Scroll to:
The role of transcription factor FoxP3 as a predictor of acute and chronic urticaria in children
https://doi.org/10.21886/2219-8075-2021-12-3-50-54
Abstract
Objective: To study the transcription factor FoxP3 in children with acute and chronic spontaneous urticaria as a possible predictor of the severity and chronicity of urticaria.
Materials and Methods: A total of 264 children of both sexes aged from 6 to 16 years old with diff erent variants of urticaria course were examined. Clinical methods of the study included the analysis of anamnestic data and an objective examination of the child with the determination of the severity of urticaria. Immunological methods of the study included the identifi cation of T-regulatory lymphocytes with the CD4+CD25+ Foxp3+CD45+immunophenotype.
Results: A signifi cant decrease in the level of transcription factor FoxP3 was found in children with severe acute urticaria and chronic urticaria compared to the control group.
Conclusion: Th e degree of reduction in the level of FoxP3 signifi cantly aff ected the likelihood of the development of a severe course of acute urticaria and possible chronization of the disease.
For citations:
Maltsev S.V., Sizyakina L.P., Lebedenko A.A. The role of transcription factor FoxP3 as a predictor of acute and chronic urticaria in children. Medical Herald of the South of Russia. 2021;12(3):50-54. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-3-50-54
Introduction
Urticaria continues to be an urgent, but not fully studied problem of childhood allergy. The prevalence of acute urticaria (AU) in the child population reaches 6.7% [1]. According to modern guidelines, AU is the spontaneous occurrence of blisters and/or angioedema for a time period of less than six weeks. Chronic spontaneous urticaria (CU) is diagnosed when symptoms persist for a period of more than six weeks [2].
Visual signs of urticaria are the same in all patients: a blister, angioedema, while the etiology and pathogenesis of their development are diverse. The algorithm for the diagnosis and treatment of urticaria is described in Russian and foreign consent documents, but most often it does not lead to the identification of the disease cause, which is a consequence of the not always determined clinical and pathogenetic characteristics of various variants of the urticaria course [3].
There is a still not fully understood question concerning the dependence of the development of the urticaria course on the functional activity of the child's immune system [4–8]. The implementation of the immune response occurs through a complex system of intercellular interactions and is carried out due to transcription factors; some mechanisms of these interactions are still unclear. Disorders in the work of immune mechanisms underlie the development of many somatic diseases, including the allergic ones. FoxP genes are an integral part of the Fox family, which includes a group of transcription factors united by the forkhead domain. At the present stage, four representatives of this subfamily have been discovered, while FoxP3 is specific for immune cells and is found in CD4+CD8-CD25+ thymocytes, is an important factor in the differentiation and implementation of the functions of CD4+CD25+T-regulatory cells. The important role of regulatory T-cells in the pathogenesis of bronchial asthma has been established [9], but this information is contradictory. The research conducted by Lee et al. (2007) showed the presence of an increased number of FoxP3 cells in children with severe bronchial asthma compared with patients with mild asthma [10]. At the same time, according to Vale-Pereira et al., the level of FoxP3 was significantly higher in healthy people than in patients with bronchial asthma [11]. In this work, the study of the FoxP3 level in allergic pathology (in particular in urticaria in children) is of serious academic interest.
Due to the ambiguity of ideas about the role of individual structures of the immune system in the urticaria pathogenesis, including transcription factors, it is of scientific interest to study the FoxP3 level and role in children with different urticaria courses, as well as its participation in the urticaria transformation into a chronic form.
The purpose of the research was to study the transcription factor FoxP3 in children with AU and CU as a possible predictor of the urticaria severity and chronicity.
Materials and methods
In order to achieve this goal, 264 children of both sexes aged from 6 to 16 years with various variants of the course of urticaria were examined. The control group included 30 children of both sexes of the same age of health groups I and II b. The examination of patients was carried out on the first day of the child admission before the beginning of therapy in the hospital. An anamnestic criterion for the inclusion of patients in the study was the presence of urticaria episodes lasting no more than six weeks for AU and more than six weeks for CU.
