Scroll to:
Complex therapy for patients with ankylosing spondylitis with mixed anxiety–depressive disorder
https://doi.org/10.21886/2219-8075-2021-12-1-38-45
Abstract
Objective. The study aimed to evaluate the effect of therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) in combination with melatonin on the dynamics of the quality of life (QOL), clinical and laboratory activity, and mixed anxiety– depressive disorder (MADD) in patients with ankylosing spondylitis (AS).
Materials and methods. The study involved 65 patients with AS and RTDS. Patients from Group I (n=32) were prescribed melatonin at a dose of 3 mg per day at night, 30–40 minutes before bedtime, in addition to standard AS therapy. Patients from Group II (n = 33) received standard therapy. 8 weeks after, the dynamics of QOL indicators, clinical and laboratory activity, and the severity of MADD in patients from both groups were evaluated.
Results. The application of melatonin in addition to standard therapy in patients with AS and MADD provided a statistically significant improvement in the functional and clinical-laboratory data (frequency and severity of anxiety and depression, pain syndrome indicators, ESR, CRP, integrative indicators of physical and psychological components of health).
Conclusion. The application of melatonin in complex therapy for patients with AS and MADD contributes to the improvement of clinical and laboratory parameters, psychoemotional state, and QOL of this category of patients.
For citations:
Blaginina I.I. Complex therapy for patients with ankylosing spondylitis with mixed anxiety–depressive disorder. Medical Herald of the South of Russia. 2021;12(1):38-45. https://doi.org/10.21886/2219-8075-2021-12-1-38-45
Introduction
The mutual effects of autoimmune inflammations and mental disorders are of interest for rheumatology today. To date, different rheumatological diseases are associated with a 7 to 15 times higher incidence of mixed anxietydepressive disorders (MADDs) compared to the general population; this trend is reflected in many research papers, most of which deal with rheumatoid arthritis and systemic lupus erythematosus [1][2].
Chronic pain syndrome associated with an immunoinflammatory process has been found to be linked to a higher incidence of comorbidities that exacerbate the underlying disease, including MADDs. Constant pain alters the psycho-emotional status of patients, giving such manifestations as anxiety, depression, apathy, fatigue, excitability, and irritability [3][4]. The conventional factors of risk of depression are: female sex, family history, social deprivation, lack of social support, chronic psychosocial stress, chronic pain, low work performance, negative thoughts about the disease found in autoimmune patients; these factors do contribute to MADDs.
There is no doubt to date that chronic inflammation is the primary pathophysiological mechanism for the development of psycho-emotional disorders including depression, the latter being considered a systemic disease associated with a higher level of inflammatory reaction markers: C-reactive protein (CRP), TNF-alpha, interleukin-1, and interleukin-6 [5][6].
Mutual pathogenesis theory suggests that immunoinflammatory diseases and MADDs have common triggers, in particular stress factors, and mostly similar pro-inflammatory pathogenesis, which means they can co-progress [7][8]. Pro-inflammatory cytokines activate the hypothalamic-pituitary-adrenal axis (HPA axis) and, combined with the effects of stressors, stimulate another release of cytokines sensitizing the HPA axis, which worsens the vegetative support of the body and disrupts its adaptive capacities, thus exacerbating the endocrine dysfunction that regulates the stress and immune responses of the body; this increases the level of pro-inflammatory cytokines, giving such clinical manifestations as poor mood, chronic pain, fatigue, and sleep disturbances [9][2].
Some research points to a high incidence and severity of MADDs in patients suffering seronegative spondyloarthritis, in particular psoriatic spondyloarthropathies and ankylosing spondylitis (AS) [10, 11]. Some studies cover the causes of psychoemotional disorder development in AS males [12]. To date, however, drug-based treatment of AS+MADD patients remains an understudied topic.
