Scroll to:
Biomarkers of the type of recurrent uterine myoma-associated growth
https://doi.org/10.21886/2219-8075-2021-12-4-6-11
Abstract
Objective: To assess the diagnostic value of biomarkers: microbiological, molecular and biological, immunological biomarkers, characteristic of various types of recurrent myoma-associated growth.
Materials and Methods: Seventy women of reproductive age with recurrent uterine myoma and its combination with adenomyosis after conservative treatment in the Clinic of Kuban State Medical University were examined. Methods: microbiological examination, sonography, Doppler sonography, histology, immunohistochemistry, morphometry.
Results: The type of recurrent myoma-associated growth was proved to be dependent on molecular and biological characteristics of tumors, the presence of infection and blood perfusion. It was indicated that women with recurrence of myoma-associated growth of a “false” type were characterized by high rates of infections (the presence of reproductive losses, chronic inflammatory diseases of the pelvic organs) and significant bacterial contamination of the genital tract biotopes. Blood perfusion features were identified for true and “false” types of recurrent myoma-associated growth based on Doppler sonography data, which were consistent with features of tumor vessel morphometry. Analysis of uterine myoma histological types and their vascularization features showed correlation of forms with a high proliferative potential of a tumor on a molecular and cellular level to moderate and high expression of steroid hormone receptors in combination with Ki67, a significant diameter of the lumen of the vessels with the highest VI and VFI values.
Conclusions: A comprehensive study of women with uterine hyperplasia determines the possibility of prediction of pathogenetic variants of recurrent myoma-associated tissue growth and adequate choice of treatment options and rehabilitation course.
Keywords
For citations:
Bashirov E.V., Krutova V.A., Kutsenko I.I. Biomarkers of the type of recurrent uterine myoma-associated growth. Medical Herald of the South of Russia. 2021;12(4):6-11. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-6-11
Introduction
The high rate of uterine myoma (UM) recurrence and its combinations with adenomyosis provide relevance to the study of the consistencies of their development with a focus on the factors that stimulate the growth of cells in the mucous membrane of the uterus and myometrium and genetic determinacy of the diseases1 [1][2]. Despite the generally acknowledged mechanisms of molecular-cellular growth of the myoma and its combination with adenomyosis, the unconsolidated and controversial data do not allow the specialists to structure them for the establishment of the tactics of management of this cohort of patients before and after various therapeutical approaches. Data on the types of nodules growth is published from the perspective of histological peculiarities of the tumor, i.e. proliferation of myogenic elements that determine the effectiveness of hormonal therapy (HT) in patients with a true type and an increase in size due to the blood supply failure, edema, and degenerative changes in patients with a “false” type2[1]. It is relevant to expand the knowledge on the etiology of recurrence of uterine myoma-associated growth variants based on a multi-aspect study of the molecular-biological peculiarities of the tumor, various types of nodule vascularization, specification of microbiota peculiarities in the genital tract, and anamnestic factors of the infectious-inflammatory etiology.
The study aimed to assess the diagnostic value of biomarkers: microbiological, molecular and biological, immunological biomarkers, characteristic of various types of recurrent myoma-associated growth.
Materials and Methods
Seventy women of reproductive age with recurrent uterine myoma and its combination with adenomyosis after conservative treatment in the Clinic of Kuban State Medical University were examined.
Patients included in the study were prospectively split into groups based on the results of clinical and instrumental methods for the verification of UM and its combination with adenomatosis.
The studied patients were split into a group with “false” nodule growth (n=32) and true nodule growth (n=38) based on the type of myoma-associated growth recurrence. The age of women ranged from 22 to 45 years old, the mean age was 36.2 ± 5.2 years old.
The criteria of inclusion in the study included myoma-associated nodules of all types with diameters from 2 to 10 cm, their combination with adenomatosis, no hormone therapy for at least 3 months before surgical treatment, and a signed form of informed consent.
