Scroll to:
Cardiovascular pathology in diff erent forms of primary hyperparathyroidism
https://doi.org/10.21886/2219-8075-2021-12-3-36-43
Abstract
Objective: To study the frequency and features of the clinical course of cardiovascular pathology in patients with diff erent clinical forms of primary hyperparathyroidism (PHPT).
Materials and Methods: A retrospective analysis of case histories of 48 patients who received inpatient treatment for PHPT in the endocrinological or surgical departments of two large hospitals in Arkhangelsk from 2005 to 2015 was performed.
Results: Among the revealed cases of PHPT, the symptomatic form of PHPT was the most common (88%). Th e analysis showed a high frequency of arterial hypertension (AH) (69%) with a more severe course in patients with a mixed form of PHPT. Th e level of parathyroid hormone (PTH) was higher in patients with AH compared with patients without hypertension and PHPT (p = 0.008). Left ventricular hypertrophy was detected in 60% of patients with a mixed form of PHPT. In a mild form, this complication was not observed. Th e relationship between the level of total blood calcium and PTH and the interventricular septum thickness was revealed by the results of echocardiography (p = 0.036 and p = 0.012). Th e inverse relationship between the duration of the QT interval and the level of ionized blood calcium was shown (p = 0.022).
Conclusions: Changes in phosphorus-calcium metabolism provoked by PHPT aff ect the state of the cardiovascular system, which determines the need for increased attention of various specialists to this type of complications in PHPT, timely indication of treatment, and improvement of the quality of patient’s life.
Keywords
For citations:
Baranova I.A., Zykova T.A., Baranov A.V. Cardiovascular pathology in diff erent forms of primary hyperparathyroidism. Medical Herald of the South of Russia. 2021;12(3):36-43. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-3-36-43
Introduction
Primary hyperparathyroidism (PHPT) is characterized by excessive secretion of parathyroid hormone (PTH) in combination with upper normal or increased blood calcium (Ca) levels due to the development of adenoma or hyperplasia of parathyroid glands [1]. Since the early 1970s, in many countries due to the introduction of routine analysis of blood Ca levels, PHPT has moved from the category of rare severe diseases to the category of common endocrine pathologies with a frequency of 1% in the population, and after 55 years, the risk of its development increased to 2% [1][2]. Currently, the majority of cases of PHPT are mild forms (80–90%); however, in Russia, PHPT remains a rare disease with a predominance of overt forms requiring surgical treatment [2][3].
Russian and foreign studies indicate an increased risk of mortality from cardiovascular diseases (CVDs) in patients with PHPT, such as myocardial infarction, stroke, and heart failure [3][4][5]. The relative risk of death from CVDs with PHPT can be 1.17–1.85 [6]. The results of a number of studies show that the frequency of arterial hypertension (AH) [5][6–9], left ventricular hypertrophy (LVH) [10–12] is increased against the background of PHPT, there is a violation of left ventricular diastolic function [7][13][14], calcifications in the myocardium and heart valves [4][7][14], rhythm and conduction disturbances [13, 15–17]. However, the presence of cardiovascular pathology in patients with PHPT is currently not considered an absolute indication for surgical treatment in accordance with international recommendations for the treatment of PHPT (2014) [18].
The cause of the occurrence of structural and functional changes in the cardiovascular system may be a direct increase in PTH or blood Ca. PTH is widely recognized as a hormone with cardiac and cardiovascular properties [19]. PTH receptors are found in the myocardium, vascular smooth muscle cells, blood cells, liver, etc. [20]. PTH can have a direct effect on cardiomyocytes through the activation of protein kinase C, which leads to their hypertrophic growth [7]. One of the effects of PTH is the activation of the renin-angiotensin-aldosterone system. Thus, according to in vitro studies, PTH stimulates the secretion of aldosterone by binding to its receptors in the adrenal glomerular zone [21]. An increased level of PTH when binding to the receptors of pacemaker cells can have a positive chronotropic effect [22]. An increase in Ca causes abnormalities on the electrocardiogram in the form of a shortening of the QT interval, lengthening of the PR and QRS intervals [3]. Hypercalcemia can lead to the development of late post-depolarizations, as well as to a shortening of the refractory period and induction of the reentry mechanism [3]. However, many causal relationships and aspects of the pathogenesis of CVD against the background of PHPT remain poorly understood at the moment.
