.D student, Assistant, Hospital Pediatrics with Postgraduate Course
Omsk
O. A. Savchenko
Russian Federation
Ol`ga A. Savchenko, Cand. Sci. (Med.), , Associate Professor, Hospital Pediatrics with Postgraduate Course
Omsk
Review
For citations:
Pavlinova E.B., Gubich A.A., Savchenko O.A. Prediction injury of the central nervous system in the neonatal period in preterm newborns. Medical Herald of the South of Russia. 2022;13(2):122-133. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-122-133







