The study design included pelvic ultrasonography, hysteroscopy, a pathomorphological study of the obtained specimens, especially mucous membrane of the uterus, enucleate myoma nodules, and dissected adenomyosis specimens. The complex study of microbiota included urogenital smear, a bacteriological study of the cervical discharge, and endometrium. The expression of estrogen receptors (clon SP-1), progesterone (clon SP-2), protein Ki-67 (clon SP-6), vimentin (clon SP20), and collagen IV type (Spring BioScience, USA) in tumor nodules were studied using diluted ready-to-use monoclonal antibodies. The variants of expression were evaluated as “no”, “weak”, “moderate”, “expressed”, “<10”, “<100”, “<200”, “<300”, respectively.
Morphometry was performed with module software Zen 2012 (blue edition). For the analysis of the blood circulation system, the total area and width of the vascular lumen, and the thickness of the vascular wall in the dissected specimens. The results were accumulated in the systemic tables and processed with calculation software.
3D-doppler sonography of UM was used to evaluate the vascularization index (VI), blood flow index (FI), and vascular-flow index (VFI).
The choice of the variant of surgical treatment was made individually (traditional laparotomy, endoscopy, uterine arteries embolization) based on objective and subjective factors.
The statistical analysis was made with IBM SPSS Statistics 23 software. The obtained data were analyzed with methods of parametric analysis according to the normality of the distribution. The evaluation of intergroup differences in the features with continuous distribution was made with Student’s t-test. The zero statistical hypothesis (on the lack of significant differences or factor-induced influence) was significant at 0.05.
Earlier, the materials included in the present study were used in the dissertation for the PhD degree “Differentiated choice of organ-sparing technologies of surgical treatment for uterine myoma and its combinations with adenomyosis”.
Results
The authors performed a thorough analysis of the gynecological anamnesis in the studied groups to reveal and systemize predicting factors for the development of the hyperplastic process in the uterus (HPU) and recurrence of myoma-associated growth. It also intended to expand the understanding of possible variants of the recurrent myoma-associated growth, in particular, true proliferative or “false” infectious-associated. The study was focused on reproductive losses and intrauterine manipulations as well as infectious-inflammatory predictors of recurrence.
In patients with a “false” phenotype of UM, the unsatisfactory reproductive outcome was registered 2 times more often than in patients with myoma in combination with adenomyosis (78.6% vs 33.3%, p<0.05). A similar situation was observed after intrauterine interventions (81.7% vs 43.2% on average in the whole HPU group, p<0.05).
Gynecological anamnesis of women with “false” type of UM contained a higher occurrence rate of chronic inflammatory processes in the pelvic organs (2 times higher than in women with true type). UM combined with adenomyosis was observed 1.5 times more often than UM with isolated tumors (64.3% vs 44.4%), however, without statistically significant differences. Structuring of the data on pathologic cervical processes showed similar results. “False” type of myoma-associated growth was observed 2 times more often (62.7% vs 34.6%). Besides, in the sampling with a phenotypically “false” variant of the growth, chronic endometritis prevailed, in particular, in combination with UM and adenomyosis (50.0% vs 10.7%, p <0.05).
The identification of microbes and their associations in myoma nodules was performed with bacterial and PCR tests in patients with a high index of genital tract infection.
The percent of cervical infections in the group of patients with UM was 23.9%, and in the case of a combination with adenomyosis, the occurrence rate of infection prevailed in patients with “false” nodule growth (71.4% vs 32.1%, p<0.05) (Fig. 1).
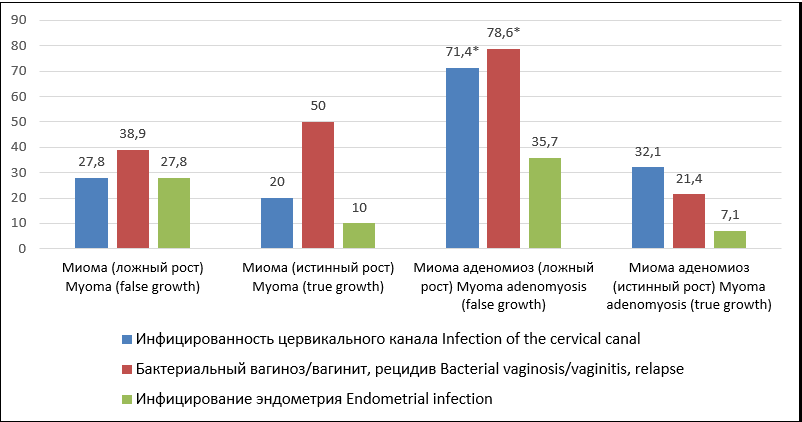
Рисунок 1. Инфицированность локусов половой сферы при рецидивах миоматозного роста — «ложном» и истинном.