The study of the pathology of the cardiovascular system against the background of PHPT is currently relevant due to the high mortality rate and increased frequency of CVD in this disease, which can worsen the prognosis and quality of life of patients.
The aim of the study was to study the frequency and features of the clinical course of the pathology of the cardiovascular system in patients with various forms of PHPT.
Materials and Methods
A retrospective analysis of 48 case histories of patients (continuous sample) who were hospitalized with the final diagnosis of PHPT in the endocrinological and surgical departments of the State Budgetary Healthcare Institution JSC "First City Clinical Hospital named after E.E. Volosevich" and State Budgetary Healthcare Institution JSC "Arkhangelsk Regional Clinical Hospital" from 2005 to 2015. The criteria for inclusion in the study were age (from 18 years) and the final diagnosis of PHPT in a patient who received inpatient treatment for PHPT in the period from 2005 to 2015. Over a ten-year period, the primary hospital incidence of PHPT was calculated based on the number of hospitalizations for PHPT diagnosed for the first time during the study year. The diagnosis of PHPT was established when an elevated blood Ca level was detected with a double measurement in combination with an increase in the PTH level. The exclusion criteria were secondary and tertiary hyperparathyroidism. When assessing the case histories, the medical history, complaints upon admission, the clinical picture, the results of physical examination, laboratory tests, and instrumental examination (electrocardiography, echocardiography, Holter monitoring) were studied. The diagnosis of overt (mixed, visceral, bone) or mild forms of PHPT and indications for surgical treatment were established on the basis of international recommendations for the treatment of PHPT (2014) [18].
Statistical analysis was performed using the SPSS 22 statistical software package (IBM SPSS Statistics, 2013). Quantitative characteristics are presented as a median, first and third quartiles. Comparison of two groups in terms of quantitative characteristics was carried out using the Mann-Whitney test, several groups – using the Kruskal-Wallis test. When analyzing the frequency of values of features, the χ2 test with Yates' correction was used to compare the two groups. The probability of random error less than 5% (p < 0.05) was chosen as a criterion of statistical significance using the Bonferroni correction.
Results
Prior to 2010, the primary hospital incidence of PHPT in the Arkhangelsk Region was 2–3 cases per 1 million population per year, and after 2012, there was a slight increase in the incidence to 11 cases per 1 million population per year. Among the identified patients with PHPT over a ten-year period (n = 48), the median and quartiles of age were 56.5 [ 53; 61] years (min 22 years, max 75 years), the peak incidence was observed at the age of over 50 years. Female patients accounted for 96% of cases (46/48), of which 76% were postmenopausal (35/46). The ratio of male to female patients averaged 1:23.
Among all cases of PHPT, the overt form (mixed, bone, and visceral) was detected in 88% (42/48), the mild form – in 12% of cases (6/48), the normocalcemic variant of PHPT was not recorded (Fig. 1).
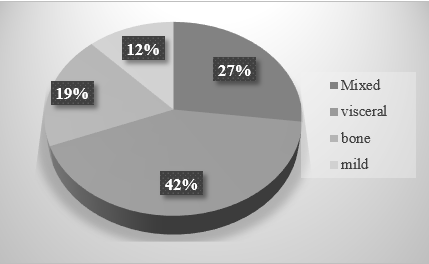
Figure 1. Distribution of clinical forms of PHPT according to the data of a retrospective analysis in the Arkhangelsk Region (n = 48), %.
Changes in the state of phosphorus-calcium metabolism and an increase in PTH against the background of the mixed form were more pronounced in comparison with other forms of PHPT (Table 1). On clinical examination, patients with a mixed form of PHPT were observed to have such rare complications of PHPT as coral nephrolithiasis, fibrocystic osteitis, and recurrent fractures, as well as acute destructive pancreatitis against a background of severe hypercalcemia. The mild form was the rarest; however, over the study period, it was found that the frequency of cases of the mild form of PHPT before 2011 was 7%, and after 2012 it increased more than 2 times and reached 15% of cases.
