Scroll to:
Thymic stromal lymphopoietin in bronchial asthma patients of different age groups: correlation with other markers, lung function results and disease control
https://doi.org/10.21886/2219-8075-2022-13-2-113-121
Abstract
Objective: to investigate correlation between thymic stromal lymphopoietin and bronchial asthma course and control in patients of different age groups.
Materials and methods: one hundred and four patients were included in 1-year long open prospective study. There were three age groups: children (6 –11 y.o., n=38), adolescents (14–17 y.o., n=35) and adults (25 –50 y.o., n=31). we used asthma duration ≥12 months, uncontrolled asthma and acute respiratory infection absence for ≥14 days as inclusion criteria. Clinical history, validated questionnaires, spirometry, common blood count, serum and nasal material to evaluate thymic stromal lymphopoietin were obtained during first visit. Patient were consequently examined twice with 6 months intervals. Statistical analyses included ANOVA (Kruskal-wallis test) and Pearson’s correlation (r). Differences accepted significant with р<0,05.
Resuts: prevalence of main risk factors of asthma control lost (poor compliance, obesity, non-atopic phenotype, fixed airway obstruction) was different in age groups. we didn’t find any thymic stromal lymphopoietin in nasal material. Thymic stromal lymphopoietin concentration correlate significantly with duration of uncontrolled asthma in previous 12 months (r=0,74). we have found greater serum thymic stromal lymphopoietin concentration in patients who demonstrated FEV1 below normal at Visit 3.
Conclusion. Serum thymic stromal lymphopoietin level can be used as risk factor of asthma future exacerbation and spirometry results decline.
For citations:
Kamaev A.V., Krivskaya S.A., Lyashenko N.L., Kamaeva I.A., Mizernitsky Yu.L., Shaporova N.L. Thymic stromal lymphopoietin in bronchial asthma patients of different age groups: correlation with other markers, lung function results and disease control. Medical Herald of the South of Russia. 2022;13(2):113-121. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-113-121
Introduction
In different age groups, from preschoolers to adult patients, bronchial asthma (BA) is widespread (up to 7% of the population). Current recommendations state that the purpose of BA treatment is the achievement of control, but not recovery1 [1]. BA pathogenesis is based on the chronic inflammation of the bronchial wall regulated by an imbalance of cytokines secreted by various immunocompetent cells. The most studied and most common variant of chronic inflammation is the T2 type, the key cytokines of which are interleukins 4, 5, and 13 [2]. One of the key factors determining the T2-polarization of the immune response is the airway epithelium damage and the spectrum of biologically active molecules released by epithelial cells and the innate immune system cells after this damage. An important immunological marker of this damage is the thymic stromal lymphopoietin molecule (TSLP), the study of which is of great importance in BA pathogenesis [3].
The leading marker of epithelial damage, TSLP, belongs to the group of "alarmins", non-specific signaling molecules, which also include interleukin-25 and interleukin-33. These proteins are synthesized by epithelial cells of both upper and lower respiratory tracts after contact with both infectious (viruses, bacteria) and noninfectious (pollutants, allergens) damaging factors [4]. Normally, TSLP acts as a chemoattractant of innate immune response cells and initiator of a protective inflammatory response; its excessive, pathological secretion leads to a chronic inflammatory focus with the involvement of multiple T2-polarized immunocompetent cells (eosinophils, dendritic cells, innate type 2 lymphoid cells, and type 2 T-helper cells) [5].
By producing TSLP, respiratory epithelial cells simultaneously activate both levels of the immune response: nonspecific (secretion of inflammatory cytokines by innate type II lymphoid cells) and adaptive (differentiation of naive type II T-helper cells, activation of dendritic cells). Thus, TSLP differs from other alarmins by its effects both at the local, tissue level, and at the organismal level — stimulation of maturation and differentiation of dendritic cells and lymphocytes in hematopoietic organs [6]. TSLP also directly activates mast cells causing the secretion of proinflammatory mediators independent of IdE. In addition to mastocytes, TSLP affects other key effectors of allergic inflammation, eosinophils (via protein kinase and NF-kB signaling pathways), by preventing their apoptosis and stimulating the secretion of mediators and enzymes from their granules [7]. Compared to healthy volunteers, patients with BA have higher levels of TSLP secretion in airway tissues [8]; in the group of patients with BA, TSLP concentration correlates with an increase in other mediators of T2 inflammation (interleukin-5, TNFa) [9]. In patients with a more severe BA according to clinical signs, as well as with a more pronounced deterioration of external respiratory function (ERF) parameters, a correlated increase in serum TSLP is described [8][9].