Immune inflammation and MADDs have common pathogenic mechanisms in such patients; this means a modified approach is needed to treat AS coupled with MADD. Melatonin is commonly used to correct circadian rhythms; however, it has other proven clinical effects and seems a promising solution to the problem above. A broad range of melatonin effects (chronobiotic, antioxidant, cytoprotective, analgesic, and antiapoptotic effects) have been studied so far. However, it has less studied yet no less important peripheral antiinflammatory effects associated with inhibiting COX2 and NO synthase [13]. Clinical trials prove the medication to be an antidepressant as well [14][15]. Noteworthy is the ability of melatonin to inhibit matrix metalloproteinase as found in patients with rheumatoid arthritis [16].
The objective hereof was to study how nonsteroidal anti-inflammatory drugs (NSAIDs) coupled with melatonin would affect the quality of life, clinical and laboratory test results, and MADDs in AS patients.
Materials and Methods
The study involved 65 AS patients, aged 25 to 58 (43.4±7.7), 41 males and 26 females, who on average had had the disease for 10.1 ± 4.6 years; 25 had university education. Thirty-four (52.3%) patients had disabilities: 21 (61.8%) with Cat. 3 disabilities, and 13 (38.2%) with Cat. 2 disabilities, mostly with Functional Class 3 disorders (69.2%). AS was diagnosed on the basis of modified New York criteria (ACR, 1984); the activity of the pathological process was estimated by the Bath AS disease activity index (BASDAI) according to the EULAR criteria. All the involved patients were registered to have the core diagnostic signs of mixed anxiety-depressive disorder (F41.2): depressed mood, loss of interest and pleasure, anxiety and unrest, as well as some additional symptoms: sleep disturbances and loss of appetite, impaired concentration, tension and fussiness, tremor, irritability, low libido. All the patients were offered psychiatric assistance since antidepressant treatment must be prescribed and monitored by a psychiatrist. However, most of the patients rejected partner treatment, i.e., co-treatment by the attending physician and a psychiatrist. For this reason, the researchers developed an alternative treatment relying on melatonin, as it has a clinically proven pleiotropic antidepressant effect [14][15].
Inclusion criteria were: informed consent, verified AS diagnosis, AS lasting at least three years, and no diagnosed central nervous system disorders that might result in MADDs.
Pain syndrome, the duration of morning stiffness, and the patient’s own health (POH) were evaluated by the patients themselves on the visual analog scale (VAS); laboratory readings were CRP and the erythrocyte sedimentation rate (ESR).
Spielberger’s State-Trait Anxiety Inventory was used to assess the psycho-emotional state of the patients with scores below 30 being classified as mild anxiety, 30 to 45 as moderate anxiety, and >45 as severe anxiety. Hamilton Depression Scale (HDS) was used to detect signs of depression; the total score of 16 to 18 in young patients, 18 to 20 in elderly patients would indicate a non-psychotic depression. Taylor Manifest Anxiety Scale (TMAS) adapted for Russian patients by Nemchinov was used to assess anxiety. The questionnaire consisted of 50 yes/no questions. The score would depend on the patient’s answers. The interpretation guideline was as follows: 0 to 5 points for mild anxiety; 5 to 15 for moderate-to-mild anxiety; 15 to 25 for moderate-tosevere anxiety; 20 to 40 for severe anxiety; 40 to 50 for very severe anxiety.
Quality of life (QoL) was tested using the Medical Outcomes Study Short Form (SF-36), a questionnaire that covers 8 concepts or scales of health: physical functioning, PF; role-physical functioning, RP; bodily pain, BP; general health, GH; vitality, VT; social functioning, SF; role-emotional, RE; and mental health, MH. SF-36 generally helps evaluate the physical status of health (PSH) and mental status of health (MSH). Each of the 8 scales is to be rated 0 to 100; the higher the score, the better the patient’s status on the given scale [17].