Table 1
Comparative characteristics of phosphorus-calcium metabolism and the frequency of arterial hypertension among patients with different forms of PHPT
|
|
Mixed (1) |
Bone (2) |
Visceral (3) |
Mild (4) |
Level of P* |
|
n |
13 |
9 |
20 |
6 |
|
|
Age, years |
58 [ 54; 61] |
61 [ 49; 65] |
56 [ 51; 62] |
55 [ 50; 58] |
NS |
|
Menopause, % (abs.) |
77% (10) |
78% (7) |
70% (14) |
66% (4) |
NS |
|
Ca total, mmol/L |
3.01 [ 2.65; 3.33] |
2.89 [ 2.77; 3.24] |
2.78 [ 2.6; 2.98] |
2.5 [ 2.37; 2.7] |
1-4=0.003 |
|
Ca++, mmol/L |
1.53 [ 1.4; 1.73] |
1.46 [ 1.36; 1.6] |
1.41 [ 1.27; 1.55] |
1.25 [ 1.17; 1.47] |
NS |
|
Parathyroid hormone, pg/ml |
538 [ 153; 1174] |
175 [ 128; 580] |
234 [ 172; 376] |
166 [ 116; 316] |
NS |
|
Blood phosphorus, mmol/L |
0.8 [ 0.56; 0.94] |
0.85 [ 0.7; 1.01] |
0.84 [ 0.7; 0.92] |
1.0 [ 0.64; 1.15] |
NS |
|
Smoking, % (abs.) |
15% (2) |
11% (1) |
10% (2) |
16% (1) |
NS |
|
Frequency of AH, % |
92% (12) |
67% (6) |
60% (12) |
50% (3) |
NS |
|
The degree of increase in blood pressure (I/II/III), abs. |
6/3/3 |
5/1/0 |
11/1/0 |
3/0/0 |
NS |
|
Frequency of AH treatment, % |
75% (9) |
83% (5) |
75% (9) |
67% (2) |
NS |
When studying the state of the cardiovascular system in a group of patients with PHPT, AH was most often observed, which was diagnosed in 69% of cases (33/48) [23], in patients with an overt form of PHPT, its frequency was 71% (30/42), and with a mild form – 50% (3/6), without statistically significant differences (p = 0.55) [24]. Against the background of the mixed form of the disease, hypertension was diagnosed in 92% of patients (12/13), in four of them, there was a persistent increase in blood pressure up to the 3rd degree (Table 1), which was not revealed in other forms. According to the case histories, 69.6% of patients with hypertension associated with PHPT (23/33) received antihypertensive therapy; no statistically significant differences in its frequency were found among various forms of PHPT. Combined three-component therapy was prescribed in 26% of patients (6/23), most of them had a mixed form of PHPT (4/6), and in patients with a mild form, only one-component therapy with ACE inhibitors (3/6) was used [23]. The PTH level was statistically significantly higher among patients with PHPT and hypertension compared with patients without hypertension (Table 2).
Table 2
Comparative characteristics of indicators of phosphorus-calcium metabolism in patients with PHPT, depending on the presence or absence of arterial hypertension
|
|
The presence of AH |
Absence of AH |
Level of P* |
|
n |
33 |
15 |
|
|
Age, years |
57 [ 54; 61] |
50 [ 46; 61] |
NS |
|
Body mass index, kg/m2 |
27.9 [ 24.6; 33.4] |
24.7 [ 22.7; 31.2] |
NS |
|
Menopausal women |
79% (26) |
60% (9) |
NS |
|
Ca total, mmol/L Hypercalcemia: 1st degree (< 3 mmol/L) 2nd degree (3–3.5 mmol/L) 3rd degree (>3.5 mmol/L) |
2.88 [ 2.65; 3.2]
21 (64%) 6 (18%) 6 (18%) |
2.75 [ 2.6; 3.01]
12 (80%) 3 (20%) 0 (0%) |
NS |
|
Ca++, mmol/L |
1.53 [ 1.4; 1.73] |
1.46 [ 1.36; 1.6] |
NS |
|
Parathyroid hormone, pg/ml |
247 [ 172; 799] |
148 [ 103; 229] |
P=0.008 |
|
Blood phosphorus, mmol/L |
0.81 [ 0.66; 0.92] |
0.89 [ 0.71; 0.99] |
NS |
Notes: NS – not significant differences. * Comparison of quantitative data in groups was carried out using the Mann-Whitney criterion, frequency comparisons were carried out using the χ2 criterion with the Yates correction. The statistically significant level of P in pairwise comparisons was assumed to be the level of P < 0.05.