Given the significant role of TSLP in the polarization of the immune response toward chronic allergic inflammation described above, as well as this protein involvement in the pathogenesis of difficult-to-control obstruction in BA, TSLP is currently considered not only a significant diagnostic marker of respiratory allergic diseases but also a therapeutic target in severe uncontrollable BA [10].
Throughout the life of a patient with BA, therapy selection can be particularly difficult at the disease onset, with the T2 endotype often occurring in the preschool period; in adolescence and at the age of "young adults," when the internal picture of the disease changes and compliance may decrease [11]. It is also important in these age groups to assess future risks during BA, for which it is rational to use the analysis of a set of anamnestic, laboratory, and instrumental criteria [12][13].
The study objective was to assess the TSLP concentration in blood serum and nasal secretions in patients with uncontrolled BA and their correlation with indicators of respiratory function, peripheral blood eosinophils, and achievement of BA control.
Materials and methods
Over a 6-month period (September 2018 to February 2019), 104 patients were enrolled in the study. Eligibility criteria were at least 1 year of BA diagnosis, uncontrolled or partially controlled disease (according to GINA criteria and AST/cAST test data), and the absence of signs of acute respiratory infection within 14 days of the inclusion date. Smokers were not included in the study. All patients and/or their legally authorized representatives filled out an informed consent for the use of personal and medical data. This work fragment is included in a long-term observational study of real-life' clinical practice (3029GP48APP7) approved by the Ethics Committee of the Pavlov First Saint Petersburg State Medical University (meeting of November 21, 2017).
For all patients, a formal case history including the data of anamnesis and medical documents, allergy examination, and a physical examination at the time of inclusion was filled out. An atopic phenotype was defined as the presence of anamnestic confirmed sensitization to inhaled allergens and/or concomitant allergic rhinitis (AR) or atopic dermatitis (AtD).
The age and demographic characteristics, as well as selected data of allergological history in the groups of enrolled patients, are presented in Table 1.
Таблица / Table 1
Демографические характеристики включённых в исследование пациентов
Demographic features of included patients
|
Группа Group Показатель Feature |
Дети Children |
Подростки Adolescents |
Взрослые Adults |
|
Возрастной интервал, годы Age interval, years |
6-11 |
14-17 |
25-49 |
|
Количество пациентов, чел. Number of patients, prs |
38 |
35 |
31 |
|
Возраст, годы Age, years Me [Q25; Q75] |
8,3 [ 6,8; 9,9] |
16,5 [ 15,1; 17,3] |
33,7 [ 27,7; 34,7] |
|
Доля мужчин, n (%) Men share, n (%) |
27 (71) |
21 (60) |
14 (45,2) |
|
Давность постановки диагноза БА, годы Asthma duration, years Me [Q25; Q75] |
1,9 [ 1,2; 2,3] |
4,7 [ 3,5; 5,1] |
8,6 [ 5,9; 17,4] |
The authors analyzed factors associated with the onset of BA exacerbations in the preceding 12 months (allergens, respiratory infections, physical activity, stress, or contact with tobacco smoke) and the volume and age dynamics of baseline BA therapy. The authors also assessed the frequency of some phenotypic characteristics previously described as risk factors of loss of asthma control (obesity, pronounced decrease in baseline FEV1 and FEV1 after a bronchodilator below 80% of the age norm, signs of bronchial hyperresponsiveness such as the onset of BA symptoms after physical activity or contact with pollutants or cold/dry air inhalation).
At each of three visits to the clinic (inclusion and twice at 6-month intervals during the case follow-up), spirometry and a test with salbutamol (200 µg in patients under 12 years old and 400 µg – over 12 years old) were performed; the results were assessed relative to age- and height-adjusted standards by Quanier (GLI-ERS, 2012) and expressed as a percentage of proper [16]. Also, at each visit, patients (and their parents at less than 12 years of age) completed an unattended asthma control questionnaire for the previous 4 weeks (AST/cAST, https://www.asthmacontroltest.com/ru-ru/welcome/).