Statistical testing of the results was done in Statistica 10.0 (Statsoft, USA). Normally distributed data is given herein as mean ± standard deviation (m±σ), else as Me (LQ-UQ), where Me is the median, LQ is the lower quartile, UQ is the upper quartile. To compare quantitative readings in groups before and after treatment, the authors used Wilcoxon’s test; to compare two independent groups – Mann-Whitney’s nonparametric U-test. Qualitative data was analyzed by means of contingency tables and χ2. Differences were deemed significant at p < 0.05.
Results
Low activity was found in 17 patients, moderate activity in 32, and high activity in 16 at the beginning of this study. BASDAI averaged 3.9 (3.3; 4.3), being >4 in 40% of the patients and >7 in 4.6%. The Bath Ankylosing Spondylitis Functional Index (BASFI) was 4.1 (3.6; 4.6), being >4 in 52.3% of all cases and below 2 in 3.6%. ESR averaged 25.9 ± 8.7 mm/h, being normal in 17 (30.4%) patients. CRP averaged 19.7 ± 20.3 mg/l; being above 10 mg/l in 35 (53.8%) and above 50 mg/l in 7.7%.
Pain syndrome and morning stiffness in AS patients were as follows: 61.2±18.0 for spine pain, 61.6±17.6 mm for morning stiffness, 60.9±17.2 mm for POH. The severity of MADDs in the patients was as follows: HDS 17 for depression (14; 19), indicating non-psychotic depression in this age group; one Spielberger’s scale, RA 32 (29; 37) and PA 40 (35; 44), 28 on Taylor’s scale (19; 36), generally indicate moderately severe anxiety in the patients.
SF-36 QoL ratings were low both for the overall physical component score (PSH), which was 28.6 (26.6; 32.7), and for the overall mental component score (MSH), which was 39.2 (34.1; 42.9). PSH dropped seemingly due to a low RP of 25 (0; 25), reflecting the effects of the patient’s physical status on their daily life, as well as the effects of their pain syndrome (BP) rated at 32 (22; 32). As for the mental component, they preserved relatively high SF values of 50 (37.5; 62.5) but had a low RE of 33.3 (33.3; 66.6) due to their emotional state.
Given the objectives hereof, all the patients were randomly sampled into two groups: Group 1 consisted of 32 patients who were treated throughout the study with an NSAID dosed equivalently to 150 mg of diclofenac (400 mg of celecoxib, 200 mg of nimesulide, 90 mg of etoricoxib, 200 mg of ketoprofen, or 200 mg of aceclofenac a day) plus 3 mg of melatonin 30 to 40 minutes before bedtime; Group II consisted of 33 patients treated the same way but without melatonin. As expected, the initial clinical and laboratory indicators of the patients did not differ significantly between the two groups (p > 0.05 in all cases).
Treatment was evaluated for effectiveness 8 weeks later using all the tested indicators (clinical and laboratory test results, anxiety, depression, and SF-36 scores). No adverse effects were reported throughout the study that would require canceling melatonin, such as morning drowsiness, fatigue, nightmares, or headaches.
All the examined patients had sleep disorders of varying nature and severity as of the start of the study. Shorter sleep was reported for 68.7% of the patients in Group I, 66.7% in Group II; nearly all the patients needed more time to fall asleep: 90.6% in Group I and 94% in Group II. Dissatisfaction with night sleep due to frequent awakenings was reported by 62.5% of the patients in Group I, 66.7% in Group II; postsleep fatigue in the morning was reported by 78.1% in Group I, 72.7% in Group II. The therapy made the sleep disorders milder and less frequent in Group I: only 21.8% still slept insufficiently long (χ2= 5.4; p = 0.02); 40.6% still reported that they needed more time to fall asleep (χ2 = 3.8; p = 0.056); sleep dissatisfaction reporting fell to 28.1% (χ2= 2.9; p = 0.08), and morning fatigue only occurred in 31.3% (χ2= 4.2; p = 0.039). No positive trends were registered in Group II. Sleep dissatisfaction rates dropped only insignificantly (χ2 = 0.6; p = 0.43), and so did post-sleep fatigue (χ2 = 1.0; p = 0.32), both still reported by 48.5% in Group II.