The clinical methods of the study included the analysis of anamnestic data, an objective examination of the child with the determination of the urticaria severity according to Zuberbier et al. (calculation of the urticaria activity index during the seven days of the patient's stay in the hospital – UAS7) [12]. In order to identify T-regulatory lymphocytes with the CD4+CD25+ Foxp3+CD45+ immunophenotype, peripheral blood was taken from the ulnar vein in the morning on an empty stomach into a test tube with a heparin sodium salt spray. Therefore, 100 µl of whole heparinized blood and 20 µl of monoclonal antibodies to CD4+ stained with fluorochrome PE (R-phycoerythrin, phycoerythrin), CD25+ stained with PC5 (Phycoerythrin Cyanine 5.1), CD45+ stained with ECD (Phycoerythrin Texas Red) of Beckman Coulter, USA manufacturer were added in a cytometric test tube. The contents of the cytometric tube were mixed on a vortex and incubated for 20 minutes at a temperature of 18–20° C in a dark place. This stage provided staining of surface markers of T-lymphocytes. Next, T-lymphocytes were treated with Intra Prep Permeabilization Reagent (Immunotech) in accordance with the manufacturer's instructions, then intracellular Foxp3 staining (FITC) was performed with appropriate monoclonal antibodies (manufacturer eBioscience). The expression of the studied T-lymphocyte antigens as a result of multicolored staining was analyzed using a Cytomics FC 500 flow cytometer (Beckman Coulter). The results were presented as a percentage of positive cells. Cell populations were identified using the CXP software (Beckman Coulter, USA), based on the obtained parameters, the lymphoid region was isolated on a dot plot for the subsequent analysis of cells subpopulations with mandatory control of the lymphoid gate allocation purity by CD45 staining. At least 25 thousand events were counted in each studied sample. All the laboratory tests were carried out once. The informed consent for conducting a clinical trial and taking blood from a vein was obtained from parents of children under 15 years of age and from adolescents of 15 years and older.
The data was checked for the normality of the distribution by means of the Shapiro-Wilk test. As descriptive statistics for quantitative indices, the average ± mean square deviations, median and quartiles, minimum and maximum values in the sample were calculated. The comparison of medians in the groups was carried out according to the Kruskal-Wallis test (pairwise a posteriori comparisons were made according to the Nemenyi method). The differences were recognized as statistically significant at the level of p < 0.05. The calculations were performed in R (version 3.2, R Foundation for Statistical Computing, Vienna, Austria). The analysis of the bond strength was performed using correlation coefficients. The relationship between qualitative and quantitative (or ordinal) indices was analyzed using the Goodman gamma correlation. Also, correlations were recognized as statistically significant at the level of p < 0.05.
Results
An analysis of anamnestic data, clinical manifestations (urticaria, itching), UAS7 values found that a mild course of AU (UAS7 7 – 15 points) was observed in 36 children, moderate (UAS7 16–27 points) — in 139 children, severe AU (UAS7 28 – 42 points) — in 61 children, and CU of varying activity degrees was recorded in 28 children.
While determining the levels of the transcription factor FoxP3 in children with various urticaria variants, a significant decrease in its level was recorded in children with severe AU and CU compared to the control group of healthy children (1.23 ± 0.3%, median 1.37% in severe AU, 0.89 ± 0.084%, median 0.88% in CU; 4.08 ± 00.41%, median 4.15% in the control group).
The FoxP3 index in all the studied and in the control groups did not show a statistically significant difference from the normal distribution law (the Shapiro-Wilk test). Thus, one can assume the normality of the distribution according to the indicator of the functioning of the immune system “FoxP3” (Table 1).