At the inclusion visit, all patients underwent venipuncture, blood was centrifuged for 10 minutes at 1500 rpm, and a frozen (-20 °C) serum bank was formed. A frozen nasal epithelium bank obtained by the method2 of Grigorieva was also formed: A sterile cytological probe was inserted sequentially into both lower nasal passages and then washed intensively in 1 ml of sterile PBS. The liquid was centrifuged (10 minutes, 1500 rpm); the supernatant was frozen. The results of a clinical blood test performed no later than 7 days after the inclusion visit, outside the episode of respiratory infection, were recorded.
After the patient’s inclusion was complete, serum and nasal epithelial samples were thawed and thymic stromal lymphopoietin concentration (pg/ml) was examined using an Abcam human ELISA kit (Cat. No. ab155444, Abcam, UK) with calibration and in-test control according to the manufacturer's recommendations, measuring range 10–800 pg/ml, bias no greater than 3 pg/ml.
The collected data were processed using the Statistica for Windows 10.0 computer software package (Statsoft Inc., USA). The normality of the obtained results distribution was checked according to the Shapiro criterion; data with a normal distribution are presented as the average (M) and its standard deviation (±SD); data with a different type of distribution as median and upper, lower quartile Me [Q 25; Q 75]. When comparing quantitative indicators between groups, the ANOVA Kruskal-Wallis test was used, followed by the Wilcoxon criterion (w-test). The prevalences (frequency of the trait in the groups) were compared using a chi-square test with a Bonferroni correction. The differences were considered statistically significant at p<0.05. The strength of correlations was estimated using the Pearson correlation coefficient (r).
Results
During one year of follow-up, 4 patients withdrew: one in each of the "Children" and "Adolescents" groups and two from the "Adults" group. Reasons for removing patients were exclusively non-medical: relocation and unwillingness to visit the center for visiting procedures; no adverse events requiring the completion of the study were reported. The retention of 96.2% of patients during the entire follow-up period allows considering the data obtained representative.
An increase in the phenotypic frequency with unconfirmed atopy with an increase in the age of patients with uncontrolled BA (5.3%, 8.6%, and 19.4% for the groups "Children", "Adolescents", and "Adults", respectively) was revealed; however, the differences reached statistical significance only when comparing the "Children" and "Adults" subgroups (p=0.02). Significant differences were found between the incidence of diagnosed obesity of any degree (2.6%, 14.3%, and 22.6% for the "Children", "Adolescents", and "Adults" groups, respectively), although the proportions of overweight patients were about equal (about 15%) between groups. In addition to this, patients with established obesity comorbid with BA in all age groups had a longer duration of the main diagnosis (asthma) than their peers with normal body weight; however, due to the small size of the obese patient subgroup, it was not possible to statistically correctly assess the correlation between the duration of BA and obesity (Figure 1).
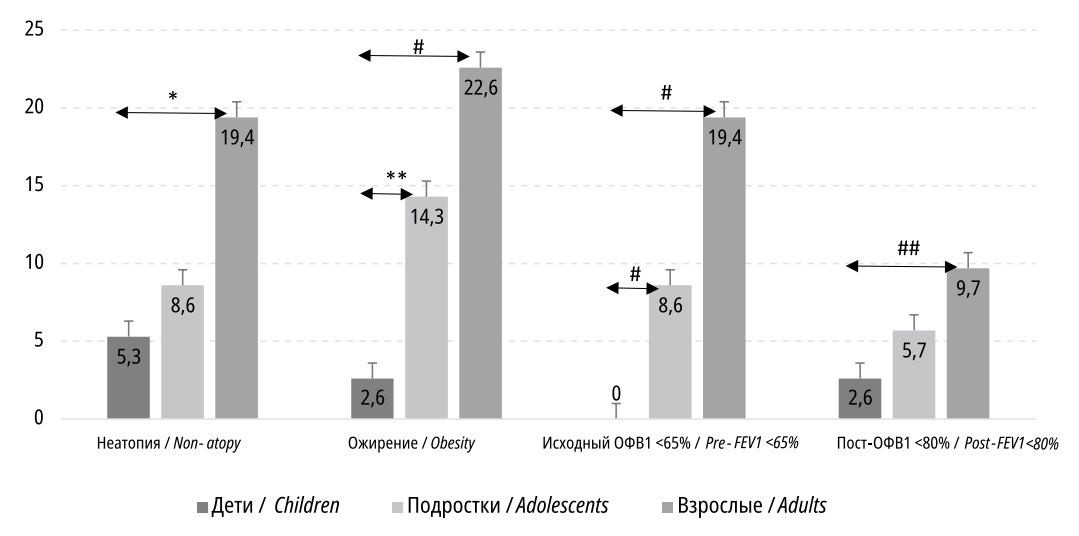
Figure 1. Patient shares (%) with peculiar phenotype and functional asthma markers, by groups
The BA functional characteristics (phenotype of severe and partially reversible obstruction) also differed significantly between age groups. The proportion of patients with an initially low level of FEV1 significantly increased with age (0%, 8.6%, and 19.4 % for the "Children", "Adolescents", and "Adults" groups, respectively); the differences are shown in Figure 1.