At the end of the study, patients on melatonin had very significantly lower (p < 0.001) incidence and severity of depression per HDS, anxiety per TMAS, RA and PA. A significant reduction in PA and TMAS scores was found in Group II. Post-treatment comparison of the groups shows a significant (p < 0.05) reduction in anxiety in Group I. See Table 1 for the data.
Table 2 shows the laboratory indicators and pain syndrome data collected from both groups before and after treatment. These indicators had positive trends in both groups. Notably, Group I had a more significant reduction in BASDAI, BASFI, and spine stiffness than Group II. However, these differences were not significant. Post-treatment comparison in terms of POH, which had positive dynamics in both groups, revealed a significant difference (p = 0.025) in favor of Group I. This proves melatonin affects patients’ emotions and personality. ESR and CRP improved significantly in both groups. Notably, Group I still had a greater improvement in both at p = 0.075 and p = 0.15, respectively.
The SF-36 questionnaire was used before and after treatment and showed that in Group I, physical health improved mainly in terms of the effects of physical condition and pain intensity on daily life as indicated by a 35.7% increase in PF, 22.6% increase in BP. Vitality, social and role functioning improved by 44%, 23%, and 49.6%, respectively, due to melatonin-associated mildening of depressive and psychovegetative disorders. These results are supported by the significant improvement in the integrative physical and mental components in Group I. In Group II, significant improvement was observed with respect to pain intensity and overall physical health. Post-treatment intergroup comparison showed significantly higher vitality mental component in melatonin-treated patients. See Table 3 for the data.
Таблица / Table 1
Динамика показателей тревоги и депрессии в группах
Dynamics of indicators of anxiety and depression in groups
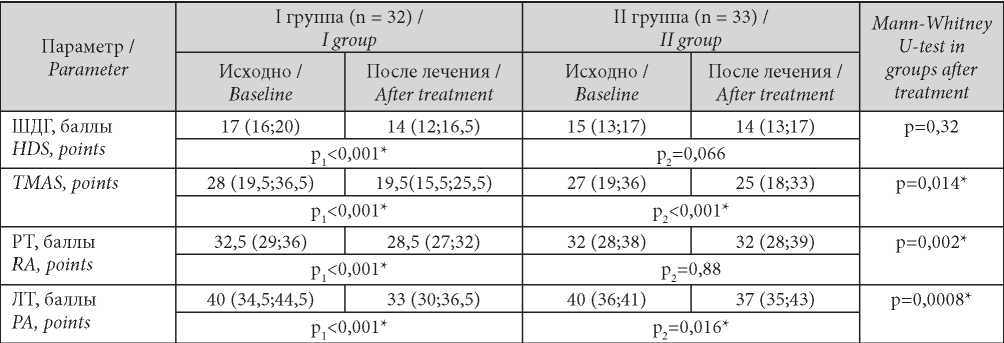
Note: * — statistical significance of the differences (p ≤ 0.05); р1 — significance of differences when comparing indicators before and after treatment of the I group; р2 — significance of differences when comparing indicators before and after treatment of the II group.
Примечание: * —показатели, при сравнении которых получены статистически значимые результаты (р ≤ 0,05); р1 — уровень статистической значимости различий при сравнении показателей до и после лечения I группы; р2 — уровень статистической значимости различий при сравнении показателей до и после лечения II группы.