According to the results of echocardiography, LVH was diagnosed in 60% of the examined patients (9/15) according to the data of case histories [24]. The median and quartiles of the interventricular septum (IVS) thickness were 10.5 mm [9; 13], the posterior wall of the left ventricle (LV PW) was 11 mm [ 9; 13]. It was noted that among patients with established LVH (n = 9), the median and quartiles of age were 61 years [ 57; 70], all patients were female, had a mixed form of PHPT, and hypertension in the anamnesis. Patients without LVH (n = 7) were also female, they had an overt form of PHPT (mixed – 29%, bone – 29%, visceral – 42%) and a history of hypertension in 57% of cases (4/7), median and the quartiles of age were 56 years [ 47; 61], without significant differences with the group of patients with LVH. According to the results of the correlation analysis, the dependence of the IVS thickness on the level of total Ca and PTH in the examined patients was revealed (r = 0.303, p = 0.036 and r = 0.444, p = 0.012, n = 15).
Calcifications in the area of the aortic valve and posterior cusp of the mitral valve according to echocardiography were detected in two cases (13%) – against the background of a mixed form of PHPT (2/15) and hypercalcemia > 3 mmol/L.
According to the electrocardiography results, the median and quartiles of the QT interval were 0.36 s [ 0.34; 0.38] (n = 48), the dependence of the QT interval duration on the blood Ca++ level was revealed (r = -0.381, p = 0.022, n = 48, Spearman's test) [23]. Grade I AV blockade was observed in a small percentage of cases (4%) in patients with a mixed form against a background of moderate hypercalcemia (total Ca > 3 mmol/L). Violations of conduction along the right bundle branch were registered in 8% of cases (4/48), along the left bundle branch (anterior branch) – in 6% of cases (3/48), complete blockade of the left bundle branch was not detected in any of cases. Supraventricular arrhythmias were observed in 6% of cases (3/48) against the background of an overt form of PHPT, ventricular extrasystole – in 15% of cases (7/48) in 5 patients with an overt form of PHPT and two patients with a mild form.
Acute coronary events were observed in the anamnesis in 6% of patients with an overt form of PHPT (3/48), three patients had a myocardial infarction, two of them had a history of ischemic stroke (4%) [24]. The authors also assessed some metabolic disorders in patients with HHPT, which may contribute to an increase in morbidity and mortality from CVD. There was a high incidence of overweight or obesity (62.5%), dyslipidemia (73%), and type 2 diabetes mellitus (20%).
Discussion
The detected low incidence of PHPT and the prevalence of overt forms (almost 90%) show that PHPT in the Arkhangelsk Region remains a rare disease detected at late stages with severe complications, which is associated with the lack of blood calcium testing in routine biochemical practice in Russia. According to epidemiological studies of the world, the incidence of PHPT is from 4 to 188 cases per 100 thousand population per year, and 80–90% of cases of PHPT are mild forms without clinical symptoms of the disease [2][25]. In the group of identified patients with PHPT, there was a predominance of women in the postmenopausal period, who belong to the main risk group for the development of PHPT, which coincides with the data of other researchers [25]. According to the results of this study, the ratio of men and women was 1:23, while in Western Europe and the United States there are 3–5 women with PHPT per 1 man [26].
The high incidence of hypertension in patients with PHPT in this study is similar to the results of other studies, where its frequency ranged from 40 to 65%, mainly among patients with clinically expressed PHPT [4][8][12]. Patients with hypertension had a statistically significantly higher level of PTH, which may have an impact on the development of this cardiovascular complication against the background of PHPT, as reported by other authors. The highest incidence of hypertension (93%) and an increase in blood pressure to the 3rd degree were observed against the background of the mixed form of PHPT, in the authors’ opinion, due to higher levels of PTH and Ca in the blood against the background of this form and a more severe clinical course. However, despite more pronounced changes in phosphorus-calcium metabolism in patients with an overt form of PHPT, there were no significant differences in the frequency of hypertension compared with the mild form against the background of mild hypercalcemia, which was observed in other studies. According to a Russian study involving 65 patients with PHPT, the frequency of hypertension was 54.8% against the background of the overt form (first group) and 50% against the background of the mild form (second group), without significant differences [13]. However, the authors noted that the first group was dominated by patients with AH of the 2nd degree of increase in blood pressure (71%), and in the second group – with AH of the 1st degree (65%). Tordjman et al. [8] found the same frequency of AH both in patients with hypercalcemia against the background of the overt form of PHPT and in the normocalcemic version of PHPT, which requires further research to study the mechanisms of the development of AH against the background of this pathology.