As a result of therapy correction (dose increase and/or change of the iGCs molecule, addition of tiotropium or intranasal GCs), elimination measures, and recommendations for regular physical activity, most patients in the "Children" and "Adolescents" age groups achieved control of BA by Visit 2; further, the proportion of patients with controllable BA also increased. In the "Adult" age group, the proportions of patients with uncontrolled BA at both visits were significantly higher than in the pediatric groups, but also tended to decrease (Table 2).
Table 2
Asthma controlled patients’ number per group, per visit
|
Group Feature |
Children (1), n=38 |
Adolescents (2), n= 35 |
Adults (3), n= 31 |
Significant differences |
|
Controlled asthma at Visit 2, n (%) |
22 (57.9) |
22 (62.9) |
13 (43.3) |
р(2-3)=0.029 |
|
Controlled asthma at Visit 3, n (%) |
28 (75.7) |
24 (71.6) |
16 (55.2) |
р(1-3)=0.018 р(2-3)=0.029 |
In any of the patients examined, biomaterial obtained by nasal brush biopsy did not contain TSLP at a concentration sufficient to be detected by the test system used.
Comparison of age groups by serum TSLP (Me [Q25; Q75] pg/ml) showed no significant differences: "Children" (715.6 [ 490.8; 883.7] pg/ml), "Adolescents" (763.3 [ 508.3; 913.5] pg/ml), and "Adults" (852.2 [ 516.4; 971.2] pg/ml). Despite the fact that there was a trend toward an increase in TSLP with increasing age, it seems statistically insignificant; further TSLP analysis was performed without dividing patients by age groups. Patients of any age interval showed a very pronounced range of values of serum TSLP, which required grouping based on another criterion to form more homogeneous patient groups. In the pooled group of patients who had not achieved BA control by Visit 3, the median serum TSLP was higher than in the pooled group of patients with control of BA by Visit 3, although this statistical significance difference was borderline: 893.3 pg/mL [ 681.9; 1031.4] versus 611.7 pg/mL [ 411.5; 697.3] (p=0.047).
The duration of uncontrolled BA by retrospective anamnestic data, in weeks per year, obtained from the Visit 1 patient questionnaire, was assessed. The range of values ranged from 18 to 39 weeks per year, and patients with a longer uncontrolled BA were found in all age groups. Most often, the reasons for a longer uncontrolled BA were indications of continued contact with the identified causative allergen (keeping pets at home) or passive smoking. Pooled analysis of data from patients of all age groups revealed a strong direct correlation between uncontrolled BA in the previous 12 months and serum TSLP (r=0.74).
An important feature of atopic BA is comorbidity with AR and/or AtD. In this study, 84 (80.8%) patients had comorbid AR; the difference in the frequency of comorbid AR statistically significantly decreased when comparing the "Children" (35 patients, 92.1%) and "Adults" (20 patients, 64.5%) subgroups (p=0.01). Concomitant AtD occurred significantly less frequently (21 (20.2%) patients), the tendency to a decrease in the frequency of the combination of AtD and BA with increasing age was the same as for AR, but due to the small sample size, statistical significance was not obtained. Comparison of TSLP concentrations in groups of patients with isolated BA (Me=489.3 [ 399.7; 631.6] pg/ml), combination of BA with AtD alone (Me=541.7 [ 415.4; 710.2] pg/ml), combination of BA with AR alone (Me=602.7 [ 511.6 834.9] pg/mL), and the simultaneous presence of the three nosologies (BA+AtD+AR) (Me=691.8 [ 554.2; 913.1] pg/mL) revealed statistically significant differences (Figure 2).