Таблица / Table 2
Динамика показателей болевого синдрома и клинико-лабораторной активности в группах
Dynamics of indicators of pain syndrome and clinical and laboratory activity in groups
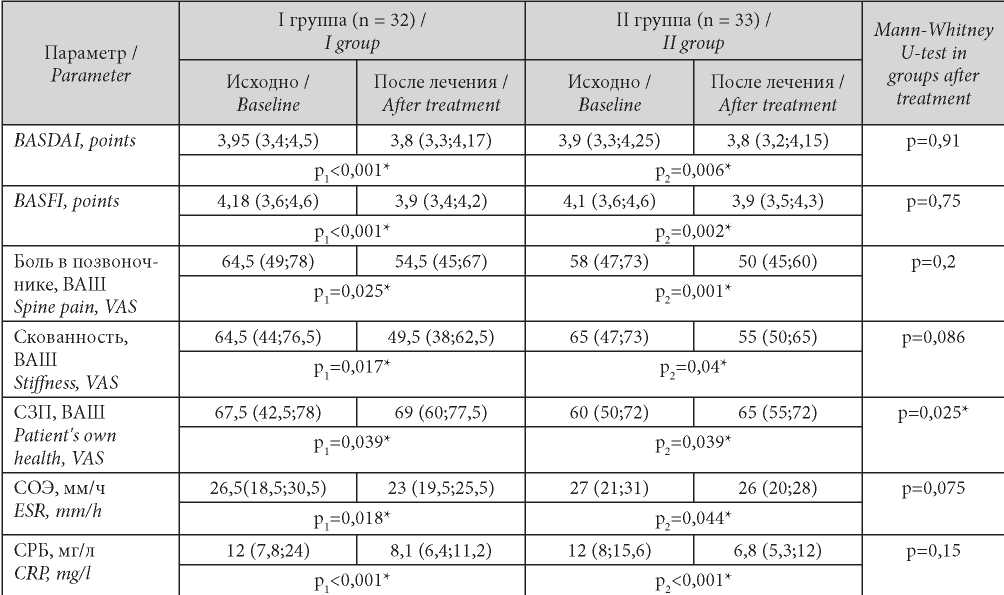
Note: * — statistical significance of the differences (p ≤ 0.05); р1 — significance of differences when comparing indicators before and after treatment of the I group; р2 — significance of differences when comparing indicators before and after treatment of the II group.
Примечание: * — знаком отмечены показатели, при сравнении которых получены статистически значимые результаты (р ≤ 0,05); р1— уровень статистической значимости различий при сравнении показателей до и после лечения I группы; р2 — уровень статистической значимости различий при сравнении показателей до и после лечения II группы.
Таблица / Table 3
Динамика показателей качества жизни в группах
Dynamics of indicators of quality of life in groups
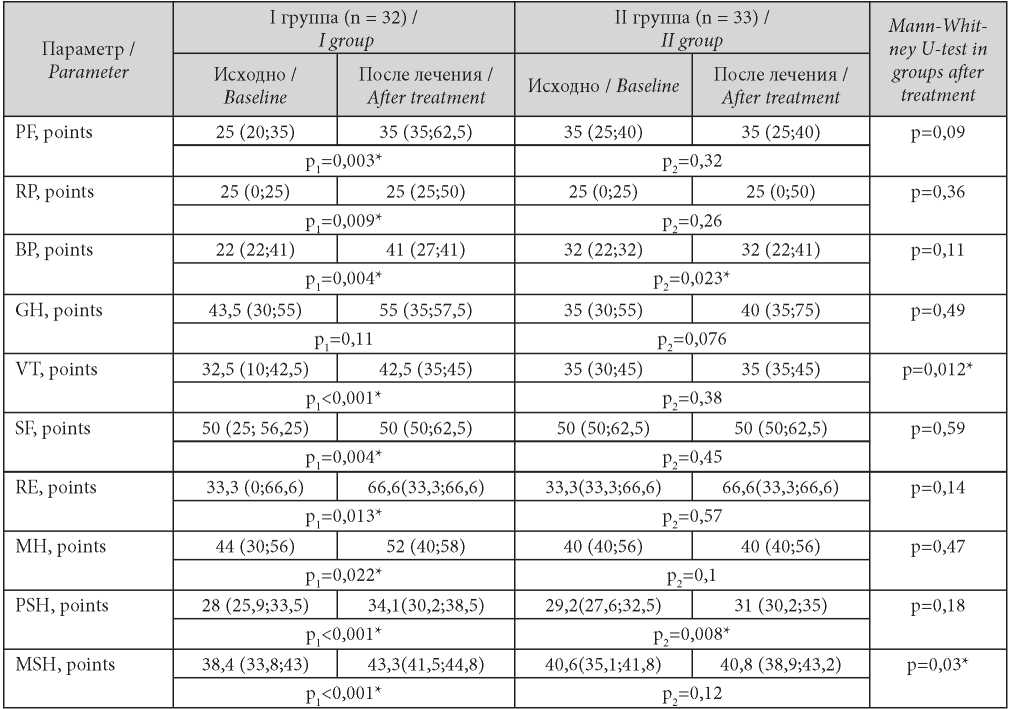
Note: * — statistical significance of the differences (p ≤ 0.05); р1 — significance of differences when comparing indicators before and after treatment of the I group; р2 — significance of differences when comparing indicators before and after treatment o the II f group.