Table 1
Descriptive statistics of the FOXP3 index in groups of children with acute and chronic urticaria
|
Indices/Form of urticaria |
Average± standard deviation |
Median |
Quartile |
Minimum value |
Maximum value |
Shapiro-Wilk test |
|
CD4+CD25+ Foxp3+ CD45+ with a light current of AU, % |
2.81±0.32 |
2.75 |
[ 2.59; 2.87] |
2.48 |
3.36 |
0.003 |
|
CD4+CD25+ Foxp3+ CD45+ with a moderate current of AU, % |
1.89±0.23 |
1.97 |
[ 1.77; 2.08] |
1.54 |
2.21 |
0.002 |
|
CD4+CD25+ Foxp3+ CD45+ with a severe course of AU, % |
1.23±0.3 |
1.37 |
[ 1.02; 1.47] |
0.73 |
1.73 |
0.08 |
|
CD4+CD25+ Foxp3+ CD45+ in CU, % |
0.89±0.084 |
0.88 |
[ 0.85; 0.93] |
0.74 |
1.04 |
0.24 |
|
CD4+CD25+ Foxp3+ CD45+ in the control group, % |
4.08±0.41 |
4.15 |
[ 3.87; 4.35] |
3.25 |
4.83 |
1 |
When comparing the levels of the FoxP3 index in children in pairs using the Nemenyi method, statistically significant differences were found in different variants of the urticaria course from children of the control group (Table 2, Fig. 1).
Table 2
Levels of statistical significance for comparing the medians of the CD4+CD25+ Foxp3+ CD45 index + in pairs in groups (CONTROL, Mild AU, Moderate AU, Severe AU, CU)
|
|
Control & Mild AU |
Control & Moderate AU |
Control & Severe AU |
Control & CU |
Mild AU & Moderate AU |
|
CD4+CD25+ Foxp3+ CD45+ |
0.6 |
0.001 |
<0.0001 |
<0.0001 |
0.2 |
|
|
Mild AU & Severe AU |
Mild AU & CU |
Moderate AU & Severe AU |
Moderate AU & CU |
Severe AU & CU |
|
CD4+CD25+ Foxp3+ CD45+ |
<0.0001 |
<0.0001 |
0.004 |
<0.0001 |
0.2 |
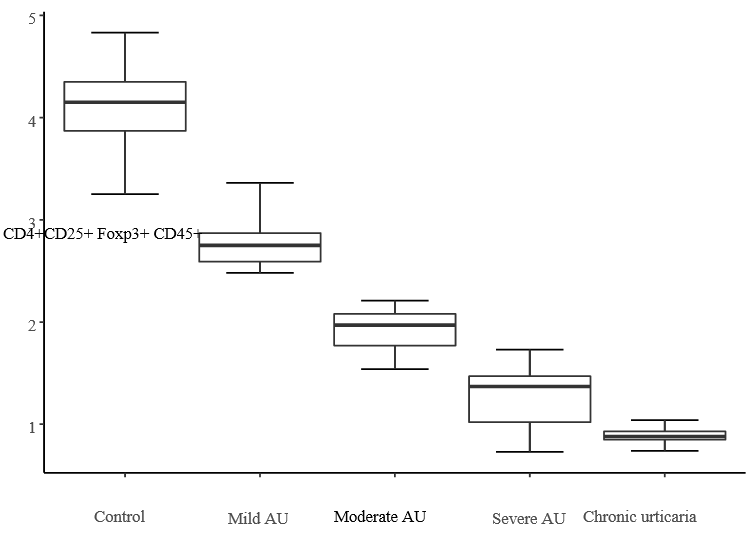
Figure 1. Span chart for “FoxP3, %”.
Statistically significant differences in the FoxP3 indices were found in the following groups of examined children: “Control/Moderate AU”, “Control/Severe AU”, “Control/CU”, “Mild AU/Severe AU”, “Mild AU/CU”, “Moderate AU/Severe AU”, “Moderate AU/CU”.
Further, in the course of the work, the relationship between the level of the FoxP3 index and the probability of developing a severe urticaria course was evaluated (Table 2).
Table 3
Statistically significant correlation between the studied indices
|
The first index |
The second index |
Correlation coefficient |
Significance level, p |
|
CD4+CD25+ Foxp3+ CD45+ |
Severity of urticaria |
0.97 |
<0.0001 |
As can be seen from Table 3, a significant strong relationship was found between the variables “CD4+CD25+ Foxp3+ CD45+” and “Severity of urticaria”.
Discussion
Thus, this work shows that all the patients with AU and CU are characterized by a significant decrease in the proportion of CD4+CD25+ Foxp3+ CD45+ in peripheral blood, which probably causes a decrease in their functional activity. At the same time, there is a statistically significant relationship between the level of the Foxp3 index and the urticaria severity.