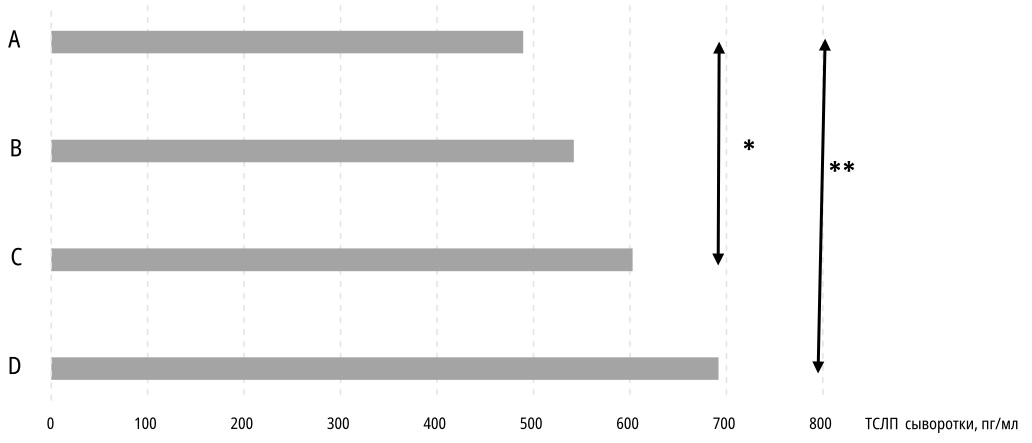
Figure 2. Thymic stromal lymphopoietin median serum concentrations in patients with isolated bronchial asthma (A), bronchial asthma with atopic dermatitis (B), bronchial asthma with allergic rhinitis (C) or all three diseases combination (D). * p=0,01; ** p=0,001
Peripheral blood eosinophils varied markedly between patients regardless of the age group. At the same time, in more than 90% of patients in each age group, these fluctuations remained within normal values (up to 350 cells/μL for the "Children" group and up to 500 cells/μL for the "Adolescents" and "Adults" groups). There was no significant relationship between serum TSLP and eosinophils in the clinical blood count.
Discussion
The BA atopic phenotype appeared to be the most common in all age groups, which was previously described repeatedly [11][15]. At the same time, even the atopic phenotype group demonstrated clinical (by the presence of control over BA, by the presence of comorbidity with AtD and/or AR) and functional (by the severity of initial obstruction and the degree of its reversibility, as well as by the stability of a decrease in FEV1 during 1 year of observation) heterogeneity, which should be taken into account when individualizing therapy in a particular patient. For the atopic BA group in different calendar periods and in patients with different baseline values of ERF, optimization of baseline therapy included adding tiotropium, increasing the dose of iGCs, and changing the delivery device. One of the important characteristics of the BA atopic phenotype is the higher probability of achieving asthma control with standard (inhaled) baseline therapy and compliance correction than with the non-atopic phenotype. Understanding both biochemical and immunological individual factors guiding and modulating chronic inflammation in the bronchi helps to optimize the asthma baseline therapy and to personalize changes in patients' drug load, especially in pediatric practice [16].
Only separate information on serum TSLP in patients with BA was published [9]. The correlation presented by the authors of this paper with the BA control indicators and the enrollment of patients of different age groups adds new information to the understanding of the inflammation pathogenesis in BA. High TSLP in the atopic BA group was unexpected. For the first time, the authors obtained results showing the relationship between this biomarker serum concentration and the sensitization type, namely, the maximum concentration in patients sensitized to house dust mites. Probably, such predominance can be caused by the pronounced proteinase activity of tick allergens and their active damaging effect on the bronchial epithelium [17]. Experimental studies showed the ability of a house dust mite extract injected endotracheally to increase TSLP secretion by the dendritic cells of the bronchial tree of mice compared to the cells of animals that were treated with a placebo (PBS) [18].
In the available literature, the authors have not found studies investigating the TSLP concentration in nasal material. The hypothesis rationale to study one of the key proinflammatory mediators of BA in nasal epithelial cells was the published experience of culturing and modeling respiratory epithelium allergic reactions and investigating various biomarkers on nasal cells, inspired by Jean Busquet's general concept of "unified airways" [19]. The authors expected to see greater activity and variability in TSLP concentrations in the airway epithelium than in serum. At the same time, it can be confidently stated that the TSLP absence in nasal biomaterial is not associated with a technological defect of biomaterial sampling, as sufficient concentrations of another important cytokine, periostin [20], and the protective protein SS-16, were found in aliquots of the same nasal supernatant (own unpublished data). Thus, the results obtained do not stack in the common hypothesis of the unity of the upper and lower respiratory tracts in relation to the allergic inflammation pathogenesis. It also remains unexplained that TCLP was not detected in nasal material, including in patients with BA comorbid with AR.