Примечание: * — знаком отмечены показатели, при сравнении которых получены статистически значимые результаты (р ≤ 0,05); р1— уровень статистической значимости различий при сравнении показателей до и после лечения I группы; р2 — уровень статистической значимости различий при сравнении показателей до и после лечения II группы.
Discussion
Immuno-inflammatory diseases and MADDs have to date been proven to have similar pathogenic mechanisms based on response to stress that triggers the HPA axis, resulting in increased catecholamine and cortisol levels, as well as in serotoninergic and noradrenergic deficiency [18]. Therefore, exposure to stress disrupts the homeostasis of neuroendocrine and immune systems, intensifying pain, activating negative perception of reality, and contributing to disadaptive behavior. Besides, constant pain and lack of adaptation to the disease, both being characteristic of AS+MADD patients, elevate the pain even further to close the ‘vicious circle’.
Treatment of these patients should seek clinical and laboratory remission by inhibiting inflammation, mitigating pain, and improving the physical and mental QoL for better psycho-emotional stability.
Melatonin has a broad range of clinical effects listed herein whilst also having no severe side effects for up to 3 months of continuous administration; this allowed using it in addition to NSAIDs in AS+MADD patients that had clinical signs of anxiety-depressive disorders.
Findings suggest that this combined therapy not only improves the psycho-emotional status of patients but also stabilizes the QoL indicators and thus speeds up recovery from chronic pain and intense inflammation in AS+MADD patients.
Conclusions
Adding melatonin to the comprehensive treatment of AS in MADD-affected patients not only improves the emotional indicators such as incidence and intensity of sleep disturbances and anxiety-depressive disorders but also alleviates bodily pain, improves laboratory test results, and therefore enhances the quality of life of such patients.