Among patients who underwent echocardiography, a fairly high incidence of LVH was identified, one of the main predictors of the risk of death from CVD. According to the results of Stefenelli et al., an even more frequent development of LVH was revealed against the background of PHPT: in 82% of cases, IVS hypertrophy was observed, in 78% – LV PW hypertrophy [11][13], although other authors report a lower incidence of this complication [12]. All patients with LVH in the studied group had a history of AH; nevertheless, some researchers note a high incidence of LVH in the presence of PHPT even in the absence of AH. Piovesan et al. demonstrated a significantly higher LV myocardial mass index among patients with PHPT compared with the control group, without differences in age, sex, and blood pressure [27]. A number of studies have shown a positive effect of surgical treatment of PHPT in the form of a decrease in LVH in patients after a decrease in Ca and PTH levels [11][13][28], which may further reduce mortality from CVD in patients with PHPT.
A number of clinical studies have found a high incidence of calcifications during echocardiography in patients with PHPT [11–13]. In one of them, calcifications in the aortic valve were recorded in 63% of cases, in the mitral valve – in 49% of cases, significantly exceeding the frequency of their detection in the control group (12% and 15%, respectively) [11]. According to Langle et al., calcifications in the valve structures or in the myocardium were observed in 78% of cases, and there was also a relationship between the severity of calcifications and LVH (p = 0.005) [12]. According to the obtained data, the incidence of calcifications was much lower than the literature data, which is most likely due to the small number of echocardiographic studies performed (n = 15).
There are not so many studies devoted to the study of rhythm and conduction disturbances against the background of PHPT. According to the data of the current study, a low frequency of cardiac conduction disturbances was revealed in patients with PHPT in comparison, for example, with the data of Voronenko (2009), where the frequency of 1st degree AV blockade was 25.8% among patients with overt PHPT and 8.8% – with mild form, the frequency of right bundle branch block was 9.7% and 8.8%, respectively, the frequency of blockade of the anterior branch of the left bundle branch was 22.6% and 8.8%, respectively [13]. Data on cardiac arrhythmias associated with PHPT are found mainly in the form of individual clinical cases [15][16]. The electrophysiological mechanisms underlying rhythm disturbances in PHPT are not yet fully understood, but, undoubtedly, hypercalcemia can cause life-threatening rhythm disturbances and require surgical intervention to prevent them [3].
Almost two-thirds of patients with PHPT in this study were overweight or obese of various degrees, had dyslipidemia, and in 20% of cases, carbohydrate metabolism disorders were observed, which is noted by other authors and can increase the risk of developing CVD, having a negative impact on the long-term prognosis of the life of patients with PHPT.
Among the study group, parathyroidectomy was performed in 90% (43/48) of patients (37 patients with overt forms and 6 patients with a mild form of PHPT). In accordance with the histological structure, in 42 cases (97%), adenoma of the thyroid gland was observed, in one case (3%) – hyperplasia of the thyroid gland, no cases of cancer were recorded. In five cases, surgery was not performed due to referral to central medical research centers (n = 4) or patient refusal (n = 1).
Conclusions
Thus, in patients with PHPT, a sufficiently high incidence of cardiovascular pathology such as AH and LVH with a prevalence against the background of a mixed form of PHPT was revealed, and calcifications of heart valves and rhythm and conduction disturbances can also be found. Further study is required of the relationship between changes in phosphorus-calcium metabolism against the background of the development of various forms of CVD in PHPT.