Figure 1. Infection of biotopes of the genital tract with recurrent myomatous growth — "false" and true.
Примечание: *(p <0,05) — различия показателей статистически значимы (от группы с ММ).
Note: *(p <0.05) — the differences in indicators are statistically significant (from the group with UM).
The occurrence rate of bacterial vaginoses and vaginitis in patients with comorbid HPU (78.6%) was 4 times higher than in patients with recurrences with phenotypically true myoma-associated growth.
Endometrium infection was revealed in 1/3 of women with UM recurrence with nodules of a “false” type in combination with adenomyosis. In women with isolated tumors, it was 84.4%.
The authors identified predicting histotypes of tumor growth based on the peculiarities of the blood supply to myoma-associated and adenomyosis-associated nodules. The analysis of the results of 3D Doppler sonography showed that true phenotype was characterized by high-velocity blood flow in the nodules. Phenotypically “false” growth was characterized by a decrease in VI and VFI parameters associated with peripheral blood supply edema3(Fig. 2).
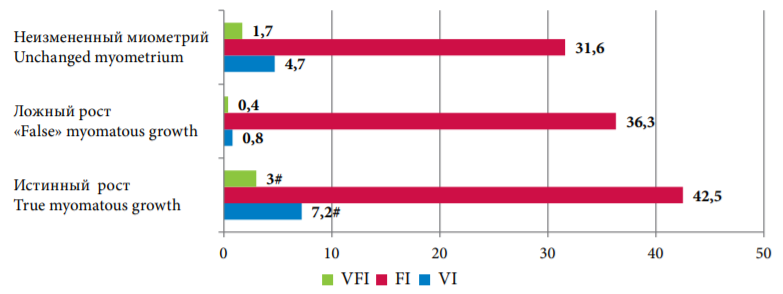
Рисунок 2. Динамика трехмерной сонографии с допплером в зависимости от фенотипа узлов — истинного или «ложного»
Figure 2. Features of 3D-power Doppler ultrasonography depending on the genesis of nodes — true or "false"
Примечание: #(p <0,05) — различия показателей статистически значимы (от всех групп).
Note: #(p <0.05) — differences in indicators are statistically significant (from all groups).
Discussion
The detected foci of chronic inflammation in the genital tract biotopes suggest its trigger role in the etiology of recurrence of myoma-associated “false” nodule growth, which agrees with some other authors [3].
Similar observations indicate a significant role of infectious agents in the processes of differentiation and apoptosis of uterine tissue and a failure of immune-endocrine homeostasis at a local level, especially in patients with recurrent genital tract dysbiosis. The changes in vaginal microflora caused by the development of vaginitis and dysbiosis associated with chronic inflammatory processes are also confirmed by other authors. They are provoked by U. Urealyticum (91.0%) as a mono variant and in combination with chlamydia, viruses, and some representatives of opportunistic pathogen microflora in diagnostically significant titers detected in various genital tract biotopes and myoma-associated nodules.
Pathomorphologic characteristics, that are typical for a “false” phenotypic myoma-associated growth, include dystrophic and necrotic alterations, cellular lymphocytic infiltration with signs of distraction, hydropic degeneration, and hyalinosis. These histological data agree with the hypothesis on the influence of infectious provoking factors with an excessive synthesis of extracellular matrix by the cells of myometrium [4].