In the Russian-language literature, the authors found only one research devoted to serum TSLP in children with allergic pathology [21]. In the cited work, the main diagnosis of patients (AtD) was different and a different set of test reagents was used, which does not allow a direct comparison of raw numbers of TSLP concentrations. Nevertheless, a clear indication was obtained of the difference in serum TSLP in patients with allergic pathology and healthy control subjects, as well as an increase in this biomarker concentration with an increase in the allergic disease severity [21]. Certain parallels can be drawn here with the data obtained in the present research: higher serum TSLP in patients with BA which remained uncontrolled after 12 months of follow-up and higher serum TSLP in multimorbid patients.
These data may be important in determining the timing of the conventional baseline therapy in patients with BA of different ages and in the selection of candidates for therapy with monoclonal antibodies, both already registered in Russia and taking into account the expected entry into clinical practice of tezepelumab [10].
Conclusion
Uncontrolled BA significantly reduces a patient's quality of life, increases the risk of adverse outcomes (reduced lung function, hospitalization, mortality), and increases the cost to the health care system as a whole and the labor costs of a particular physician observing such a patient. Based on current scientific data, the prognosis for such patients is determined by regular personalized anti-inflammatory therapy and monitoring inflammation in the bronchi. Highly invasive bronchoalveolar lavage and bronchial biopsy techniques are not applicable in clinical, especially outpatient, practice; the practitioner needs tools for the gentle monitoring of allergic inflammation. In combination with a detailed anamnestic evaluation, a dynamic study of functional indices, determination of serum TSLP can be considered as a promising monitoring and prognostic tool for long-term BA management in patients of different ages.
1. Federal Clinical Guidelines – Bronchial Asthma. 2021, Moscow, Russian Respiratory Society/Russian Association of Allergology and Clinical Immunology/Union of Pediatricians of Russia, 114 p.
2. RADAR. Allergic rhinitis in children: Recommendations and algorithm for pediatric allergic rhinitis. Moscow: Original-Maket, 2015. 80 p.
References
1. Batozhargalova B.T., Mizernitsky Yu.L., Podolnaya M.A. Meta-analysis of the prevalence of asthma-like symptoms and asthma in Russia (according to the results of ISAAC). Rossiyskiy Vestnik Perinatologii i Pediatrii (Russian Bulletin of Perinatology and Pediatrics). 2016;61(4):59-69. (In Russ.) DOI: 10.21508/1027-4065-2016-61-4-59-69
2. Koczulla AR, Vogelmeier CF, Garn H, Renz H. New concepts in asthma: clinical phenotypes and pathophysiological mechanisms. Drug Discov Today. 2017;22(2):388-396. DOI: 10.1016/j.drudis.2016.11.008
3. Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3(2):138-47. DOI: 10.1038/mi.2009.134
4. Loxham M, Davies DE. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J Allergy Clin Immunol. 2017;139(6):1736-1751. DOI: 10.1016/j.jaci.2017.04.005
5. Hellings Pw, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145(6):1499-1509. DOI: 10.1016/j.jaci.2020.04.010
6. Lin SC, Cheng FY, Liu jj, Ye YL. Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma. Int J Mol Sci. 2018;19(4):1231. DOI: 10.3390/ijms19041231
7. Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129(4):1441-1451. DOI: 10.1172/jCI124606
8. Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130(4):845-52. DOI: 10.1016/j.jaci.2012.07.010
9. Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):104-11.e1-9. DOI: 10.1016/j.jaci.2011.08.031
10. Dorey-Stein ZL, Shenoy KV. Tezepelumab as an Emerging Therapeutic Option for the Treatment of Severe Asthma: Evidence to Date. Drug Des Devel Ther. 2021;15:331-338. DOI: 10.2147/DDDT.S250825