References
1. Dedov I.I., Rozhinskaya L.Ya., Mokrysheva N.G., Vasilyeva T.O. Etiology, pathogenesis, clinical presentation, diagnostics and treatment of the primary hyperparathyroidism. Osteoporosis and Bone Diseases. 2010;13(1):13-18 (in Russ.) DOI: 10.14341/osteo2010113-18
2. Rozhinskaya L.Ya., Rostomyan L.G., Mokrysheva N.G., Mirnaja S.S., Kirdjankina N.O. Epidemiologic aspects of primary hyperparathyroidism in Russia. Osteoporosis and Bone Diseases. 2010;13(3):13-18. (in Russ.) eLIBRARY ID: 16972569
3. Voronenko I.V., Syrkin A.L., Rozhinskaya L.Ya., Melnichenko G.A. Hyperparathyroidism and cardiovascular pathology. Osteoporosis and Bone Diseases. 2006;9(2):33-41 (in Russ.) eLIBRARY ID: 12881459
4. Pepe J., Cipriani C., Sonato C., Raim O., Biamonte F., Minisola S. Cardiovascular manifestations of primary hyperparathyroidism: a narrative review. European Journal of Endocrinology. 2017;177(6): R297–R308. DOI: 10.1530/EJE-17-0485
5. Walker M.D., Silverberg S.J. Cardiovascular aspects of primary hyperparathyroidism. J Endocrinoll Invest. 2008; 31(10): 925–931. DOI: 10.1007/BF03346443
6. Hedbäck G, Odén A. Increased risk of death from primary hyperparathyroidism--an update. Eur J Clin Invest. 1998;28(4):271-6. doi: 10.1046/j.1365-2362.1998.00289.x
7. Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease--a review. Eur Heart J. 2004;25(20):1776-87. doi: 10.1016/j.ehj.2004.07.010
8. Tordjman K.M., Yaron M., Izkhakov E., Osher E., Shenkerman G., et al. Cardiovascular risk factors and arterial rigidity are similar in asymptomatic normocalcemic and hypercalcemic primary hyperparathyroidism. European Journal of Endocrinology. 2010;162: 925–933. DOI:10.1530/EJE-09-1067
9. Kalla A., Krishnamoorthy P., Gopalakrishnan A., Garg J., Patel N.C., Figueredo V.M. Primary hyperparathyroidism predicts hypertension: results from the National Inpatient Sample. International Journal of Cardiology. 2017; 227: 335–337. DOI: 10.1016/j.ijcard.2016.11.080
10. Silverberg S.J. Cardiovascular disease in primary hyperparathyroidism. // J Clin Endocrinol and Metabolism. – 2000. – V. 85(10). – P. 3513–3514. DOI: 10.1210/jcem.85.10.6927
11. Stefenelli T., Mayr H., Bergler-Klein J., Globits S., Woloszczuk W., Niederle B. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility aft er successful parathyroidectomy. Am J Med. 1993; 95(2):197–202. DOI: 10.1016/0002-9343(93)90260-v
12. Längle F, Abela C, Koller-Strametz J., Mittelböck M., Bergler-Klein J., et al. Primary hyperparathyroidism and the heart: cardiac abnormalities correlated to clinical and biochemical data. World J Surg. 1994;18(4): 619-624. DOI: 10.1007/BF00353780
13. Voronenko I.V., Mokrysheva N.G., Rozhinskaya L.Ya., Syrkin A.L. Th e cardiovascular system in patients with symptomatic and mild primary hyperparathyroidism. Problems of Endocrinology. 2009;55(3):25-29 (in Russ.) DOI: 10.14341/probl200955325-29
14. Nilsson I.L., Aberg J., Rastad J., Lind L. Maintained normalization of cardiovascular dysfunction 5 years aft er parathyroidectomy in primary hyperparathyroidism. Surgery. 2005; 137(6):632–8. DOI: 10.1016/j.surg.2005.02.001
15. Kiewiet R.M., Ponssen H.H., Janssens E.N., Fels P.W. Ventricular fi brillation in hypercalcaemic crisis due to primary hyperparathyroidism. Netherland Journal of Medicine. 2004; 62: 94–96. PMID: 15209475.