References
1. Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int. J. Clin. Rheumtol. 2011;6(6):617-23. DOI: 10.2217/IJR. 11. 6
2. Lisitsyna T.A., Veltischev D.Yu., Nasonov E.L. Stress factors and depressive disorders in rheumatic diseases. Scientific and practical rheumatology. 2013;51(2):98-103. (In Russ.) DOI: 10.14412/1995-4484-2013-634
3. Barulin A.E., Kurushina O.V., Kalinchenko B.M., Chernovolenko E.P. Chronic pain and depression. Medicinal Herald. 2016;10(1):3-10. (In Russ.) eLIBRARY ID: 27316712
4. Miller AH, Raison ChL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology. 2016;16:22-34. DOI: 10.1038/nri.2015.5
5. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171-86. DOI: 10.1097/PSY.0b013e3181907c1b
6. Schmidt FM, Lichtblau N, Minkwitz J, Chittka T, Thormann J, et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J Psychiatr Res. 2014;55:29-34. DOI: 10.1016/j.jpsychires.2014.04.021
7. Lisitsyna T.A., Veltischev D.Yu., Krasnov V.N., Nasonov E.L. Clinical and pathogenetic relationships of immuneinflammatory rheumatic diseases and mental disorders. Clinical Medicine. 2014;92(1):12-20. (In Russ.) eLIBRARY ID: 21179822
8. Abbott R, Whear R, Nikolaou V, Bethel A, Coon JT, et al. Tumour necrosis factor-α inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res. 2015;79(3):175-84. DOI: 10.1016/j.jpsychores.2015.04.008
9. Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems --2011 Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37(3):307-16. DOI: 10.1016/j.psyneuen.2011.12.015
10. Filimonova O.G., Simonova O.V. Features of the quality of life and vegetative status in patients with psoriatic arthritis with anxiety-depressive disorders. Pharmateca. 2016;7:59–62. (In Russ.) eLIBRARY ID: 26010197
11. Afanasyeva T.Yu., Gimadeeva A.M., Afanasyeva M.A., Sukhorukova E.V., Abdulganieva D.I., Lapshina S.A. Assessment of the psychological state of patients with ankylosing spondylitis and its impact on the quality of treatment. Issues of organization and informatization of healthcare. 2016;S:36-38. (In Russ.) eLIBRARY ID: 29855318
12. Peláez-Ballestas I, Boonen A, Vázquez-Mellado J, ReyesLagunes I, Hernández-Garduño A, et al. Coping strategies for health and daily-life stressors in patients with rheumatoid arthritis, ankylosing spondylitis, and gout: STROBEcompliant article. Medicine (Baltimore). 2015;94(10):e600. DOI: 10.1097/MD.0000000000000600
13. Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, et al. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165(1-2):139-49. DOI: 10.1016/j.jneuroim.2005.05.002
14. den Boer JA, Bosker FJ, Meesters Y. Clinical efficacy of agomelatine in depression: the evidence. Int Clin Psychopharmacol. 2006;21 Suppl 1:S21-4. DOI: 10.1097/01.yic.0000195661.37267.86
15. Lôo H, Daléry J, Macher JP, Payen A. Etude pilote comparant en aveugle l’effet thérapeutique de deux doses d’agomélatine - agoniste des récepteurs de la mélatonine et antagoniste des récepteurs 5HT2c - chez 30 patients [Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatoninergic agonist and selective 5HT2C receptors antagonist, in the treatment of major depressive disorders]. Encephale. 2002;28(4):356-62. (In French). PMID: 12232545.
16. Rudra DS, Pal U, Maiti NC, Reiter RJ, Swarnakar S. Melatonin inhibits matrix metalloproteinase-9 activity by binding to its active site. J Pineal Res. 2013;54(4):398-405. DOI: 10.1111/jpi.12034
17. Law L, Beckman Rehnman J, Deminger A, Klingberg E, Jacobsson LTH, Forsblad-d’Elia H. Factors related to healthrelated quality of life in ankylosing spondylitis, overall and stratified by sex. Arthritis Res Ther. 2018;20(1):284. DOI: 10.1186/s13075-018-1784-8
18. Muslimova E.V. Strategies for overcoming chronic pain in rheumatoid arthritis. Practical Medicine. 2014;4(80):72-74. (In Russ.) eLIBRARY ID: 21845038
About the Author
I. I. BlagininaUkraine
Irina I. Blaginina, Cand. Sci. (Med.), associate professor; associate professor, Department of internal medicine, Faculty of Postgraduate Education
Luhansk, LPR
Review
For citations:
Blaginina I.I. Complex therapy for patients with ankylosing spondylitis with mixed anxiety–depressive disorder. Medical Herald of the South of Russia. 2021;12(1):38-45. https://doi.org/10.21886/2219-8075-2021-12-1-38-45







