Histological description of the tumor with excessive mitotic activity and primarily cellular type of structure corresponded to phenotypically true growth. Clinical manifestations in this group included menometrorrhagia that anematizes women and decreases their quality of life. Frequently, these symptoms were explained by the rapid growth of the dominant nodule and a combination of hyperplastic processes of the mucosa. Conclusion on the excessive proliferative activity of the uterine tissues is formed based on the facts of genetic determination of the mechanisms of local steroidogenesis in more than 1/3 of women [5][6]. Such observations correspond with the characteristics of the group, i.e. the identification of large myoma-associated nodules in the early reproductive age, their combination with adenomyosis, hyperplasia of the endometrium, and breasts.
The results of the study on the peculiarities of blood supply to myoma-associated and adenomyosis-associated nodules showed high sensitivity of 3D| Doppler sonography in the evaluation of the tumor vascular network [7] and predicting of HPU recurrences. Similar results on the association between 3D Doppler sonography findings and morphological structure and characteristics of myoma-associated growth are also confirmed by other authors [8][9].
The multifactorial nature (expression of estrogen and progesterone receptors, type of blood flow, proliferative index) of the true recurrence of myoma-associated growth etiology was shown during the structuring of nodule immune-histochemical growth (Table 1).
Таблица / Table 1
Молекулярно-биологические характеристики миоматозных узлов различного генеза
Molecular and biological characteristics of myoma nodules of various etiology
|
Группы Groups
|
N |
Экспрессия рецепторов к прогестерону (выраженная) Expression of progesterone receptors (pronounced) |
Экспрессия рецепторов к эстрогенам (выраженная) Expression of estrogen receptors (pronounced) |
Диаметр сосудов Diameter of vessels |
Уровень Ki-67 высокий (6-10%) High Ki-67 level (6-10%) |
||
|
Миома матки Uterine myoma |
«Ложный» рост узла “False” myoma-associated growth |
N |
18 |
4 |
3 |
4587 |
0 |
|
% |
22,2 |
16,7 |
|||||
|
Истинный рост узла True myoma-associated growth |
N |
10 |
7∞ |
5 |
6287∞ |
6,3∞ |
|
|
% |
70,0 |
50,0 |
|||||
|
Миома матки в сочетании с аденомиозом Uterine myoma with adenomyosis |
«Ложный» рост узла “False” myoma-associated growth |
N |
14 |
4 |
5 |
4390 |
0,3 |
|
% |
28,6 |
35,7 |
|||||
|
Истинный рост узла True myoma-associated growth |
N |
28 |
21∞ |
13 |
8049∞ |
7,3∞ |
|
|
% |
75,0 |
46,4 |
|||||
Note: ∞ (P <0.05) — the differences are statistically significant (intragroup comparison).
Elevated values of the steroid receptor expression, which were more pronounced in patients with myoma in combination with adenomyosis, indicated proliferative effect in the cases of true recurrence. This fact agreed with a hypothesis on an increase in the area of growth zones with damaged stromal-mesenchymal contacts [10].
Thus, an elevated index of proliferation Ki-67 indicated recurrence of UM, multiple tumors (more often), and a rapid growth of the dominant nodule, combined lesion with adenomyosis, elevated expression of steroid hormones receptors, sensitivity to vimentin, and collagen [1].
Morphometric evaluation of the vascular lumen parameters (with the external coating and without it) indirectly showed the level of blood supply to the loci of myometrium proliferation that was more expressed in patients with HPU recurrence. Reduced vascularization was observed in simple types of leiomyoma with mature stroma and foci of hyalinosis. A larger area of the vascular network (2021 vs 1654 µm²) due to vascular diameters (37.8 vs 25.3 µm) indicated the activity of the process of formation of the vascular network and corresponded to a true phenotype of the growth, which confirmed the possibility of UM recurrence, especially of combined forms, in the conditions of high cellular activity, excessive metabolism, and a proved role of the local hyperestrogenism in the endometrial heterotopy [2].
Conclusion
A multi-aspect preoperative examination of patients with hyperplastic uterine processes that includes a combination of markers and anamnestic risk factors allows specialists to model various phenotypes of recurrence of myoma-associated growth and select an adequate therapy and a course of rehabilitation.