16. Pepe J., Curione M., Morelli S., Colotto M., Varrenti M., et al. Arrhythmias in primary hyperparathyroidism evaluated by exercise test. European Journal of Clinical Investigation. 2013; 43(2): 208–214. DOI:10.1111/eci.12038
17. Chang C.J., Chen S.A., Tai C.T., Yu W.C., Chen Y.J., et al. Ventricular tachycardia in a patient with primary hyperparathyroidism. Pacing Clin Electrophysiol. 2000; 23 (4 Pt 1): 534-537. DOI: 10.1111/j.1540-8159.2000.tb00842.x
18. Eastell R., Brandi M. L., Costa A. G., D'Amour P., Shoback D.M., Th akker R.V. Diagnosis of asymptomatic primary hyperparathyroidism: Proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014; 99: 3570–3579. DOI: 10.1210/jc.2014-1414
19. Schluter K.D., Piper H.M. Cardiovascular actions of parathyroid hormone and parathyroid hormone-related peptide. Cardiovascular Research. 1998; 37: 34–41. DOI:10.1016/S0008-6363(97)00194-6
20. Rashid G., Bernheim J., Green J., Benchetrit S. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. American Journal of Physiology: Renal Physiology. 2007; 292: F1215–F1218. DOI: 10.1152/ ajprenal.00406.2006
21. Runova G.E., Fadeev V.V. Asymptomatic primary hyperparathyroidism and cardiovascular pathology. Arterial hypertension. 2017;23(4):282–293 (in Russ.) DOI: 10.18705/1607-419X-2017-23-4-282-293
22. Shimoyama M., Ogino K., Furuse Y, Uchida K., Kinugasa Y., et al. Signaling pathway and chronotropic action of parathyroid hormone in isolated perfused rat heart. J Cardiovasc Pharmacol. 2001; 38: 491–499. DOI: 10.1097/00005344-200110000-00001
23. Baranova I.A. Primary hyperparathyroidism: morbidity, clinical picture and treatment in the Arkhangelsk region: dis. ... cand.med. 14.01.02. – Northern State Medical University, Arkhangelsk, 2020 – 159 p. (in Russ.)
24. Baranova I.A., Zykova T.A., Sergeeva O.A. Comparative analysis of clinical manifestations of primary hyperparathyroidism by results of hospitalizations and screening for hypercalcemia in the Arkhangelsk region. Medical Herald of the South of Russia. 2019;10(4):36-42. (In Russ.). doi: 10.21886/2219-8075-2019-10-4-36-42
25. Rozhinskay LYa, Rostomyan LG, Mokrysheva NG, Mirnaja S.S., Kirdjankina N.O. Epidemiology of primary hyperparathyroidism. Lechashii vrach. 2010; 11: 50-56. (in Russ.) eLIBRARY ID: 21671686
26. Miller B.S., Dimick J., Wainess R., Burney R.E. Ageand sex-related incidence of surgically treated primary hyperparathyroidism. World J Surg. 2008;32(5):795-9. DOI: 10.1007/s00268-007-9427-2
27. Piovesan A., Molineri N., Casasso F., Emmolo I, Ugliengo G, et al. Left ventricular hypertrophy in primary hyperparathyroidism. Eff ects of successful parathyroidectomy. Clin Endocrinol. 1999; 50 (3): 321–328. DOI: 10.1046/j.1365-2265.1999.00651.x
28. McMahon D.J., Carrelli A., Palmeri N., Zhang C., Di Tullio M., et al. Eff ect of parathyroidectomy upon left ventricular mass in primary hyperparathyroidism: a meta-analysis. Journal of Clinical Endocrinology and Metabolism. 2015; 100(12): 4399–4407. DOI:10.1210/jc.2015-3202
About the Authors
I. A. BaranovaРоссия
Irina A. Baranova, endocrinologist, laboratory assistant at the Central Research Laboratory
Arkhangelsk
T. A. Zykova
Россия
Tatyana A. Zykova, Dr. Sci. (Med.), Professor of the Department of Faculty Th erapy with the course of endocrinology
Arkhangelsk
A. V. Baranov
Россия
Alexander V. Baranov, Cand. Sci. (Med.), researcher of the Central Research Institute
Arkhangelsk
Review
For citations:
Baranova I.A., Zykova T.A., Baranov A.V. Cardiovascular pathology in diff erent forms of primary hyperparathyroidism. Medical Herald of the South of Russia. 2021;12(3):36-43. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-3-36-43
JATS XML







