Conclusion
- It was established for the first time that children with AU and CU were characterized by a decrease in the peripheral blood of functionally active CD4+CD25+ Foxp3+CD45+.
- It was determined that the reduction degree in the amount of FoxP3 affected the likelihood of developing a more severe course of AU and possible disease chronization.
- For the first time, FoxP can be proposed as an immunological index as a predictor of the severe course of AU in children and its transformation into a chronic form.
References
1. Goryachkina LA, Nenasheva NM, Borzova EYU. Urticaria. Attending physician. 2003;(9):43-45. (In Russ)
2. Federal clinical guidelines for the care of children with urticaria. Union of pediatricians of Russia; 2015. (In Russ).
3. Yalovega G.E., Lebedenko A.A., Mal’tsev S.V., Kalmykova T.S., Averkina L.A., et al. Features of the microelement status in children with acute urticaria. Pediatric pharmacology. 2016;13(2):101-104. (In Russ.). DOI: 10.15690/pf.v13i2.1550
4. Malcev S.V., Sizyakina L.P., Lebedenko A.A. Features of adaptive and innate immunity in children with diff erent variants of acute urticarial. Cytokines and infl ammation. 2014;(3): 117-118. (In Russ). eLIBRARY ID: 22840551
5. Sizyakina LP, Andreeva II. Handbook of clinical immunology. Rostov-on-Don; 2005. (In Russ).
6. Sizyakina L.P., Lebedenko A.A., Malcev S.V., Posevina A.N., Averkina L.A. Murticaria in children: a modern view on the problem. Medical Herald of the South of Russia. 2015;(4):5-13. (In Russ.) DOI: 10.21886/2219-8075-2015-4-5-13
7. Lin YR, Liu TH, Wu TK, Chang YJ, Chou CC, Wu HP. Predictive factors of the duration of a fi rst-attack acute urticaria in children. Am J Emerg Med. 2011;29(8):883-9. DOI: 10.1016/j.ajem.2010.04.004
8. Mathur AN, Mathes EF. Urticaria mimickers in children. Dermatol Ther. 2013;26(6):467-75. DOI: 10.1111/dth.12103.
9. Mineev V.N., Sorokina L.N., Eremeeva A.V., Nema M.A., Bedenko A.S. Pathogenetic role of cooperative interactions of transcription factors FoxP3, GATA-3, PAX-5 in bronchial asthma. Medical immunology. 2013;(4):303-312. (In Russ). eLIBRARY ID: 20143594
10. Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148(1):53-63. DOI: 10.1111/j.1365-2249.2007.03329.x
11. Vale-Pereira S, Todo-Bom A, Geraldes L, Schmidt-Weber C, Akdis CA, Mota-Pinto A. FoxP3, GATA-3 and T-bet expression in elderly asthma. Clin Exp Allergy. 2011;41(4):490-6. DOI: 10.1111/j.1365-2222.2010.03640.x
12. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, et al. EAACI/GA(2)LEN/EDF/WAO guideline: defi nition, classifi cation and diagnosis of urticaria. Allergy. 2009;64(10):1417-26. DOI: 10.1111/j.1398-9995.2009.02179.x
About the Authors
S. V. MaltsevРоссия
Stanislav V. Maltsev, Сand. Sc. (Med.), associate Professor, head of pediatric Department of clinic
Rostov-on-Don
L. P. Sizyakina
Россия
Lyudmila P. Sizyakina, Dr. Sc. (Med.), Professor, head of the Department of clinical immunology and Allergology
Rostov-on-Don
A. A. Lebedenko
Россия
Alexander A. Lebedenko, Dr. Sc. (Med.), Professor, head of the Department of children’s diseases №2
Rostov-on-Don
Review
For citations:
Maltsev S.V., Sizyakina L.P., Lebedenko A.A. The role of transcription factor FoxP3 as a predictor of acute and chronic urticaria in children. Medical Herald of the South of Russia. 2021;12(3):50-54. (In Russ.) https://doi.org/10.21886/2219-8075-2021-12-3-50-54
JATS XML






