11. Semernik OE, Lebedenko AA, Appoeva AA, Kobtseva DY, Rudenko VR. Frequency of inpatient care for children with bronchial asthma and atopic dermatitis in a large industrial city. Allergology and immunology in Pediatrics. 2020;63(4):23-28. (In Russ.). DOI 10.24411/2500-1175-2020-10015
12. Lebedenko A.A., Shkurat T.P., Mashkina E.V., Semernik O.E., Dreyzina T.K. An analysis of association between growth factor gene polymorphisms and a risk of bronchial asthma in children. PULMONOLOGIYA. 2018;28(1):7-12. (In Russ.). DOI: 10.18093/0869-0189-2018-28-1-7-12
13. Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) II. J Allergy Clin Immunol. 2018;141(5):1590-1597.e9. DOI: 10.1016/j.jaci.2017.07.014
14. Quanjer PH, Brazzale Dj, Boros Pw, Pretto jj. Implications of adopting the Global Lungs Initiative 2012 all-age reference equations for spirometry. Eur Respir J. 2013;42(4):1046-54. DOI: 10.1183/09031936.00195512
15. Nenasheva N.M. Т2-high and T2-low bronchial asthma, endotype characteristics and biomarkers. PULMONOLOGIYA. 2019;29(2):216-228. (In Russ.) DOI: 10.18093/0869-0189-2019-29-2-216-228
16. Lebedenko A.A., Semernik O.E., Tyurina E.B., Appoeva A.A., Musiychuk N.S., Donskova N.S. The role of macroelements in the pathogenesis of bronchial asthma in children. Medical Herald of the South of Russia. 2021;12(2):43-47. (In Russ.) DOI: 10.21886/2219-8075-2021-12-2-43-47
17. jacquet A, Robinson C. Proteolytic, lipidergic and polysaccharide molecular recognition shape innate responses to house dust mite allergens. Allergy. 2020;75(1):33-53. DOI: 10.1111/all.13940
18. Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard wj. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187(3):1207-11. DOI: 10.4049/jimmunol.1100355
19. Stokes AB, Kieninger E, Schögler A, Kopf BS, Casaulta C, et al. Comparison of three different brushing techniques to isolate and culture primary nasal epithelial cells from human subjects. Exp Lung Res. 2014;40(7):327-32. DOI: 10.3109/01902148.2014.925987
20. Kamaev A.V. Periostin as a predictor of uncontrolled asthma and lung function decrease in patient of different age groups. The Scientific Notes of the Pavlov University. 2020;27(4):71-79. (In Russ.) DOI: 10.24884/1607-4181-2020-27-4-71-79
21. Zaynullina O.N., Pechkurov D.V., Hismatullina Z.R., Gankovskaya L.V. The pilot study of Toll-like receptor level 2 and thymic stromal lymphopoietin in children with atopic dermatitis. Pediatria n.a. G.N. Speransky. 2021;100(2):64–71. (In Russ.). DOI: 10.24110/0031-403x-2021-100-2-64-71
About the Authors
A. V. KamaevRussian Federation
Andrey V. Kamaev, Cand. Sci. (Med.), allergist, pediatrician, associated professor at general practice (family medicine) department
Saint Petersburg
S. A. Krivskaya
Russian Federation
Svetlana A. Krivskaya, allergist
Saint Petersburg
N. L. Lyashenko
Russian Federation
Natalia L. Lyshenko, allergist, pediatrician, assistant professor at general practice (family medicine) department
Saint Petersburg
I. A. Kamaeva
Russian Federation
Irina A. Kamaeva, Cand. Sci. (Med.), allergist, pediatrician, associated professor at general practice (family medicine) department
Saint Petersburg
Yu. L. Mizernitsky
Russian Federation
Yury L. Mizernitsky, Dr. Sci. (Med.), Prof., pulmonologist, pediatrician, full professor, head of chronic, inflammatory and allergic lung diseases
Moscow
N. L. Shaporova
Russian Federation
Natalia L. Shaporova, Dr. Sci. (Med.), Prof., pulmonologist, head of general practice (family medicine) department
Saint Petersburg
Review
For citations:
Kamaev A.V., Krivskaya S.A., Lyashenko N.L., Kamaeva I.A., Mizernitsky Yu.L., Shaporova N.L. Thymic stromal lymphopoietin in bronchial asthma patients of different age groups: correlation with other markers, lung function results and disease control. Medical Herald of the South of Russia. 2022;13(2):113-121. (In Russ.) https://doi.org/10.21886/2219-8075-2022-13-2-113-121
JATS XML







