1. Krasnova I.A., Aksenova V.B., Krasnova A.S. The site for ultrasonic investigation in patients with uterine myoma before and after embolization of uterine arteries. Ultrasonic and functional diagnostic. 2015;(4S):93b. eLIBRARY ID: 25611446
2. Tichomirov A.L. Pathogenetic grounds for organ-sparing treatment for myoma. Monography. Мoscow;2013.
3. Krasnova I.A., Aksenova V. B., Krasnova A.S. The site for ultrasonic investigation in patients with uterine myoma before and after embolization of uterine arteries. Ultrasonic and functional diagnostic. 2015;(4S):93b. eLIBRARY ID: 25611446
References
1. Sidorova I.S., Kogan Ye.A., Unanyan A.L. Clinical and morphological parallels and molecular mechanisms of stromal-parenchymal relationships in uterine myoma. Molecular medicine. 2009;(1):9–15. (In Russ.). eLIBRARY ID: 12111184
2. Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. 2014; 20(3):386-402. DOI: 10.1093/humupd/dmt052.
3. Sparic R, Mirkovic L, Malvasi A, Tinelli A. Epidemiology of Uterine Myomas: A Review. Int J Fertil Steril. 2016; 9(4):424-35. DOI: 10.22074/ijfs.2015.4599.
4. Sosin S.A., Zazerskaya I.E. Uterine artery embolization for treatment of uterine leyomioma. Effectiveness and safety concerns. The Bulletin of Almazov Federal Heart, Blood and Endocrinology Centre. 2011;(6):20–22. (In Russ.). eLIBRARY ID: 17800207
5. Styer AK, Rueda BR. The Epidemiology and Genetics of Uterine Leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016; 34:3-12. DOI: 10.1016/j.bpobgyn.2015.11.018.
6. Wise LA, Laughlin-Tommaso SK. Epidemiology of Uterine Fibroids: From Menarche to Menopause. Clin Obstet Gynecol. 2016; 59(1):2-24. DOI: 10.1097/GRF.0000000000000164.
7. Naumova N.V., Boldovskaya E.A., Makukhina V.V., Krutova V.A. Intraoperative ultrasound navigation in surgical treatment of uterine myomas. Kuban Scientific Medical Bulletin. 2018; 25(1):30-33. (In Russ.) DOI: 10.25207/1608-6228-2018-25-1-30-33/
8. Buyanova S.N., Titchenko L.I., Kareva E.N., Gasparyan N.D., Titchenko L.P., Chechneva M.A. The clinical value of estimation of intratumor blood flow values in the diagnosis of estrogen- and progesterone-dependent uterine myoma. Russian bulletin of obstetrician-gynecologist. 2006; 6(3):42–45. (In Russ.). eLIBRARY ID: 9247532
9. Buianova S.N., Mgeliashvili M.V., Petrakova S.A. Current views of the etiology, pathogenesis, and morphogenesis of uterine myoma. Russian bulletin of obstetrician-gynecologist. 2008; 8(6):45–51. (In Russ.). eLIBRARY ID: 13333253
10. Ciavattini A, Di Giuseppe J, Stortoni P, Montik N, Giannubilo SR, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int. 2013; 2013:173184. DOI: 10.1155/2013/173184.
About the Authors
E. V. BashirovRussian Federation
Eduard V. Bashirov, Dr. Sci. (Med.), Associated Professor, Kuban State Medical University
Krasnodar
V. A. Krutova
Viktoria A. Krutova, Dr. Sci. (Med.), head physician
Krasnodar
I. I. Kutsenko
Irina I. Kutsenko, Dr. Sci. (Med.), department of obstetrics, gynecology and perinatology
Krasnodar
Review
For citations:
Bashirov E.V., Krutova V.A., Kutsenko I.I. Biomarkers of the type of recurrent uterine myoma-associated growth. Medical Herald of the South of Russia. 2021;12(4):6-11. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-4-6-11






































